Laboratory medicine and precision medicine
The past decades have witnessed the dramatic changes in laboratory medicine. Laboratory medicine, initially focused on analytic technology in clinical laboratory, has now further been involved in the relationships between laboratory tests and diseases. Currently, laboratory medicine has extended their role in nearly every aspects of clinical practice and innovated the strategies for management of certain diseases, such as cardiovascular diseases and cancers.
What is laboratory medicine: from my point of view
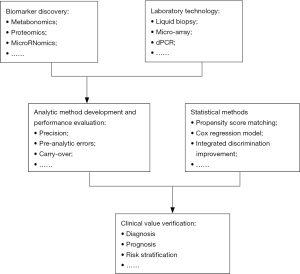
Overall, the laboratory medicine has a wide scope (Figure 1):
Upstream: to verify novel disease markers by using a variety of omics such as metabonomics, genomics, proteomics, and microRNAomics;
Midstream: this part belongs to the scope of traditional laboratory medicine, which includes the research, development and evaluation of various test assays; exploring the pre- and intra-analytical errors of a test, and establishing various test assays that are feasible for clinical application.
Downstream: in this stage, a variety of statistical methods are used to evaluate the clinical values of laboratory tests, including their roles in diagnosis, prognosis, and risk prediction.
Integration and refinement are two major characteristics of laboratory medicine. Laboratory medicine needs to integrate the conclusions from the translational research, chemistry and physics, to verify novel disease markers, and establish clinical feasible assays; meanwhile, by using cohort studies, cross-section studies, case-control studies and diagnostic accuracy test studies, the clinical values of laboratory tests are refined to different populations and thus to explore its values in the diagnosis, prognostic estimation, risk prediction, and treatment monitoring.
For a new marker, the translation from discovery to clinical application has to face a long journey. For instance, atrial natriuretic peptide (ANP) was discovered by de Bold et al. in 1981 (1). The subsequent studies demonstrated that peripheral blood ANP level was a potential marker for heart failure (2). However, there had been no breakthrough in the peripheral blood ANP detection technology for many years. Although some ANP detection techniques had been established (3,4), the detection performance was not satisfactory, mainly due to the short half-life of ANP in vitro. It was not until 2004 that a clinical feasible ANP detection method was established (5). This new technique used an antibody targeting a relatively stable segment in the middle region of the ANP, which is now known as the mid-regional pro-atrial natriuretic peptide (MR-proANP). Subsequently, many studies have evaluated the role of MR-proANP in the diagnosis and prognostication of heart failure (6-10). In the guidelines published by the European Society of Cardiology (ESC) in 2012 and 2016, MR-proANP is recommended as a diagnostic marker for heart failure (11,12).
However, not all of the markers can be as lucky as MR-proANP, and eventually appear in the disease guidelines. For many markers, even if the test assay has been clinical feasible, their values remain controversial. The interferon-gamma release assay (IGRA), for example, was once considered as a promising marker for tuberculosis; however, the results of some meta-analyses showed that its ability in diagnosing tuberculosis is inferior to traditional markers (13-15). Of course, for some markers, although proved to be with high clinical values, their imperfect analytical performance hampered their application in clinical practice. For example, although studies have confirmed that glial fibrillary acidic protein (GFAP) is a promising marker for differential diagnosis of cerebral hemorrhage and cerebral infarction (16,17), a point-of-care testing of GFAP is still not realized. Therefore, there is still a long way to go for its clinical application.
Laboratory medicine should advance the precise medicine
The current trends of laboratory medicine include: First, the new clinical values of traditional markers are constantly being verified. For example, traditionally the red cell distribution width (RDW) is a blood disease marker, mainly for the diagnosis of the cause of anemia; in recent 10 years, however, an increasing number of studies have found that RDW is a promising prognostic factor for cardiovascular diseases and cancers (18,19). Gamma-glutamyl transferase (GGT) is traditionally regarded as a test for evaluating liver function; however, a recent study has shown that it is associated with the prognosis of coronary heart disease (20). Second, new markers are emerging, providing new options for clinicians during disease management. For example, presepsin, a new marker of sepsis, has a high diagnostic value for sepsis (21,22). Circulating microRNA, as a marker of cancer, has also attracted the attention of clinicians (23). Third, the continuous improvement of detection technology, including the emerging of many high-throughput, low-cost tests (e.g., next-generation sequencing), has enabled us to obtain many detection results within a short time (24). Notably, in the management of certain diseases, if a disease marker is used to guide the interventions, the effectiveness is superior to the intervention strategy guided by clinical experiences. For example, studies have shown that in patients with acute upper respiratory tract infections, procalcitonin (PCT)-guided antibiotic therapies can significantly reduce antibiotic exposure in patients (compared with the empirical antibiotic treatment) without increasing the risk of death or treatment failure in patients (25). In another interesting study, for patients with dyspnea, if the clinicians diagnosed the heart failure based on clinical features (including ECG, chest X-ray, physical examination, and B-type natriuretic peptide), the diagnostic accuracy was inferior to the diagnosis made based on MR-proANP (26). These results urge us to rethink: were the clinical values of laboratory tests usually underestimated?
In future, when the new clinical values of biomarkers are constantly being revealed, new markers are emerging, and a large number of genetic or biological marker information becomes available within a short time, how can we utilize these information to manage the patients? In 2016, Google’s AlphaGo beat Go world champion Lee Sedol, which may provide us some inspiration (27). When there is a large number of laboratory test information available for clinicians to choose from, clinicians have been unable to interpret the clinical value of each test. Thus, we need to introduce the computer-assisted decision-making systems, which are based on machine learning. Just like alphaGo, the computer-assisted decision-making system can be directly input with data from laboratory information system. By using a certain algorithm, the system can provide the clinicians with the best conclusion about a disease diagnosis or intervention options. For example, for a patient presenting with acute headache, the system can calculate the risk and prognosis of ischemic cerebrovascular disease. Or, for an individual without any symptom, the probability of ischemic cerebrovascular disease within the coming 10 years can be calculated.
In my opinion, in addition to further improve the laboratory technology, the mission of the laboratory medicine is to further assess the clinical values of these laboratory markers; in other words, they will provide most primitive data for the algorithms of computer-assisted decision-making systems. Currently, clinical decisions are usually based on the disease guidelines supported by evidence-based medicine. Since the guidelines are not likely to cover every aspect of the disease, some decisions still need to be based on the personal experiences of clinicians. However, since all experience is subjective, the efficiency of the experience depends on an individual’s ability to sum up their work. While we have now entered the Big Data era (28-30), most of the data are from clinical laboratory. Consider this: if we can use Big Data to establish a reliable algorithm and continuously improve it, such a “real world” derived conclusion obviously has good clinical application; then, can it replace part of the role played by clinicians? If an alphaGo-like system is input with the laboratory test results of tens of thousands of patients presenting with dyspnea, will its capability in managing populations with dyspnea be inferior to an experienced clinician (27)? I believe the answer will be “No”. As mentioned before, in patients with acute respiratory tract infections, the use of antibiotics based on PCT has shown a good potential. In fact, this protocol can be easily addressed in a computer program. If we input the data of tens of thousands of patients with acute upper respiratory tract infection into an alphaGo-like computer and allow the computer program analyze and summarize these cases and find out the best scheme to guide antibiotic therapy, the computer is likely to output a similar treatment scheme as PCT guided therapy. Unfortunately, we have never tried to leave the chance of analyzing/summering these experiences to a computer.
Assessment of the prognosis and risk of a disease is the basis for developing intervention measures. In the current clinical practices, the disease classification is still not precise enough. For example, although clinical trials have demonstrated that a chemotherapy agent has better efficacy than conventional therapies in patients with stage II non-small-cell lung cancer, the problem is: it can only benefit a limited number of patients. It is therefore assumed that, for the same stage II non-small-cell lung cancer patients, if we could further divide these patients according to the huge amount of markers and genetic data, the treatment protocol might be further optimized and patients who could really benefit from the treatment might be identified. This is now known as the precision medicine in clinical oncology (31). Of course, the phenomenon that “(a specific treatment) benefit for a small proportion of patients” also exist in the interventions for patients with cardiovascular disease, metabolic disease, and autoimmune diseases; therefore, it is urgently needed that the precise treatment of these diseases should be promoted from the perspective of laboratory tests.
Therefore, the AME Publishing Company launched this new journal titled the Journal of Laboratory and Precision Medicine. I fully believe that, in an era of Big Data, we can make full use of the laboratory data to explore the mechanisms governing the development of diseases and to advance precision medicine.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2016.11.01). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Bold AJ, Borenstein HB, Veress AT, et al. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 1981;28:89-94. [Crossref] [PubMed]
- Hartter E, Weissel M, Stummvoll HK, et al. Atrial natriuretic peptide concentrations in blood from right atrium in patient with severe right heart failure. Lancet 1985;2:93-4. [Crossref] [PubMed]
- Missbichler A, Hawa G, Schmal N, et al. Sandwich ELISA for proANP 1-98 facilitates investigation of left ventricular dysfunction. Eur J Med Res 2001;6:105-11. [PubMed]
- Hartter E, Khalafpour S, Missbichler A, et al. Enzyme immunoassays for fragments (epitopes) of human proatrial natriuretic peptides. Clin Chem Lab Med 2000;38:27-32. [Crossref] [PubMed]
- Morgenthaler NG, Struck J, Thomas B, et al. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem 2004;50:234-6. [Crossref] [PubMed]
- Gegenhuber A, Struck J, Dieplinger B, et al. Comparative evaluation of B-type natriuretic peptide, mid-regional pro-A-type natriuretic peptide, mid-regional pro-adrenomedullin, and Copeptin to predict 1-year mortality in patients with acute destabilized heart failure. J Card Fail 2007;13:42-9. [Crossref] [PubMed]
- Mueller T, Dieplinger B, Gegenhuber A, et al. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem 2008;54:752-6. [Crossref] [PubMed]
- Dieplinger B, Gegenhuber A, Kaar G, et al. Prognostic value of established and novel biomarkers in patients with shortness of breath attending an emergency department. Clin Biochem 2010;43:714-9. [Crossref] [PubMed]
- Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010;55:2062-76. [Crossref] [PubMed]
- Eckstein J, Potocki M, Murray K, et al. Direct comparison of mid-regional pro-atrial natriuretic peptide with N-terminal pro B-type natriuretic peptide in the diagnosis of patients with atrial fibrillation and dyspnoea. Heart 2012;98:1518-22. [Crossref] [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Zhou Q, Chen YQ, Qin SM, et al. Diagnostic accuracy of T-cell interferon-γ release assays in tuberculous pleurisy: a meta-analysis. Respirology 2011;16:473-80. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Gupta D, et al. Interferon Gamma Release Assays for Diagnosis of Pleural Tuberculosis: a Systematic Review and Meta-Analysis. J Clin Microbiol 2015;53:2451-9. [Crossref] [PubMed]
- Yu J, Wang ZJ, Chen LH, et al. Diagnostic accuracy of interferon-gamma release assays for tuberculous meningitis: a meta-analysis. Int J Tuberc Lung Dis 2016;20:494-9. [Crossref] [PubMed]
- Sun Y, Qin Q, Shang YJ, et al. The accuracy of glial fibrillary acidic protein in acute stroke differential diagnosis: A meta-analysis. Scand J Clin Lab Invest 2013;73:601-6. [Crossref] [PubMed]
- Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 2012;58:237-45. [Crossref] [PubMed]
- Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 2011;50:635-41. [PubMed]
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86-105. [Crossref] [PubMed]
- Ndrepepa G, Braun S, Schunkert H, et al. Gamma-glutamyl transferase and prognosis in patients with coronary artery disease. Clin Chim Acta 2016;452:155-60. [Crossref] [PubMed]
- Zhang X, Liu D, Liu YN, et al. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care 2015;19:323. [Crossref] [PubMed]
- Zhang J, Hu ZD, Song J, et al. Diagnostic Value of Presepsin for Sepsis: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e2158 [Crossref] [PubMed]
- He Y, Lin J, Kong D, et al. Current State of Circulating MicroRNAs as Cancer Biomarkers. Clin Chem 2015;61:1138-55. [Crossref] [PubMed]
- van Nimwegen KJ, van Soest RA, Veltman JA, et al. Is the $1000 Genome as Near as We Think? A Cost Analysis of Next-Generation Sequencing. Clin Chem 2016;62:1458-64. [PubMed]
- Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012;55:651-62. [Crossref] [PubMed]
- Potocki M, Breidthardt T, Reichlin T, et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure. J Intern Med 2010;267:119-29. [Crossref] [PubMed]
- Zhang Z. When doctors meet with AlphaGo: potential application of machine learning to clinical medicine. Ann Transl Med 2016;4:125. [Crossref] [PubMed]
- Wang SD, Shen Y. Big-data Clinical Trial (BCT): the third talk. J Thorac Dis 2015;7:E243-4. [PubMed]
- Wang SD, Shen Y. Redefining big-data clinical trial (BCT). Ann Transl Med 2014;2:96. [PubMed]
- Wang SD. Opportunities and challenges of clinical research in the big-data era: from RCT to BCT. J Thorac Dis 2013;5:721-3. [PubMed]
- Millner LM, Strotman LN. The Future of Precision Medicine in Oncology. Clin Lab Med 2016;36:557-73. [Crossref] [PubMed]
Cite this article as: Hu ZD. Laboratory medicine and precision medicine. J Lab Precis Med 2016;1:2.