
Trimethoprim-sulfamethoxazole associated rhabdomyolysis in an immunocompetent patient: case report and review of the literature
Introduction
Trimethoprim-sulfamethoxazole (TMP-SMX) is a frequently used antibiotic for the treatment of infections such as urinary tract infections (UTI). It is considered to be a safe drug with infrequent side effects. Some well-known but rare adverse effects of TMP-SMX are Stevens-Johnson syndrome, toxic epidermal necrolysis, various cytopenias and hepatotoxicity (1). Sometimes, these uncommon side effects lead to lethal consequences especially due to its sulfa component. Therefore, caution must be exercised when prescribing this medication and patients should be educated about these potential side effects. Rhabdomyolysis is an unusual and largely unknown side effect of TMP-SMX. There have been a few reported cases of TMP-SMX induced rhabdomyolysis and most of them occurred in immunocompromised patients (2). Rhabdomyolysis due to TMP-SMX has only been reported in three immunocompetent patients in the past (3-5). Herein, we report a very rare case of TMP-SMX-induced rhabdomyolysis in an immunocompetent female.
Case presentation
An 18-year-old African American female with past history of Arnold-Chiari malformation post decompression surgery presented to her primary care physician (PCP) with complaints of severe generalized weakness and diffuse muscle pain in all four extremities for 2 days. Her PCP referred her to the local emergency room (ER) because of the presence of tachycardia and low blood pressure. In the local ER, the patient was resuscitated with intravenous fluids. Initial laboratory findings were significant for serum sodium of 125 mEq/L, bicarbonate 20 mEq/L, and corrected (with albumin) Ca of 6.4 mEq/L. Given the patient’s presenting symptoms, laboratory abnormalities, and hemodynamic instability, she was later transferred to our institution for further care.
At our institution, history & physical were sought again and patient revealed that she had recently completed a course of TMP-SMX for possible UTI/cystitis which she received from her PCP. The patient denied history of seizures, alcoholism and taking any other medications. There was no family history of autoimmune diseases and malignancies. On physical examination, she was found to have a temperature of 39 °F, heart rate of 145/min, and blood pressure of 150/70 mmHg. Remainder of the physical exam was normal except significant proximal weakness in upper and lower extremities along with diffusely tender muscles. Sensation to fine touch was intact in all extremities. Initial laboratory testing in our institution was notable as in Table 1. Depending on patient’s presenting symptoms and laboratory findings she was suspected to have inflammatory myopathies and/or rhabdomyolysis. She was started on aggressive intravenous (IV) fluid hydration with daily monitoring of creatine phosphokinase (CPK) levels.
Table 1
| Laboratorial data | Admission labs | Reference ranges |
|---|---|---|
| Sodium | 129 meq/L | 136–144 meq/L |
| Potassium | 4.0 meq/L | 3.5–5.1 meq/L |
| Magnesium | 1.6 mg/dL | 1.6–2.6 mg/dL |
| Calcium | 6.2 mg/dL | 8.5–10.3 mg/dL |
| Corrected calcium (with albumin) | 7.3 mg/dL | 8.5–10.3 mg/dL |
| Blood urea nitrogen | 6 mg/dL | 7–23 mg/dL |
| Creatinine | 0.64 mg/dL | 0.57–1.11 mg/dL |
| Creatine phosphokinase (CPK) | 20,418 IU/L | 43–237 IU/L |
| Aspartate aminotransferase (AST) | 450 IU/L | 5–40 IU/L |
| Alanine aminotransferase (ALT) | 74 IU/L | 5–49 IU/L |
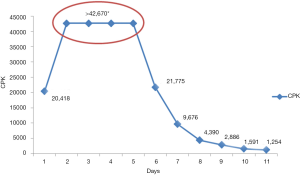
The Next day, the patient’s CPK level rose to level greater than 42,670 IU/L, the maximum quantifiable level at our hospital laboratory, and continue to remain elevated. She received aggressive IV hydration but her muscle weakness did not improve. Guillain-Barré syndrome was ruled out by normal cerebrospinal fluid analysis. A 4th generation HIV screen was negative. Antinuclear antibody profile (which includes ANA, SS-A IgG Ab, SS-B IgG Ab, ribonuclear protein IgG Ab, SCL-70 IgG Ab, JO-1 IgG Ab, dsDNA Ab, Histon IgG Ab) was negative ruling out autoimmune process as a possible cause. On day 5 of the admission, IV immunoglobulin G (IVIG) was started due to lack of improvement in her muscle strength. Finally, after receiving IVIG, her CPK started to trend down (Figure 1) with improvement in muscle strength. She received IVIG for a total of 5 days. Skeletal muscle biopsy results indicated occasional degenerating fibers and multiple small foci of chronic perivascular inflammation consistent with rhabdomyolysis. The patient was diagnosed with TMP-SMX induced rhabdomyolysis and she was discharged home in the stable condition with an advice to avoid use of TMP-SMX in the future.
Discussion
With disintegration of myocytes, rhabdomyolysis can lead to multisystem failure due to leakage of myocyte contents and resulting intravascular fluid reallocation into damaged muscles. Multiple etiologies of rhabdomyolysis exist, which can be grouped into eight common categories: trauma, exertion, muscle hypoxia, genetic defects, infections, body-temperature changes, metabolic and electrolytic disorders, and drugs and toxins. Regardless of the etiology, patients usually present with symptoms of muscle pain, weakness, and dark-colored urine.
Review of literature shows that TMP-SMX induced rhabdomyolysis occurs mostly in immunocompromised patients who are either HIV (2) or on immunosuppressant medications (6). Moreover, only three cases of TMP-SMX associated rhabdomyolysis have been reported in immunocompetent patients (Table 2). Our patient was HIV negative and had no other indications to suggest immunocompromised state. Furthermore, she was a young healthy adult with no past medical history other than an unrelated congenital malformation. When evaluated with Naranjo adverse drug probability scale, her score was 5 which signify a probable drug reaction (7). Moreover, symptoms of our patient did not improve with conservative management and she had undergo IVIG treatment for improvement in rhabdomyolysis.
Table 2
| Cases | Age (years)/sex | Initial CPK (U/L) | Highest CPK (U/L) | Renal dysfunction | Risk factors | Treatment outcome |
|---|---|---|---|---|---|---|
| Ainapurapu et al. (3) | 40/female | 20,063 | 20,063 | Yes | None | Favorable |
| Schmidt et al. (4) | 24/male | 22,717 | 22,717 | Yes | Epstein virus infection | Favorable |
| Petrov et al. (5) | 64/male | 1,524 | 64,691 | No | Concomitant NSAID use | Favorable |
| Our patient | 18/female | 20,418 | >42,670 | No | None | Favorable |
TMP-SMX, trimethoprim-sulfamethoxazole; CPK, creatine phosphokinase; NSAID, nonsteroidal anti-inflammatory drug.
Acute kidney injury (AKI) from rhabdomyolysis accounts for about 7% to 10% of all causes of AKI’s in the United States. If present, renal failure from rhabdomyolysis turns the prognosis of rhabdomyolysis from good to potentially poor. Thus, it is imperative that not only do physicians need to recognize rhabdomyolysis but also to identify the precipitating cause of rhabdomyolysis. TMP-SMX can cause rhabdomyolysis and early recognition and management can prevent or minimize the extent of AKI (8). In conclusion, TMP-SMX has various adverse effects including rhabdomyolysis, which physicians need to be aware of, as the diagnosis is not always evident. A thorough and complete history inclusive of medication intake can point the health care providers in the right direction.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.10.01). Hemant Goyal serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from May 2017 to April 2019. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
-
Drug information on Sulfamethoxazole/Trimethoprim - Singer SJ, Racoosin JA, Viraraghavan R. Rhabdomyolysis in human immunodeficiency virus--positive patients taking trimethoprim-sulfamethoxazole. Clin Infect Dis 1998;26:233-4. [Crossref] [PubMed]
- Ainapurapu B, Kanakadandi UB. Trimethoprim-sulfamethoxazole induced rhabdomyolysis. Am J Ther 2014;21:e78-9. [Crossref] [PubMed]
- Schmidt TW, Garfinkle M, Battafarano DF. Rash and elevated creatine kinase in a deployed soldier. Mil Med 2014;179:e245-8. [Crossref] [PubMed]
- Petrov M, Yatsynovich Y, Lionte C. An unusual cause of rhabdomyolysis in emergency setting: challenges of diagnosis. Am J Emerg Med 2015;33:123.e1-3. [Crossref] [PubMed]
- Augustyn A, Lisa Alattar M, Naina H. Rhabdomyolysis due to Trimethoprim-Sulfamethoxazole Administration following a Hematopoietic Stem Cell Transplant. Case Rep Oncol Med 2015;2015:619473
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. [Crossref] [PubMed]
- Cervellin G, Comelli I, Benatti M, et al. Non-traumatic rhabdomyolysis: Background, laboratory features, and acute clinical management. Clin Biochem 2017;50:656-62. [Crossref] [PubMed]
Cite this article as: Goyal H, Hang M, Singla U, Chiranjeevi S. Trimethoprim-sulfamethoxazole associated rhabdomyolysis in an immunocompetent patient: case report and review of the literature. J Lab Precis Med 2017;2:85.


