Quality in laboratory medicine: an unfinished journey
Introduction
Laboratory medicine is a relatively young discipline, regardless its historical roots (1) that underwent major advancements in the last four decades. Over the past 50 years, the clinical laboratory and its professionals have been at the heart of facing the challenges of rapid technologic development, scientific advances, and the changing medical landscape of disease and our approach to diagnosis and therapy. Clinical laboratories have recently been defined as “the nerve center of diagnostic medicine” because they provide essential information for screening, prevention, early diagnoses, tailored monitoring, and effective monitoring of human diseases (2). The evolving landscape of clinical laboratories had an impact not only on menu and volume of tests, but also on their accuracy and quality of laboratory information. The concept of quality in laboratory medicine has hence evolved in parallel over time, from focusing only on analytical accuracy to a broader and more comprehensive picture, which takes into consideration all steps of the total testing process (TTP) to ultimately provide an effective and valuable information to the clinical decision-making process, and to patient care (3).
Quality in medicine
The US Institute of Medicine (IOM) has defined quality as “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” (4). Good quality means providing patients with appropriate services in a technically competent manner, with good communication, shared decision making, and cultural sensitivity. According to Donabedian, quality can be evaluated based on structure, process, and outcomes (5). Structural quality evaluates health system capacities, process quality assesses interactions between clinicians and patients, and outcomes offer evidence about changes in patients’ health status. All three dimensions can provide valuable information for measuring quality, but most of the quality-of-care literature focuses on measuring processes of care. Another way to measure process quality is to determine whether care meets or adheres to professional standards. This assessment can be done by creating a list of quality indicators (QIs) aimed to describing a process of care that should occur for a particular type of patient or clinical circumstance and by evaluating whether patients’ care is consistent with the indicators. QIs are based on standards of care, which are either found in the research literature and in statements of professional medical organizations, or can be determined by an expert panel. In 2001, the IOM has released a document called “Crossing the quality chasm: a new health system for the 21st century” which proposed six aims for improvement to address key dimensions in which today’s health care system functions (6). According to these domains, health care should be safe, effective, patient-centered, timely, efficient and equitable. Table 1 shows the six domains of quality as described by the IOM. The six aims for improving health care have been successfully accepted by the scientific community and still represent the main areas of work to assure quality in medicine, but the focus is now shifting to provide better clinical and economical outcomes, moving from process measures to outcomes indicators. According to Porter and Colleagues “health care is shifting from the volume of the services delivered to the value created for patients, with “value” defined as the outcomes achieved relative to the costs” (7).
Table 1
| Domain | Description |
|---|---|
| Safe | Avoiding injuries to patients from the care that is intended to help them |
| Effective | Providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit (avoiding underuse and overuse, respectively) |
| Patient-centered | Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions |
| Timely | Reducing waits and sometimes harmful delays for both those who receive and those who give care |
| Efficient | Avoiding waste, including waste of equipment, supplies, ideas, and energy |
| Equitable | Providing care that does not vary in quality because of personal characteristics such as gender, ethnicity, geographic location, and socio-economic status |
Quality in laboratory medicine
The evolving landscape of clinical laboratories affects not only the menu and volume of tests, but also their accuracy and the quality of laboratory information. In particular, the evolving landscape of quality and errors in clinical laboratories moved first from analytical errors to all errors performed within the laboratory walls, later to errors in laboratory medicine (including errors in test request and result interpretation), to finally focus on errors more frequently associated to adverse events (laboratory-associated errors). Historically, efforts to improve diagnosis and therapy in laboratory medicine have been directed toward improving diagnostic technology—higher volumes and more accurate laboratory tests—but previously reported data emphasized the need to re-evaluate the seminal concept of the “brain-to-brain-loop” (8). According to this concept, the generation of laboratory test results consists of 9 steps, including ordering, collection, identification (at several stages), transportation, separation (or preparation), analysis, reporting, interpretation, and action. Twenty years later, in a seminal editorial, the father of this concept, Lundberg emphasized that even the final step, i.e., the action undertaken on the patient and based on laboratory information, is not far enough because “clinicians and laboratorians should all be concerned about the effects of that laboratory test and whether the performance of it was useful for the patient or for the public’s health,” thus stressing the need for an outcomes research agenda (9). More recently, Plebani et al. reviewed this influential concept by adding two other brains, in addition to the physician brain, those of laboratory professional and patient, respectively, so emphasizing again the need for evaluating the value of laboratory tests according to patient’s outcomes (10). The concept of quality in laboratory medicine is hence evolving from the focus on internal processes to the real impact of laboratory information in patient care and/or in assuring a healthy status to any individual and the whole population. Taking into consideration the brain-to-brain-loop framework, Plebani defined quality in clinical laboratories as “the guarantee that each and every step in the total TTP is correctly performed, thus assuring valuable medical decision making and effective patient care” (11). In this sense, the TTP has been defined as “a set of interrelated or interacting activities that transform biologic patient sample materials into laboratory results and information to ultimately assure the most appropriate clinical outcomes”.
Changing the paradigm
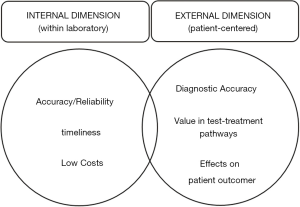
The paradigm adopted in laboratory medicine in the past 50 years has generated a gap between the laboratory and the clinical side, with a main focus on analytical quality and productivity, to increase the risk of inappropriateness, consolidate the analytical work in megastructures only oriented to volumes and decreased cost per test, so contributing to generate misleading perception of laboratory services as simple commodities. Currently, the business model involved in delivery of laboratory services is primarily designed, managed, and carried out in individual units or silos. Such units are driven by internal activities of the discipline and managed according to performance metrics that match the discipline itself—rather than the product of services to improve clinical pathways, clinical and economical outcomes and patient safety. Many drivers, however, should change the current paradigm. First, there is increasing awareness about the evidence that the value of a diagnostic test cannot be simply measured by its accuracy, but depends on how it affects patient health, and by evaluating the downstream consequences of testing on patient outcomes (12,13). Second, the recently released document by IOM “Improving Diagnosis in Health Care” emphasizes that diagnostic errors represent an important and underestimated safety concern, and that addressing this issue represents a professional and moral imperative for all health care operators, including laboratorians. In particular, it calls for laboratory professionals to be actively involved as partner on diagnostic team (14), and for payment reform that would reward the time spent on clinical advice and consultation. The take-home message for laboratory professionals is largely based on the framework of the total TTP, and the realization that so many diagnostic errors reflect problems in the pre- and post-analytical phases of testing (15,16). Third, the shift of payment models from volume to value and outcomes and especially for clinical laboratories—from fee-for-service (FFS) to bundled payment (BP) systems (DRGs and APCs) is changing the nature of laboratory services. Fourth, regardless the importance of other categories, accuracy and reliability are increasingly recognised by physicians as the most important category of laboratory services, much more than routine and Stat turnaround times (17). The dimensions of quality in medicine are shown in Figure 1. Changing the paradigm seems to be necessary to assure the survival of our discipline in the context of the dramatic changes in healthcare and the increasing economic pressures. Moreover, breakdowns in the testing cycle, in particular test ordering and interpretation along with poor follow-up and tracking of diagnostic information may ultimately generate wrong, delayed or missed diagnoses and can be associated with the risk of moderate to severe harm.
QIs
In laboratory medicine, the use of appropriate analytical performance specifications has led to impressive improvement of quality and error reduction. While these QIs have been available for more 50 years so far, development and utilization of reliable QIs is still in its infancy in the extra-analytical phase.
This may explain, at least in part, the greater vulnerability to errors of extra-analytical phases. The development and use of harmonized performance indicators may represent, therefore, a valuable tool for allowing clinical laboratory to documenting and monitoring all procedures and processes in the testing cycle, and to provide inter-laboratory comparative performances (benchmark). Using performance QIs, collecting regularly data and receiving reports, US laboratories attending a QI program documented significant decrease in defect rates (18).
The model of quality indicators (MQI) developed by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) working group on “Laboratory errors and patient safety” is providing a valuable tool for harmonising at an international level the list of QIs and the reporting system. This in turn may provide objective information for both internal improvement projects and a between-laboratories benchmark based on indicators covering all steps of the testing cycle (19-22).
The journey toward quality in laboratory medicine continues and new efforts are needed to provide evidence of accuracy, reliability and safety in all steps of the brain-to-brain-loop.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “International Conference on Laboratory Medicine”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.08.04). The series “International Conference on Laboratory Medicine” was commissioned by the editorial office without any funding or sponsorship. Mario Plebani served as an unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berger D. A brief history of medical diagnosis and the birth of the clinical laboratory. Part 1-Ancient times through the 19th century. MLO Med Lab Obs 1999;31:28-30,32,34-40. [PubMed]
- Cortelyou-Ward K, Rotarius T, Liberman A, et al. Hospital in-house laboratories: examining the external environment. Health Care Manag (Frederick) 2010;29:4-10. [Crossref] [PubMed]
- Plebani M. Quality in laboratory medicine: 50 years on. Clin Biochem 2017;50:101-4. [Crossref] [PubMed]
- Lohr KN. editor. Medicare: a strategy for quality assurance, Volume I. Washington, DC: The National Academies Press, 1990.
- Donabedian A. Quality assurance. Structure, process and outcome. Nurs Stand 1992;7:4-5. [PubMed]
- Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press, 2001.
- Porter ME. What is value in health care? N Engl J Med 2010;363:2477-81. [Crossref] [PubMed]
- Lundberg GD. Acting on significant laboratory results. JAMA 1981;245:1762-3. [Crossref] [PubMed]
- Lundberg GD. The need for an outcomes research agenda for clinical laboratory testing. JAMA 1998;280:565-6. [Crossref] [PubMed]
- Plebani M, Laposata M, Lundberg GD. The brain-to-brain loop concept for laboratory testing 40 years after its introduction. Am J Clin Pathol 2011;136:829-33. [Crossref] [PubMed]
- Plebani M. Quality indicators to detect pre-analytical errors in laboratory testing. Clin Biochem Rev 2012;33:85-8. [PubMed]
- Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, et al. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ 2012;344:e686 [Crossref] [PubMed]
- Trenti T, Schünemann HJ, Plebani M. Developing GRADE outcome-based recommendations about diagnostic tests: a key role in laboratory medicine policies. Clin Chem Lab Med 2016;54:535-43. [Crossref] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC: The National Academies Press, 2015.
- Plebani M, Sciacovelli L, Aita A, et al. Performance criteria and quality indicators for the pre-analytical phase. Clin Chem Lab Med 2015;53:943-8. [PubMed]
- Sciacovelli L, Aita A, Padoan A, et al. Performance criteria and quality indicators for the post-analytical phase. Clin Chem Lab Med 2016;54:1169-76. [Crossref] [PubMed]
- McCall SJ, Souers RJ, Blond B, et al. Physician satisfaction with clinical laboratory services: a college of american pathologists Q-Probes study of 81 institutions. Arch Pathol Lab Med 2016;140:1098-103. [Crossref] [PubMed]
- Meier FA, Souers RJ, Howanitz PJ, et al. Seven Q-Tracks monitors of laboratory quality drive general performance improvement: experience from the college of American pathologists Q-Tracks program 1999-2011. Arch Pathol Lab Med 2015;139:762-75. [Crossref] [PubMed]
- Plebani M, Astion ML, Barth JH, et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52:951-8. [Crossref] [PubMed]
- Sciacovelli L, Lippi G, Sumarac Z, et al. Quality Indicators in Laboratory Medicine: the status of the progress of IFCC Working Group "Laboratory Errors and Patient Safety" project. Clin Chem Lab Med 2017;55:348-57. [Crossref] [PubMed]
- Sciacovelli L, Panteghini M, Lippi G, et al. Defining a roadmap for harmonizing quality indicators in laboratory medicine: a consensus statement on behalf of the IFCC working group "Laboratory Error and Patient Safety" and EFLM task and finish group "Performance specifications for the extra-analytical phases". Clin Chem Lab Med 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Lippi G, Sciacovelli L, Simundic AM, et al. Innovative software for recording preanalytical errors in accord with the IFCC quality indicators. Clin Chem Lab Med 2017;55:e51-3. [Crossref] [PubMed]
Cite this article as: Plebani M. Quality in laboratory medicine: an unfinished journey. J Lab Precis Med 2017;2:63.