
Evaluation of preliminary screening strategies for human immunodeficiency virus: a single center experience
Introduction
Acquired immunodeficiency syndrome (AIDS) is a global infectious disease caused by the human immunodeficiency virus (HIV). As there is no effective preventative vaccine, early diagnosis is particularly important for its control (1). Laboratory diagnosis of HIV mainly relies on serological testing, which is suitable throughout the disease course, i.e., from the window period after initial HIV infection until the death of patients. HIV antibody detection is a routine serological testing strategy for HIV. Antibody-based tests are divided into screening and confirmatory tests (2-5). At present, the common HIV antibody-based clinical screening tests are enzyme-linked immunosorbent assay (ELISA) and point-of-care test. ELISA was the first antibody-based serological screening test for HIV, and since then ELISA reagents have progressed to the fourth-generation (4G). Most hospitals in developing countries currently use 3G ELISA reagents (5), which exhibits higher sensitivity and specificity than those of the previous two generations; however, there have been cases of misdiagnosis (5). The 4G ELISA builds on 3G ELISA by adding p24 antigen detection which increases its sensitivity for early detection of HIV infection in window period thereby ultimately reducing the risk of transmission (6-8). The 4G ELISA reagents have been gradually promoted for clinical testing, but their actual clinical performance is unknown. Comparative analyses were performed using the third-generation (3G) and 4G ELISA reagents with or without colloidal gold-immunochromatography assay (GICA) as well as confirmatory HIV western blot (WB) tests.
Methods
Study sample
This observational retrospective study received ethical approval from the Ethics Committee at the First Affiliated Hospital of Nanjing Medical University. Because this is a retrospective study, patients’ consents were waived. A retrospective analysis of 331,968 HIV screening samples which were collected at the First Affiliated Hospital of Nanjing Medical University in inpatient, outpatient and well visits from January 2010 to April 2014 was performed.
Sample collection and result interpretation
All samples were initially screened for HIV by the 3G (Kehua, China) or 4G (BioMerieux, France) ELISA and colloidal GICA kits (Xinchuang, China). According to manufacturer’s instructions, the result of a sample is given either as positive or negative, according to a signal-to-cutoff (S/CO) ratio. The cutoff based on the reagent instruction was defined. Samples having an S/CO in the range ≥0.90 to <1.0 were in the gray zone (borderline) and ≥1.0 were considered positive. All initially positive or borderline samples were retested in duplicate and repeatedly another sample and GICA. HIV results of individual samples were recorded after readings by two different laboratory personnel and according to manufacturer’s criteria for interpretation of positive or negative results.
The confirmatory tests pattern
Samples with at least two positive results were further confirmed with HIV WB analysis (MP Diagnostics, Singapore). In China, HIV-1 WBs are usually interpret by execution of the National Guideline for Detection of HIV/AIDS (2009 edition), which require detection of gp41 and gp120/160 (p24 and gp41/gp120/gp160) for positive results. HIV-associated bands that are present, but do not meet the criteria for positivity, are in indeterminate pattern.
Statistical analysis
The statistical software package SPSS17.0 (SPSS Inc., Chicago, USA) was used to perform Chi-square test to assess the difference between two generations of ELISA reagents. A P value of <0.05 was considered statistically significant.
Results
As shown in Figure 1, a total of 331, 968 subjects were enrolled and 330 subjects were found to be positive by the 3G ELISA kit (S/CO ≥1), and one subject was in grey-zone (S/CO =0.94). Besides, 3G ELISA resulted in one false negative test. These 332 subjects were further tested by the 4G ELISA kit, GICA and WB. Among 332 subjects who were tested positive by 3G ELISA kit, 189 subjects were confirmed to have HIV infection by WB, 14 were found to be in indeterminate range, and 129 subjects had negative test result. A total of 212 subjects were screened positive for HIV by 4G ELISA kit and 213 were screened positive by GICA testing.
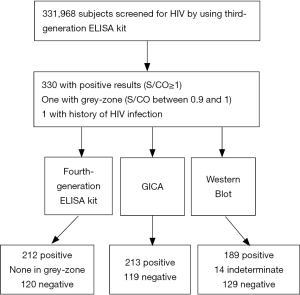
Comparison of HIV detection outcomes using 3G and 4G ELISA reagents
Significant differences in the confirmed positive rate (57.27% to 89.15%) were detected between the results of 3G & 4G ELISA reagents. In combination with GICA, the confirmed positive rate using the 4G ELISA reagents was 3.91% higher than what was obtained using the 3G ELISA reagents (Table 1)
Table 1
| Positive results, n | False positive, n | False negative, n | True positive (%) | |
|---|---|---|---|---|
| 3G ELISA | 330 | 141 | 1 | 57.27 |
| 4G ELISA | 212 | 23 | 0 | 89.15 |
| 3G ELISA + GICA | 213 | 24 | 0 | 88.73 |
| 4G ELISA + GICA | 204 | 15 | 0 | 92.64 |
HIV, human immunodeficiency virus; ELISA, enzyme-linked immunosorbent assay; GICA, gold-immunochromatography assay.
Distribution of S/CO values for positive screened samples
Table 2 lists the positive rate of HIV infection among subjects who were positive by 3G ELISA kit. Notably, for both 3G and 4G ELISA kits, the positive rates of HIV infection were significantly higher in subjects with S/CO more than 6 (P<0.01). The combination tests of GICA and 4G ELISA prevented the misdiagnosis observed by 3G ELISA.
Table 2
| S/CO range | n | Western blots (n=332) | ||
|---|---|---|---|---|
| Negative (n=129) | Unknown (n=14) | Positive (n=189) | ||
| 3G ELISA | ||||
| ≥6 | 162 | 7 (4.3%) | 7 (4.3%) | 148 (88.1%) |
| 1–5.99 | 168 | 122 (72.6%) | 6 (3.6%) | 40 (23.8%) |
| <1 | 2 | 0 (0%) | 1 (50%) | 1 (50%) |
| 4G ELISA | ||||
| ≥6 | 202 | 5 (2.5%) | 9 (4.5%) | 188 (93.1%) |
| 1–5.99 | 10 | 4 (40%) | 5 (50%) | 1 (10%) |
| <1 | 120 | 120 (100%) | 0 (0%) | 0 (0%) |
| GICA | ||||
| Positive | 213 | 13 (6.1%) | 11 (5.2%) | 189 (88.7%) |
| Negative | 119 | 116 (97.5%) | 3 (2.5%) | 0 (0%) |
| Combination tests | ||||
| 3G ELISA positive + 4G ELISA positive + GICA positive | 202 | 3 (1.5%) | 11 (5.4%) | 188 (93.1%) |
| 3G ELISA positive + 4G ELISA positive + GICA negative | 9 | 6 (66.7%) | 3 (33.3%) | 0 (0%) |
| 3G ELISA positive + 4G ELISA negative + GICA positive | 9 | 9 (100%) | 0 (0%) | 0 (0%) |
| 3G ELISA positive + 4G ELISA negative + GICA negative | 110 | 110 (0%) | 0 (0%) | 0 (0%) |
| 3G ELISA negative + 4G ELISA positive + GICA positive | 2 | 0 (0%) | 1 (50%) | 1 (50%) |
S/CO, signal-to-cutoff.
Two typical patients
Table 3 lists two subjects’ results with HIV infection but found to be in grey-zone or negative by 3G ELISA kit. Notably, both of them were found to be positive by the 4G ELISA kit and tested positive for p24 protein on WB.
Table 3
| Third generation (S/CO) | Fourth generation (S/CO) | Western blot | |
|---|---|---|---|
| Sample 1 | 0.94 | 1.81 | gp160, p24(±) |
| Sample 2 | 0.25 | 17.65 | gp160, gp120, p66, p55, p51, gp41, p39, p31, p24, p17 |
Discussion
Detection reagents are key factors affecting the accuracy of ELISA test. With the development of new technology, the detection of coated antigen in HIV ELISA reagents has evolved from the whole viral lysate in the first-generation reagents to the 3G double-antigen sandwich reagents based on gene recombination and peptide antigen labeling. The 4G ELISA test includes the addition of the p24 antigen detection system designed to detect the HIV p24 antigen and HIV-1/2 antibody. In comparison with the 3G reagents, the window period has been shortened to 5–6 days for 4G ELISA tests (6).
As shown in Table 1, the number of false-positive results was significantly lower for 4G ELISA reagents than for 3G ELISA reagents. Additionally, the confirmation rate for positive samples was significantly higher for the 4G ELISA than for 3G ELISA reagents. The difference between these two generations of reagents was statistically significant. When 3G ELISA was used in combination with GICA, the positive rate increased from 57.27% to 88.73%, and this rate was not significantly different from that obtained using the 4G ELISA reagents. Among 189 confirmed positive samples, 3G ELISA produced false-negative result for one sample, which indicates that the 3G reagents could yield inaccurate results (9,10). With respect to the distribution of S/CO values shown in Table 2, the difference between the 3G & 4G ELISA reagents for detection of HIV was significant. When GICA was used in conjunction with 3G ELISA reagents, errors in diagnosis of HIV were prevented.
As shown in Table 3, for sample 1, 3G ELISA reagent yielded a highly negative S/CO value, while 4G ELISA reagent yielded a low, positive S/CO value. The WB confirmatory test showed the presence of a p24 protein band, suggesting a low specificity of the HIV antibody. Since, 3G ELISA reagents can only detect antibodies, S/CO <1 was obtained. The addition of p24 antigen detection in 4G ELISA improved its detection sensitivity resulting in S/CO >1 and increased sensitivity is beneficial for a screening test. Sample 1 was a young male who was treated at the Department of Infectious Diseases for fever of unknown origin. His serum CD4+ T-lymphocytes concentration was 434 cells/µL. In early stages of HIV infection amount of HIV antibody could remain below the detection level irrespective of viral titer (11,12). Sample 2 was a middle-aged homosexual male, who was being treated for tuberculosis. His serum CD4+ T-lymphocytes concentration was 90 cells/µL. However, 3G ELISA reagents yielded a low, negative S/CO value for this patient. During the treatment period, this patient was repeatedly tested at different times in order to exclude human and accidental errors. The 4G ELISA reagents yielded a high, positive S/CO value and WB confirmed that the sample was HIV-1 positive with presence of all bands. Further studies are required to explain and characterize the false negative results obtained using 3G ELISA reagents.
ELISA is the most commonly used type of screening test for HIV infection because it is inexpensive, has stable performance and uses simple methodology with high sensitivity, making it suitable for testing large numbers of samples, particularly in blood testing centers (13,14). The 4G ELISA reagents are based on 3G ELISA reagents with a “double antigen sandwich” setup to simultaneously detect HIV-specific IgG and IgM antibodies. The 4G ELISA reagents additionally detects p24 antigen making it more effective in identification of HIV infection during window period (7,15).
The colloidal GICA is a rapid test for HIV antibody screening. The sensitivity and specificity of GICA are relatively high with easy interpretation of results and reagents are easy to obtain and store. Therefore, GICA method is suitable for the detection of diseases in which sample volumes are low, such as sexually transmitted diseases. GICA can also facilitate portable testing. As GICA is simple and easy to master, individuals who are at high risk can perform self-testing under permitting conditions (16). In addition, GICA can also be used for confirmation for suspicious positive samples obtained in a large-scale analysis. These results can be confirmed on the basis of the S/CO value of the ELISA test and the results of GICA.
In this study, we have attempted to identify the best test or combination of tests with optimal sensitivity and specificity which could be used for screening of HIV. Based on this study, the combination of 4G ELISA and GICA is the optimal screening method for HIV. Although the combination of 3G ELISA and GICA can reduce false positives and prevent false negatives, the testing process is prone to technical difficulties and required extra care. For suspicious samples, results should be discussed with clinicians in order to avoid misdiagnosis (17).
This retrospective analysis has some limitations. First, not all 331,968 samples were tested using the 4G ELISA reagents, and not all were screened using GICA. Furthermore, not all patients with uncertain outcomes undergo follow-up studies. Accordingly, further studies are needed, particularly for samples with unconventional outcomes.
In conclusion, we found that the combination of 4G ELISA and GICA can significantly improve the sensitivity of HIV screening and it is beneficial for the detection of HIV infection during window period. The combination of 4G ELISA and GICA is a potentially useful HIV clinical screening tool in regions with low HIV prevalence and large number of samples. We would also like to note that that each method has its own advantages, disadvantages, and unique characteristics; therefore, a scientific, rational, and precise analysis of results is extremely important to prevent production of erroneous results.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81302531), Natural Science Foundation of Jiangsu Province of China (BK20131018), the Talents Planning of Six Summit Fields of Jiangsu Province (2013-WSN-037), the National Key Clinical Department of Laboratory Medicine of China in Nanjing, Key laboratory for Laboratory Medicine of Jiangsu Province (XK201114) and by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.06.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This observational retrospective study received ethical approval from the Ethics Committee at the First Affiliated Hospital of Nanjing Medical University (No. 2017-SR-212). Because this is a retrospective study, patients’ consents were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 2011;378:256-68. [Crossref] [PubMed]
- Wang J, Liu J, Yao F, et al. Prevalence, incidence, and residual risks for transfusion-transmitted human immunodeficiency virus Types 1 and 2 infection among Chinese blood donors. Transfusion 2013;53:1240-9. [Crossref] [PubMed]
- Zeng P, Liu J, Wang J, et al. Parallel enzyme-linked immunosorbent assay screening for human immunodeficiency virus among blood donors in five Chinese blood centres: a retrospective analysis. Transfus Med 2015;25:259-64. [Crossref] [PubMed]
- Liu MQ, Zhu ZR, Kong WH, et al. High rate of missed HIV infections in individuals with indeterminate or negative HIV western blots based on current HIV testing algorithm in China. J Med Virol 2016;88:1462-6. [Crossref] [PubMed]
- Han X, Xu J, Chu Z, et al. Screening acute HIV infections among Chinese men who have sex with men from voluntary counseling & testing centers. PLoS One 2011;6:e28792 [Crossref] [PubMed]
- Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology 2013;10:56. [Crossref] [PubMed]
- Malm K, von Sydow M, Andersson S. Performance of three automated fourth-generation combined HIV antigen/antibody assays in large-scale screening of blood donors and clinical samples. Transfus Med 2009;19:78-88. [Crossref] [PubMed]
- Robb ML, Eller LA, Kibuuka H, et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N Engl J Med 2016;374:2120-30. [Crossref] [PubMed]
- Muthukumar A, Alatoom A, Burns S, et al. Comparison of 4th-Generation HIV Antigen/Antibody Combination Assay With 3rd-Generation HIV Antibody Assays for the Occurrence of False-Positive and False-Negative Results. Lab Med 2015;46:84-9; quiz e28-9.
- Li J, Zhang H, Shen Z, et al. Screening for acute HIV infections and estimating HIV incidence among female sex workers from low-grade venues in Guangxi, China. PLoS One 2014;9:e99522 [Crossref] [PubMed]
- Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med 2005;352:1873-83. [Crossref] [PubMed]
- Krajden M, Cook D, Mak A, et al. Pooled nucleic acid testing increases the diagnostic yield of acute HIV infections in a high-risk population compared to 3rd and 4th generation HIV enzyme immunoassays. J Clin Virol 2014;61:132-7. [Crossref] [PubMed]
- Bi X, Ning H, Wang T, et al. Comparative performance of electrochemiluminescence immunoassay and EIA for HIV screening in a multiethnic region of China. PLoS One 2012;7:e48162 [Crossref] [PubMed]
- Zhang R, Sun Y, Wang L, et al. Blood screening for human immunodeficiency virus: a new algorithm to reduce the false-positive results. Transfus Med 2013;23:260-4. [Crossref] [PubMed]
- Pandori MW, Hackett J Jr, Louie B, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol 2009;47:2639-42. [Crossref] [PubMed]
- Yan H, Yang H, Raymond HF, et al. Experiences and correlates of HIV self-testing among men who have sex with men in Jiangsu province, China. AIDS Behav 2015;19:485-91. [Crossref] [PubMed]
- Li Y, Zhao JK, Wang M, et al. Current antibody-based immunoassay algorithm failed to confirm three late-stage AIDS cases in China: case report. Virol J 2010;7:58. [Crossref] [PubMed]
Cite this article as: Wu ZQ, Liu YY, Ni F, Song WJ, Xie E, Goyal H, Xu HG. Evaluation of preliminary screening strategies for human immunodeficiency virus: a single center experience. J Lab Precis Med 2017;2:50.


