
Cardiac troponin testing in acute coronary syndrome: personalized thresholds or generalized cut-offs?
The paradigm of cardiac troponin testing
The diagnostics of the acute coronary syndrome (ACS) has engaged the minds of many clinicians and laboratory professionals for decades (1). The introduction of cardiac troponin testing and the further refinements of commercial immunoassays have revolutionized the diagnostic approach to this condition, since the measurement of these biomarkers by means of high-sensitivity immunoassay should now be regarded as the mainstay for early and efficient diagnosis of myocardial ischemia, as well as for safely ruling out ACS. As endorsed by most guidelines and recommendations (2-4), the most reliable and efficient strategy for using cardiac troponin in patients with suspected ACS is based on a dichotomous approach, entailing a baseline measurement followed by a second test, 1 to 3 hours thereafter, according to the value obtained on the former assessment (5). This strategy would allow to achieve an optimal degree of both sensitivity (i.e., in patients with values exceeding the lower diagnostic cut-offs) and specificity (i.e., by observing a characteristic kinetics of the biomarker concentration over time, which eventually reflects the presence of an acute and ongoing myocardial injury).
Most of us would still agree that serial measurement at fixed time points remains an unquestionably meaningful policy for increasing the diagnostic specificity of cardiac troponin testing. However, irrespective of the ongoing debate around the optimal timing of repeated testing, what has not been definitely clarified is whether the use of personalized thresholds may be superior over the use of generalized cut-offs in terms of diagnostic efficiency.
The enigma of the decision limit
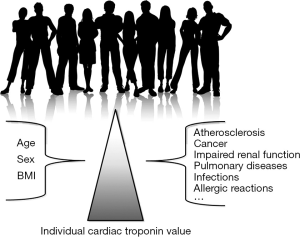
The enigma basically originates from the evidence that the concentration of cardiac troponins may be enhanced in many non-ischemic and extra-cardiac conditions, so that the use of the traditional Upper Reference Limit (URL; conventionally the 99th percentile) of a population of ostensibly healthy subjects may be inappropriate or even misleading (6). There are many doubts as to whether the use of the 99th percentile URL may be really suitable to be used as diagnostic threshold. Conventionally, the cardiac troponin URL is set on a population of healthy subjects (not less than 300). This aspect is quite problematic since “healthy” means all or nothing. The term “healthy” in cardiovascular risk assessment not only implies that the subjects are not suffering (or have not suffered) from myocardial ischemia, but also that they should be actually free from significant atherosclerotic involvement of the coronary tree, since atherosclerotic disease is a major determinant of cardiac troponin concentration (7). Even more importantly, dyslipidaemias can contribute themselves to increase the measurable value of circulating troponin (8). The presence and degree of coronary atherosclerosis can only be established with invasive or expensive and time-consuming imaging techniques (e.g., coronary angiography, cardiac magnetic resonance imaging), but the feasibility and ethical issues underlying these investigations in healthy subjects are questionable due to the unjustified iatrogenic risk. The suitable application in the real world scenario of an URL derived from a reference population of healthy subjects is also questionable. The incidence of myocardial ischemia increases in parallel with ageing and with the number of putative risk factors. The vast majority of patients with chest pain or other symptoms suggestive of ACS are aged 50 years or older and usually suffer from a number of comorbidities which may impact the actual cardiac troponin concentration (9). Therefore, the assumption that an URL calculated on healthy subjects could be efficiently used in patients with suspected myocardial ischemia can be seriously questioned.
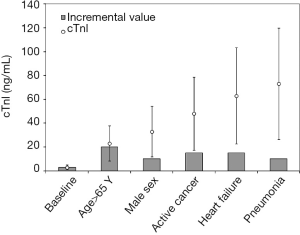
The conditions other than myocardial ischemia in which circulating cardiac troponins may be increased include “organ-specific” cardiac diseases such as myocarditis, pericarditis, heart failure, atrial fibrillation or other severe dysrhythmias, cardiac trauma and/or concussion, strenuous exercise, cardiotoxicity (e.g., chemotherapy or due to other drugs), amyloidosis, sarcoidosis and rheumatic diseases among others, as well as a kaleidoscope extra-cardiac pathologies which may trigger indirect cardiac injury (e.g., advanced cancer, impaired renal function and uraemia, chronic obstructive pulmonary disease, massive pulmonary embolism, cerebrovascular ischemia, severe systemic infections and/or sepsis, severe allergic reactions and/or anaphylaxis). The relative increase of both cardiac troponin T (cTnT) and I (cTnI) in patients with these conditions can be hardly predicted, since it mostly depends on the severity of the underlying condition, the general condition of the patient (i.e., sex, age, comorbidity, cardiac and renal function), the degree of local involvement of cardiac tissue, the timing of sampling (e.g., cardiac troponins also exhibit a circadian variation), the technique used for measuring cTnT or cTnI, and even the type of myocardial infarction (10). The various combination of these factors would make it hazardous to identifying a large number of cut-offs tailored according to the presence or absence of one or more of the physiological and pathological determinants. To put it simply, and assuming that the established inter-individual variability of cTnI is around 64%, the uncertainty of the personalized reference range is expected to grow in parallel with the measured value and with the number of potential determinants of increased concentration (Figure 1). As a paradigmatic example, the theoretical cTnI threshold of a 67-year old male patient with active cancer, heart failure and pneumonia, would raise from a virtually immeasurable value up to 73 ng/mL, with a confidence of interval (due to inter-individual variability) ranging between 26 and 120 ng/mL. This would make the use of personalized cut-offs rather unsafe due to the large (predicted) heterogeneity of cTnI variation and would also contribute to confuse clinicians, especially those working in frequently overcrowded and chaotic short stay units.
Searching for solutions
So, how can we deal with this issue? Indeed, the personalized approach does not appear to be suitable even when applied to the longitudinal monitoring of individual patient data. Incidentally, cardiac troponins are increased in advanced cancer (11) and in patients with impaired renal function (12). Yet, the individual value exhibit a dramatic variation among cancer patients and poorly correlates with the reduction of glomerular filtration rate (GFR), thus making it impossible to develop models, algorithms or nomograms that may accurately predict the impact of certain demographical or pathological variables on the measurable troponin concentration (Figure 2). The range of uncertainty would still remain so high, as in Figure 1, that no definitive conclusions could be made. So, is there a chance to get out of the maze? Yes, and the best solution is probably the simplest. There are at least two aspects that make the kinetics of cardiac troponin virtually unique in ACS. The former is the unquestionable assurance that the concentration of these biomarkers measured with high-sensitivity immunoassays is elevated above the limit of the detection (LOD) of the specific immunoassay in patients presenting with an ACS (13,14). Unlike using the 99th percentile URL, this cut-off would allow to reach up to 1.0 sensitivity and 1.0 negative predictive value at presentation, although the diagnostic efficiency would still be plagued by a considerable number of false positive results. Short-time serial testing, likely between 2–3 hours after baseline (this timing is dependent upon analytical and organizational issues), would then enable to identify a kinetics compatible with an acute myocardial ischemia in the setting of a high pre-test probability, which helps increase considerably the specificity and the positive predictive value (15).
Conclusions
Although the circle seems to be now closing, one should still consider that a significant gap remains between the number of acute myocardial infarctions (AMIs) “officially” diagnosed based on the International Classification of Disease (ICD)-Coding system and the number of AMIs diagnosed according to the currently accepted definition (16), thus paving the way to a paradoxical scenario for diagnosing AMI. By “reinventing the wheel”, we will get back when it all started, i.e., to a slightly modified definition of myocardial infarction as endorsed nearly 20 years ago by the European Society of Cardiology and the American College of Cardiology (17), according to which acute, evolving or recent myocardial infarction could now be defined as “an increased value of cardiac troponin (defined as a measurement exceeding the LOD) with a high clinical index of suspicion and/or a typical rise of cardiac troponin concentration in the following 2–3 hours”. Always remembering to include the definition in the ICD-Coding system.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2016.11.04). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cervellin G, Lippi G. Of MIs and men--a historical perspective on the diagnostics of acute myocardial infarction. Semin Thromb Hemost 2014;40:535-43. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Casagranda I, Cavazza M, Clerico A, et al. Proposal for the use in emergency departments of cardiac troponins measured with the latest generation methods in patients with suspected acute coronary syndrome without persistent ST-segment elevation. Clin Chem Lab Med 2013;51:1727-37. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Lippi G, Montagnana M, Aloe R, et al. Highly sensitive troponin immunoassays: navigating between the scylla and charybdis. Adv Clin Chem 2012;58:1-29. [Crossref] [PubMed]
- Laufer EM, Mingels AM, Winkens MH, et al. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol 2010;30:1269-75. [Crossref] [PubMed]
- Lippi G, Lo Cascio C, Brocco G, et al. High-density lipoprotein cholesterol values independently and inversely predict cardiac troponin T and I concentration. Ann Transl Med 2016;4:188. [Crossref] [PubMed]
- Goch A, Misiewicz P, Rysz J, et al. The clinical manifestation of myocardial infarction in elderly patients. Clin Cardiol 2009;32:E46-51. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Cervellin G. Cardiac troponins and mortality in type 1 and 2 myocardial infarction. Clin Chem Lab Med 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Danese E, Montagnana M, Giudici S, et al. Highly-sensitive troponin I is increased in patients with gynecological cancers. Clin Biochem 2013;46:1135-8. [Crossref] [PubMed]
- Lippi G, Cervellin G. High-sensitivity troponin T is more susceptible than high-sensitivity troponin I to impaired renal function. Am J Cardiol 2013;112:1985. [Crossref] [PubMed]
- Shah AS, Anand A, Sandoval Y, et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386:2481-8. [Crossref] [PubMed]
- Lippi G. Biomarkers: Novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol 2016;13:9-10. [Crossref] [PubMed]
- Galli C, Lippi G. High-sensitivity cardiac troponin testing in routine practice: economic and organizational advantages. Ann Transl Med 2016;4:257. [Crossref] [PubMed]
- Díaz-Garzón J, Sandoval Y, Smith SW, et al. Discordance between ICD-Coded Myocardial Infarction and Diagnosis according to the Universal Definition of Myocardial Infarction. Clin Chem 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-69. [Crossref] [PubMed]
Cite this article as: Lippi G, Cervellin G. Cardiac troponin testing in acute coronary syndrome: personalized thresholds or generalized cut-offs? J Lab Precis Med 2016;1:6.


