
Usefulness and limitations of neutrophil gelatinase-associated lipocalin in the assessment of kidney diseases
Introduction
Human neutrophil gelatinase-associated lipocalin (NGAL), also known as neutrophil glucosaminidase-associated lipocalin, 24p3 and α1-microglobulin related protein, is a 25 kDa secreted glycoprotein, which was firstly discovered as a complex protein with human neutrophil gelatinase in 1993 (1). NGAL shares a common secondary and tertiary structural feature—called as the “lipocalin fold” with other lipocalin family proteins (2), and plays critical roles in bacteria defense, signal transduction, neutrophil maturation and renal tubular damage (3). NGAL is mainly secreted by activated neutrophils, and is also released by epithelial cells, kidney tubular cells, and hepatocytes under pathological conditions, like epithelial cells damage, inflammation and bacterial infection (4).
So far, NGAL has been investigated extensively and considered as one of the most promising renal disease biomarkers, especially as an early biomarker of acute kidney injury (AKI) (5). Systemic and urinary NGAL both respond to the onset of renal tubular damage, however, many researches revealed that systemic and urinary NGAL provided different information in disease diagnosis and prognosis (3). This review summarizes current knowledge about the usefulness of systemic and urinary NGAL in diagnosis, risk stratification and prognosis of acute and chronic renal diseases. The different performances of systemic and urinary NGAL are carefully examined and the underlying reasons of this discrepancy are also discussed.
NGAL is a biomarker which can be detected in both circulation and urine
Being a small glycosylated protein, NGAL is freely filtered through the glomerular membrane, and its reabsorption mainly depend on endocytosis by the proximal tubule (6), which makes NGAL a potential biomarker for renal tubular damage. NGAL was firstly discovered as a urinary biomarker of renal injury in the mouse model of renal ischemia-reperfusion injury (7). Later, systemic and urinary NGAL were proved to be sensitive, specific, and highly predictive early biomarkers of AKI in patient undergoing cardiopulmonary bypass surgery (8), kidney transplantation (9) and coronary angiography (10). Following intensive studies revealed that the levels of NGAL protein in circulation and urine were low, and upon the damage of proximal tubule, NGAL protein was upregulated and secreted by the loop of Henle and collecting ducts (11), which then entered both the urine and the circulation. Systemic NGAL is filtrated in glomerulus and uptake by proximal tubular epithelia via endocytosis (6). Thus, detectable concentration of NGAL protein emerges, and the concentrations of NGAL protein in urine and circulation reflect disease severity. However, due to different mechanisms of appearances, they may behave differently and result in confused diagnosis, which was observed in many studies. Moreover, the kidney cells seem not to be the only source of NGAL protein in response to renal damage. Patients underwent bilateral nephrectomy showed significant higher level systemic NGAL than healthy people, suggesting an extrarenal production of NGAL protein (12).
The role of NGAL in renal damage response is believed to be protective. NGAL protein transfers iron to the proximal tubule by forming siderophore: iron-NGAL complex, which may contribute to NGAL’s tissue-protective effects (6). In line with this, NGAL was isolated from a ureteric bud cell line as a factor which could induce the differentiation of kidney progenitors in the metanephric mesenchyme into renal epithelia (13). Thus, NGAL contributes to the recovery of tubular epithelia after damage, making it a biomarker of renal injury, but not renal function (14).
Besides renal tubular protection, NGAL is also a critical component of innate immunity in defending bacteria. NGAL protein is critical to the maturation of neutrophil. The expression of NGAL was initiated in the early stage and stopped at the end stage of neutrophil maturation, then NGAL protein was eliminated from the neutrophil by secretion (3). Circulating NGAL protein inhibited bacterial growth by binding siderophore: iron, which is the major source of iron in iron-limited environment. In line with this, NGAL expression could be induced by several cytokines and growth factors including interleukin-1, IL-17, IL-22, insulin like growth factor-1, transforming growth factor alpha and tumor necrosis factor alpha, facilitating its protective function (3). Genetically modified mice, with both copies of the NGAL gene deficient, were more susceptive to bacterial infection and more vulnerable to sepsis than wild-type mice (15). Therefore, NGAL is one of the major contributors in response to bacterial infection. However, the rising of NGAL concentration in circulation upon bacterial infection and sepsis may limit the utilization of NGAL as a renal damage biomarker, which will be discussed later.
Systemic and urinary NGAL as biomarkers of acute renal diseases
Systemic and urinary NGAL predict cardiac surgery associated AKI
Cardiac surgery is one of the most common aetiology of hospital-acquired AKI. Depending on the definition of AKI, the incidence rate of the cardiac surgery associated AKI was reported to range from 3% to 30%. Since the first discovery of NGAL as the early biomarker of AKI in 2005, the usefulness of both systemic and urinary NGAL in early diagnosis of AKI in patients undergoing cardiac surgery has been proved by many clinical studies. A systemic review published in 2014 which enrolled more than 7,000 patients undergoing cardiac surgery showed NGAL predicted the subsequent development of AKI with area under receiver operating characteristic curve (AUC-ROC) of 0.82–0.83 (16). Recent meta-analysis with 4,066 patients enrolled revealed that when sample (including serum, plasma, urine and whole blood) was collected within 12 h post-operatively, the sensitivity of NGAL for the diagnosis of AKI was 0.68, and the specificity was 0.79 (17). These studies show that NGAL preforms even better than commonly used AKI diagnostic criteria, including kidney disease: improving global outcomes (KDIGO), Risk, Injury, Failure, Loss of kidney function, and End-stage renal failure (RIFLE) and Acute Kidney Injury Network (AKIN) criteria (18), suggesting NGAL is a promising candidate to be an important part of clinical management protocols. Moreover, analysis showed that NGAL had better performance in neonates/children compared to adults (AUC-ROC 0.89 vs. 0.83), making NGAL the optimal biomarker for diagnosing AKI in young patients receiving cardiac surgery. Specifically, among other potential AKI biomarker, only NGAL has predictive value as early as 2 h post-operatively (19), highlighting its huge potential in the early diagnosis of AKI.
Several meta-analysis showed systemic and urinary NGAL were both competent in early diagnosis of AKI after cardiac surgery (17,20), howbeit, a few clinical studies measuring systemic and urinary NGAL simultaneously showed somehow contradictory results. A study enrolled 60 patients undergoing cardiac surgery found that the concentration of urinary NGAL was significantly higher in AKI patient than non-AKI patient 24 h post-operatively, but systemic NGAL was not different between groups (21). Another study enrolled 1,219 adults undergoing cardiac surgery showed the AUC-ROC of systemic NGAL to predict AKI was 0.7, and when combining with clinical model, the AUC-ROC reached 0.75 (22). Systemic NGAL also improved risk classification in addition to clinical model. However, urinary NGAL did not improve the AUC-ROC above that of the clinical model, no matter for AKI prediction or risk classification. Although these studies didn’t find the underlying reason of the discrepancy, there may be unnoticed conditions affecting the accuracy of systemic or urinary NGAL differently, and care must be taken in the further utilization of systemic and urinary NGAL.
Systemic and urinary NGAL predict AKI of critically ill
Critically ill patients are another population vulnerable to AKI, despite the aetiology of these patients are various. NGAL has good overall predictive performance for intensive care unit (ICU) patients and patients admitted in the emergency department with an AUC-ROC of 0.79–0.80 (16). A study recruiting 151 patients admitted to ICU after major or ultra-major non-cardiac surgery found systemic NGAL was useful in AKI prediction and risk classification (23). Moreover, NGAL also significantly contributed to the accuracy of the RIFLE criteria to predict AKI in critically ill patients by improving the AUC-ROC (24). These results show that NGAL is able to predict AKI caused by various aetiology, not limiting to cardiac surgery. Systemic and urinary NGAL could also distinguish oliguria due to the functional adaptation from AKI (25), suggesting the predictive value of NGAL is not significantly influenced by urine volume. All current evidence shows that NGAL is a sensitive early biomarker for AKI in critically ill patients.
The different performances of systemic and urinary NGAL are also observed in predicting AKI of critically ill patients. In two studies measuring systemic and urinary NGAL simultaneously, urinary NGAL was proved to have better sensitivity and specificity. In one study recruited 65 patients, urinary NGAL was a good predictor (AUC-ROC 0.86) whereas systemic NGAL was a poor predictor (AUC-ROC 0.67) for AKI within the next 12 h (26). In another more detailed study enrolled 632 patients, both systemic and urinary NGAL values at ICU admission were significantly related to AKI severity. The AUC-ROC of systemic and urinary NGAL were for RIFLE R (0.776±0.05 and 0.806±0.04, respectively), RIFLE I (0.806±0.06 and 0.856±0.04, respectively), and RIFLE F (0.866±0.06 and 0.886±0.04, respectively) (24). Urinary NGAL performs much better than systemic NGAL in predicting less severe AKI, but in predicting renal failure, they are almost the same.
The defect of systemic NGAL in predicting AKI of critically ill patients may attribute to the function of NGAL in bacterial defense. Most critically ill patients were concomitant with systemic inflammatory response syndrome, sepsis or septic shock. Upon bacterial infection, neutrophils significantly upregulate the expression of NGAL protein, then large amount of NGAL protein is released into the circulation to inhibit the growth of bacteria. In septic patients with less severe AKI, a relatively small amount of NGAL protein is released by kidney cells, thus the concentration of systemic NGAL is readily affected by NGAL protein secreted by neutrophils. But most urinary NGAL is possibly originated from kidney cells, which is less likely affected by immune response.
Systemic and urinary NGAL as early biomarkers of graft function after kidney transplant
Although NGAL is a biomarker reflecting renal damage, it can also serve as a biomarker to monitoring the recovery of kidney function after transplant (27). The ischemia and reperfusion injury are considered as the main reason of kidney injury and failure in transplant. NGAL may reflect the extent of injury after reperfusion, which determines the probability of renal function recovery. Delayed graft function is defined as the necessity for dialysis within the first week post-transplant. A review enrolled 1,079 kidney transplant patients demonstrated that measuring systemic and urinary NGAL 6–12 hours after transplantation could predict delayed graft function with 82% sensitivity and 82% specificity (16). Another study also showed that NGAL level measured 24 h post-transplant, was an early accurate predictor of delayed graft function after kidney transplant with an AUC-ROC of 0.82 (28).
Interestingly, urinary NGAL, but not systemic NGAL can predicts long-term prognosis after transplant, especially for immediate graft function patients. By measuring urinary NGAL concentration fourth and seventh days post-surgery, NGAL predicts 1-year graft function, independent of donor characteristics, acute rejection episodes and re-hospitalizations (29). Another research found that urinary NGAL measured at day 2 post-operatively was a significant, independent factor for predicting poor long-term graft function, with 95% sensitivity and 65% specificity (30). These results demonstrate that urinary NGAL is useful in predicting adverse 1-year outcome for kidney transplant patients. However, systemic NGAL measured at day 2 post-operatively was not associated with 3 months outcome, although systemic NGAL concentration raised significantly in some patients (31). The reason is still remained to be discovered, however the immune response after kidney transplant may account for the elevated systemic NGAL concentration. Anyhow, urinary NGAL is a useful prognostic biomarker for subclinical tubular ischemic injury post-surgery.
Factors affecting systemic and urinary NGAL as biomarkers of AKI
Although several meta-analysis show that both systemic and urinary NGAL are effective in predicting AKI and renal function recovery after transplant, there are still limitations for their usefulness in certain circumstances. These limitations may be the main reasons for the discrepancy of systemic and urinary NGAL described above, and should be considered carefully before disease diagnosis.
NGAL is one of the critical component of bacterial defense system in the circulation, and functions in neutrophil maturation and bacterial growth inhibition (3). NGAL concentration in circulation markedly increases in response to infection, systemic inflammatory response syndrome and sepsis, which is observed in both AKI and non-AKI patients (32,33). As systemic NGAL is mostly filtered by glomerulus and then reabsorbed by proximal tubular, markedly elevated NGAL concentration may overload the absorption capacity of tubular epithelia, and excess NGAL protein emerges in the urine. Thus, the utilization of NGAL in predicting AKI in sepsis patients has been questioned by some studies, especially systemic NGAL, which is more readily affected by NGAL protein released from immune cells.
A research enrolled 45 septic patients found that peak level of systemic NGAL was elevated in 40 patients, and peak level of systemic NGAL were not significantly different between septic patients with and without AKI (26). However, the peak level of urinary NGAL were mostly below the upper reference limit in patients without AKI. Another research reported that systemic and urinary NGAL levels increased with biomarker of inflammation, but both of them failed in differentiating AKI from no AKI in patients with sepsis, although NGAL concentration was higher in AKI patients (33). Those results reveal that the usefulness of NGAL in sepsis patients should be carefully examined. The combination of NGAL with inflammation biomarkers, like procalcitonin and C-reaction protein, may provide further information on AKI prediction and risk classification.
Another factor affecting systemic and urinary NGAL concentrations is glomerular filtration rate (GFR). Oliguria and anuria occur frequently after kidney transplant, and patients with end stage renal damage and renal failure may exhibit oliguria, anuria and diuresis at different stages of disease. The collection of urine from oliguria and anuria patients may be impractical, which limits the utilization of urinary NGAL. Oliguria and anuria may also lead to the accumulation of NGAL in the circulation. These are supported by the observation that in patients with baseline estimated GFR <60 mL/min, urinary NGAL failed to distinguish AKI from non-AKI patients (34). And urinary NGAL best identified AKI in patients with baseline estimated GFR 90 to 120 mL/min. Diuresis disable the utility of urine output as the marker of kidney injury, and may also lead to the dilution of NGAL protein in case of using diuretic, which causes false-negative result. Thus, in patients with impaired renal function, especially those with very low GFR, NGAL is likely to lose its predictive value.
Systemic and urinary NGAL as biomarkers of chronic renal diseases
Tubular epithelia damage is associated not only with AKI, but also with various kinds of chronic kidney disease (CKD), like cardiorenal syndrome, diabetic nephropathy, lupus nephritis, glomerulonephritis, obstruction, dysplasia, polycystic kidney disease and IgA nephropathy. Growing literatures suggest that NGAL is also a marker of disease severity and clinical outcome for CKD patients. In 45 child patients with CKD stages 2–4, systemic NGAL concentrations were significantly associated with GFR (35). In 92 non-diabetic patients with CKD stages 2–4, both systemic and urinary NGAL correlated with estimated GFR, and the systemic NGAL/urinary NGAL ratio showed the best correlation (36). In a 9-year follow-up study enrolled 143 healthy controls and 143 patients with estimated GFR ≥60 mL/min/1.73 m2 and urinary albumin-creatinine ratio ≤30 mg/g, patients with urinary NGAL concentrations in the fourth quartile had more than 2-fold higher odds of incident CKD stage 3 compared to those in the first quartile (37), suggesting higher urinary NGAL levels are prognostic marker for the progression of CKD. Moreover, a prospective-cohort study of 1,245 older women with 10-year follow-up found that women with above-median systemic NGAL had significantly higher risk of renal function decline and renal disease (38). Systemic NGAL also provided additional information on estimated GFR for predicting 10-year risk of renal disease events (AUC-ROC without and with NGAL 0.64 and 0.71, respectively) and risk reclassification. These results illustrate that both systemic and urinary NGAL are useful biomarkers for the risk classification and prediction of clinical outcome in CKD patients. However, due to the various aetiology of CKD, close attention has been paid to the usefulness of NGAL in different kinds of CKD.
Cardiorenal syndrome
Renal damage and dysfunction are commonly associated with heart failure (HF), which would cause grim prognosis. NGAL was firstly found to have predictive value for the short-term and long-term outcome of HF patients in 2009 (39,40). In a study with 46 elderly chronic HF patients, the concentrations of systemic NGAL were significantly higher, and increased in parallel with the clinical severity of CHF [New York Heart Association (NYHA) classification] (39). And patients with higher baseline NGAL concentrations also had a significantly higher mortality in 2-year follow-up. In the other study with 236 patients with acute post-myocardial infarction HF and 150 chronic HF patients, elevated systemic NGAL concentrations were strongly associated with adverse outcomes in 27-month follow-up (40). Urinary NGAL is also related to a poor clinical outcome in HF patients. In a study enrolled 2,130 HF patients, urinary NGAL concentrations were strongly associated with the combined endpoint of all-cause mortality and HF hospitalizations in 3-year follow-up (41). And even in patients with normal estimated GFR, urinary NGAL was also related to a poorer outcome. Recent study found that in acute decompensated HF patients, elevation urinary NGAL level on the first day of admission was associated with poor prognosis independently (42).
B-type natriuretic peptide (BNP) or N-terminal pro b-type natriuretic peptide (NT-proBNP) is a well-established marker for the diagnosis of acute HF, and BNP levels at discharge is highly predictive of short-term outcomes (43). A study with 186 acute HF patients showed that NGAL was even better than BNP in predicting 30 days outcome in patients with acute HF (44). Subjects with high BNP/high NGAL value had the worst outcomes; while subjects with low BNP/high NGAL group had significant risk, and the risk of other patients was low, suggesting that NGAL provides additional information on BNP in risk classification. Another study also found that for acute HF patients, the combination of NT-proBNP or BNP plus NGAL at presentation had the best prediction value for the occurrence of worsening renal function (45).
HF is considered as a disorder at the level of both the heart and the kidneys. The findings that NGAL is an independent biomarker for adverse outcome for HF patients and combination of NGAL with BNP/NT-proBNP provides more information on risk stratification highlight the values of systemic and urinary NGAL as biomarkers of HF associated chronic renal disease.
Diabetic nephropathy
Both systemic and urinary NGAL was raised in diabetic nephropathy, and were associated with albuminuria, suggesting that NGAL may play important role in the development of nephropathy in diabetes (46,47). A 1-year follow-up study with 74 type 2 diabetic patients found that systemic NGAL positively correlated with albumin concentration in the urine, but the concentration of urinary NGAL was just the opposite (48). Thus systemic NGAL may reflect renal damage at the early stage of diabetic nephropathy, and urinary NGAL may be more meaningful in renal function assessment at the late stage of disease. Another study found that urinary NGAL was a sensitive marker of microalbuminuria and early renal damage with 70.6% sensitivity and 83.3% specificity (49). And systemic NGAL discriminated diabetic nephropathy and diabetic without nephropathy groups with 87% sensitivity and 74% specificity (50).
Lupus nephritis
Lupus nephritis is a great challenge in the management of systemic lupus erythematosus (SLE) patients because of the difficulty in its diagnosis. A study enrolled 107 patients found that urinary NGAL was a good predictor of renal damage in all kinds of SLE patients, with 67% sensitivity and 38% specificity (51). However, systemic NGAL was inferior to urinary NGAL in predicting renal disease activity in a prospective study with 123 lupus nephritis patients (52), and was even shown to be useless in child lupus nephritis patients (53). High levels of systemic NGAL was only associated with faster progression to CKD in patients with lupus nephritis (52). One possible reason for the useless of systemic NGAL in predicting lupus nephritis is that not only renal source of NGAL is involved in lupus nephritis, but also extra renal sources (53).
The accuracy of NGAL as renal disease biomarker is limited under certain pathological conditions
Besides bacterial infection and kidney injury, NGAL protein also upregulates and functions under many other pathological conditions, like cancer, atherosclerosis and Alzheimer’s disease, which also limits the utilization of NGAL as accurate renal disease biomarker. And as extrarenal sources of NGAL protein, they may affect systemic and urinary NGAL differently.
In tumor samples from breast carcinomas patients, NGAL expression was upregulated in 1/3 of the samples, and was associated with decreased disease-specific survival (54). In ovarian cancer, NGAL was downregulated in ovarian cancer cell lines undergoing epithelio-mesenchymal transition induced by epidermal growth factor, and systemic NGAL concentration was significantly higher in patients with malignant ovarian tumors (55). The concentration of systemic NGAL was also elevated in chronic myeloid leukemia patients (56).
NGAL was highly expressed in atheromatous human plaques and associated with increased matrix metalloproteinase-9 activity (57). Later research proved that NGAL involved in atherogenesis by promoting polarization and migration of monocytic cells and development of macrophages towards foam cells (58). The elevation of NGAL concentration in atherosclerosis may reflect the severity of vessel inflammation. Interestingly, systemic NGAL was also significantly higher in patients with Down syndrome, which was characterized by the accumulation of amyloid-β deposits and the presence of activated immune response (59).
As a protein with multiple function, it’s reasonable that NGAL responds to various pathological activities, thus the usefulness of NGAL as renal injury biomarker of patients with cancer, atherosclerosis and Alzheimer’s disease should be carefully examined, and studies should be carried out to further evaluate whether the expression of NGAL is upregulated in other diseases. Although studies discussed above reported that only systemic NGAL was interfered, the possibility that the concentration of urinary NGAL was also altered under these disease conditions cannot be excluded, which needs further investigation as well.
Analytical limitations of NGAL measurements
NGAL has been extensively studied as a biomarker of kidney injury for nearly 10 years. Most researches focused on the diagnostic value of NGAL for AKI, however, the analytical factors limiting its accuracy in practical application were neglected to some extent. Those analytical factors, including biological variation, reagent analytical performance, consistency and interference tolerance, should be explored in depth before NGAL become the “gold standard” of kidney injury diagnosis.
The reported intra-individual biological variation of urinary NGAL ranged from 27% to approximately 180%, and most studies revealed that normalizing NGAL with urinary creatinine would further enlarge the biological variation (60-64). Thus, urinary NGAL concentration was more reliable than NGAL/creatinine ratio in assessing kidney injury, which was also proved by a meta-analysis (65). The concentration of urinary creatinine is affected by gender, age, metabolism and muscle content, which may conversely blur the accuracy of NGAL. The biological variation of systemic NGAL was about 40% (64), indicating that systemic NGAL measurement may perform better in diagnosing kidney injury. However, more attention should be paid to the biological variation of both urinary and systemic NGAL as those studies only tested the biological variation of healthy individuals, but not kidney injury patients.
Due to the lack of commercial NGAL testing reagent (especially in the early stage of clinical investigation) and reference material for NGAL, the reported cut-off values for diagnosing AKI varied greatly in different papers (17,66). In addition, the self-made reagents were not validated by clinical trial, thus the analytical performances of these reagents were questionable. Even commercial reagents could give false-positive results if samples contain hemoglobin at physiological concentration (67), indicating that the results of some published clinical studies were not reliable. These practical issues bring great challenges to the standardization of NGAL testing.
Altogether, standardization of NGAL measurement based on the reference material, and further determination of cut-off value would greatly improve the accuracy of NGAL in diagnosing kidney injury and promote the clinical application of NGAL.
Future direction
Nowadays, it has been generally acknowledged that NGAL is a sensitive early biomarker for the prediction of AKI in cardiac surgery, critically ill and kidney transplant patients, and it also provides important information in the risk stratification and prediction of short-term and long-term outcome for CKD patients with various aetiology (Figure 1). Further studies into the detailed utilization of NGAL in different kinds of renal injury could well improve knowledge, and, fundamentally, improve patient diagnosis, risk stratification and prognosis.
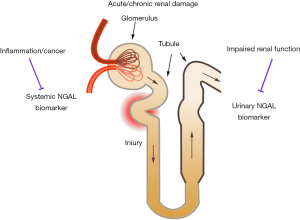
There are still a few factors affecting the accuracy of NGAL as early renal damage biomarker. And because both systemic and urinary NGAL are shown to be effective, the question that whether they reflect the same disease state arises. All studies measured systemic and urinary NGAL simultaneously reported that their concentrations correlated weakly (33,36,48,52,68). Two factors have been proved to limit the accuracy of NGAL (Figure 1): one is damaged kidney function prior to NGAL measurement, especially the impaired glomerular filtration capacity. Upon chronic injury, the baseline NGAL remains at a high level, thus kidney fails to response to acute injury. And impaired GFR leads to accumulation of NGAL in the circulation, as well as abnormal NGAL concentration in the urine. Extrarenal sources of NGAL protein released from neutrophils and other cells contributes to the other factor. Sepsis, inflammation and cancer could upregulate the expression of NGAL protein in neutrophils and cancer cells, which may alter the concentration of systemic and urinary NGAL differently.
In China, NGAL testing is usually much more expensive than other biomarkers of kidney function, and is not covered by medical insurance, bringing a great challenge in the application of NGAL for routine measurement of outpatients. Further studies should focus on the kinetics of systemic and urinary NGAL in different diseases and identify their specific field as renal disease biomarkers, maximizing the benefits of patients. For diseases compromise the accuracy of NGAL, a combination of biomarkers may help in the accurate prediction and risk stratification of renal damage, which still require minute investigations.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.12.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kjeldsen L, Johnsen AH, Sengelov H, et al. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425-32. [PubMed]
- Coles M, Diercks T, Muehlenweg B, et al. The solution structure and dynamics of human neutrophil gelatinase-associated lipocalin. J Mol Biol 1999;289:139-57. [Crossref] [PubMed]
- Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta 2012;1826:129-69. [PubMed]
- Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917-21. [Crossref] [PubMed]
- Schrezenmeier EV, Barasch J, Budde K, et al. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219:554-72. [Crossref] [PubMed]
- Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005;115:610-21. [Crossref] [PubMed]
- Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14:2534-43. [Crossref] [PubMed]
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231-8. [Crossref] [PubMed]
- Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 2006;21:856-63. [Crossref] [PubMed]
- Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007;22:2089-95. [Crossref] [PubMed]
- Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens 2006;15:442-9. [Crossref] [PubMed]
- Rau S, Habicht A, Kauke T, et al. Neutrophil gelatinase-associated lipocalin and end-stage renal disease: it is not all about the kidneys! Eur J Clin Invest 2013;43:816-20. [Crossref] [PubMed]
- Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell 2002;10:1045-56. [Crossref] [PubMed]
- Ronco C, Legrand M, Goldstein SL, et al. Neutrophil gelatinase-associated lipocalin: ready for routine clinical use? An international perspective. Blood Purif 2014;37:271-85. [Crossref] [PubMed]
- Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2006;103:1834-9. [Crossref] [PubMed]
- Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem 2014;51:335-51. [Crossref] [PubMed]
- Zhou F, Luo Q, Wang L, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg 2016;49:746-55. [Crossref] [PubMed]
- Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014;18:R144. [Crossref] [PubMed]
- Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 2011;58:2301-9. [Crossref] [PubMed]
- Ho J, Tangri N, Komenda P, et al. Urinary, Plasma, and Serum Biomarkers' Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. Am J Kidney Dis 2015;66:993-1005. [Crossref] [PubMed]
- Gaipov A, Solak Y, Turkmen K, et al. Serum uric acid may predict development of progressive acute kidney injury after open heart surgery. Ren Fail 2015;37:96-102. [Crossref] [PubMed]
- Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22:1748-57. [Crossref] [PubMed]
- Shum HP, Leung NY, Chang LL, et al. Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care unit patients after major non-cardiac surgery. Nephrology (Carlton) 2015;20:375-82. [Crossref] [PubMed]
- de Geus HR, Bakker J, Lesaffre EM, et al. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011;183:907-14. [Crossref] [PubMed]
- Egal M, de Geus HR, Groeneveld AB. Neutrophil gelatinase-associated lipocalin as a diagnostic marker for acute kidney injury in oliguric critically Ill patients: a post-hoc analysis. Nephron 2016;134:81-8. [Crossref] [PubMed]
- Martensson J, Bell M, Oldner A, et al. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med 2010;36:1333-40. [Crossref] [PubMed]
- Ramirez-Sandoval JC, Herrington W, Morales-Buenrostro LE. Neutrophil gelatinase-associated lipocalin in kidney transplantation: A review. Transplant Rev (Orlando) 2015;29:139-44. [Crossref] [PubMed]
- Mahdavi-Mazdeh M, Amerian M, Abdollahi A, et al. Comparison of serum neutrophil gelatinase-associated lipocalin (NGAL) with serum creatinine in prediction of kidney recovery after renal transplantation. Int J Organ Transplant Med 2012;3:176-82. [PubMed]
- Fonseca I, Oliveira JC, Almeida M, et al. Neutrophil gelatinase-associated lipocalin in kidney transplantation is an early marker of graft dysfunction and is associated with one-year renal function. J Transplant 2013;2013:650123 [Crossref] [PubMed]
- Choi HM, Park KT, Lee JW, et al. Urine neutrophil gelatinase-associated lipocalin predicts graft outcome up to 1 year after kidney transplantation. Transplant Proc 2013;45:122-8. [Crossref] [PubMed]
- Hall IE, Doshi MD, Poggio ED, et al. A comparison of alternative serum biomarkers with creatinine for predicting allograft function after kidney transplantation. Transplantation 2011;91:48-56. [Crossref] [PubMed]
- Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care 2015;19:223. [Crossref] [PubMed]
- Vanmassenhove J, Glorieux G, Lameire N, et al. Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 2015;16:18. [Crossref] [PubMed]
- McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol 2010;5:211-9. [Crossref] [PubMed]
- Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 2007;22:101-8. [Crossref] [PubMed]
- Malyszko J, Bachorzewska-Gajewska H, Sitniewska E, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2-4 chronic kidney disease. Ren Fail 2008;30:625-8. [Crossref] [PubMed]
- Bhavsar NA, Kottgen A, Coresh J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2012;60:233-40. [Crossref] [PubMed]
- Lim WH, Lewis JR, Wong G, et al. Plasma neutrophil gelatinase-associated lipocalin and kidney function decline and kidney disease-related clinical events in older women. Am J Nephrol 2015;41:156-64. [Crossref] [PubMed]
- Bolignano D, Basile G, Parisi P, et al. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res 2009;12:7-14. [Crossref] [PubMed]
- Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J 2009;30:1229-36. [Crossref] [PubMed]
- Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J 2011;32:2705-12. [Crossref] [PubMed]
- Nakada Y, Kawakami R, Matsui M, et al. Prognostic value of urinary neutrophil gelatinase-associated lipocalin on the first day of admission for adverse events in patients with acute decompensated heart failure. J Am Heart Assoc 2017;6. [PubMed]
- Santaguida PL, Don-Wauchope AC, Oremus M, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 2014;19:453-70. [Crossref] [PubMed]
- Maisel AS, Mueller C, Fitzgerald R, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail 2011;13:846-51. [Crossref] [PubMed]
- De Berardinis B, Gaggin HK, Magrini L, et al. Comparison between admission natriuretic peptides, NGAL and sST2 testing for the prediction of worsening renal function in patients with acutely decompensated heart failure. Clin Chem Lab Med 2015;53:613-21. [Crossref] [PubMed]
- Nielsen SE, Schjoedt KJ, Astrup AS, et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med 2010;27:1144-50. [Crossref] [PubMed]
- Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 2011;34:975-81. [Crossref] [PubMed]
- Yang YH, He XJ, Chen SR, et al. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine 2009;36:45-51. [Crossref] [PubMed]
- Assal HS, Tawfeek S, Rasheed EA, et al. Serum cystatin C and tubular urinary enzymes as biomarkers of renal dysfunction in type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 2013;6:7-13. [Crossref] [PubMed]
- Mahfouz MH, Assiri AM, Mukhtar MH. Assessment of neutrophil gelatinase-associated lipocalin (NGAL) and retinol-binding protein 4 (RBP4) in Type 2 diabetic patients with nephropathy. Biomark Insights 2016;11:31-40. [Crossref] [PubMed]
- Rubinstein T, Pitashny M, Levine B, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford) 2010;49:960-71. [Crossref] [PubMed]
- Torres-Salido MT, Cortes-Hernandez J, Vidal X, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for lupus nephritis. Nephrol Dial Transplant 2014;29:1740-9. [Crossref] [PubMed]
- Hammad A, Mosaad Y, Elhanbly S, et al. Urinary neutrophil gelatinase-associated lipocalin as a marker of severe lupus nephritis in children. Lupus 2013;22:486-91. [Crossref] [PubMed]
- Bauer M, Eickhoff JC, Gould MN, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat 2008;108:389-97. [Crossref] [PubMed]
- Lim R, Ahmed N, Borregaard N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer 2007;120:2426-34. [Crossref] [PubMed]
- Villalva C, Sorel N, Bonnet ML, et al. Neutrophil gelatinase-associated lipocalin expression in chronic myeloid leukemia. Leuk Lymphoma 2008;49:984-8. [Crossref] [PubMed]
- te Boekhorst BC, Bovens SM, Hellings WE, et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc Res 2011;89:680-8. [Crossref] [PubMed]
- Oberoi R, Bogalle EP, Matthes LA, et al. Lipocalin (LCN) 2 Mediates Pro-Atherosclerotic Processes and Is Elevated in Patients with Coronary Artery Disease. PLoS One 2015;10:e0137924 [Crossref] [PubMed]
- Naude PJ, Dekker AD, Coppus AM, et al. Serum NGAL is Associated with Distinct Plasma Amyloid-beta Peptides According to the Clinical Diagnosis of Dementia in Down Syndrome. J Alzheimers Dis 2015;45:733-43. [PubMed]
- Delanaye P, Rozet E, Krzesinski JM, et al. Urinary NGAL measurement: biological variation and ratio to creatinine. Clin Chim Acta 2011;412:390. [Crossref] [PubMed]
- Zhang X, Gibson B Jr, Mori R, et al. Analytical and biological validation of a multiplex immunoassay for acute kidney injury biomarkers. Clin Chim Acta 2013;415:88-93. [Crossref] [PubMed]
- Grenier FC, Ali S, Syed H, et al. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem 2010;43:615-20. [Crossref] [PubMed]
- Helmersson-Karlqvist J, Arnlov J, Larsson A. Day-to-day variation of urinary NGAL and rational for creatinine correction. Clin Biochem 2013;46:70-2. [Crossref] [PubMed]
- Bataille A, Tiepolo A, Robert T, et al. Reference change values of plasma and urine NGAL in cardiac surgery with cardiopulmonary bypass. Clin Biochem 2017;50:1098-103. [Crossref] [PubMed]
- Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol 2012;23:322-33. [Crossref] [PubMed]
- Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012-24. [Crossref] [PubMed]
- Kift RL, Messenger MP, Wind TC, et al. A comparison of the analytical performance of five commercially available assays for neutrophil gelatinase-associated lipocalin using urine. Ann Clin Biochem 2013;50:236-44. [Crossref] [PubMed]
- Shrestha K, Shao Z, Singh D, et al. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol 2012;110:1329-35. [Crossref] [PubMed]
Cite this article as: Ning M, Mao X, Niu Y, Tang B, Shen H. Usefulness and limitations of neutrophil gelatinase-associated lipocalin in the assessment of kidney diseases. J Lab Precis Med 2018;3:1.


