Are we getting better at the preanalytical phase or just better at measuring it?
The total testing process
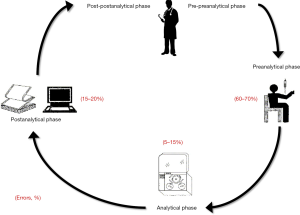
The total testing process can be conventionally split into five principal areas, only one of which is directly related to analytical testing. Briefly, the testing process begins with the pre-preanalytical phase (mainly related to diagnostic tests ordering), then continues with the preanalytical phase (involving patient preparation before testing along with collection, handling, transportation and storage of biological specimens), the analytical phase (i.e., sample analysis), the post-analytical phase (test results reporting), and finally ends with the post-postanalytical phase (pertaining result interpretation and the ensuing clinical decisions) (Figure 1) (1). Notably, the first and clear division of the total testing process in 3 to 5 phases, as currently accepted, can be dated back to 1981, when Gorge D. Lundberg, the former editor of the Journal of American Medical Association (JAMA), coined the term “brain-to-brain turnaround loop” (2). Since then, this brilliant representation has been widely used to illustrate the inherent complexity of the testing process, which is no longer uniquely identified with the analytical phase (3).
The concept of preanalytical errors
In English dictionaries, laboratory error is defined as “an error made by the personnel in a clinical laboratory in performing a test, interpreting data, or reporting or recording the results” (4). According to the ISO Technical Report 22367, the same concept is reported as “a defect occurring in any part of the laboratory cycle, from ordering tests to reporting results and appropriately interpreting and reacting in these” (5). These two definitions, which appear substantially identical, both emphasize that a laboratory error can be seen as any type of mistake occurring throughout the total testing process, i.e., from the pre-preanalytical to the post-postanalytical phase, so dispelling a widespread clinicians’ perception that diagnostic errors are essentially or exclusively “analytical” (6).
As for many other human activities, the possibility that an error may occur throughout the total testing process is not irrelevant. Reliable studies attest that the overall frequency of laboratory errors can be as high as 0.31% of all tests performed (7). It is worthwhile mentioning here, however, that the burden of human errors is directly related to error probability per opportunity and to the number of opportunities for an error to be made (8). Therefore, although it is not difficult to believe that clinical laboratories may actually make 10- to 100-fold more errors than radiologists, the overall number of tests performed by a clinical laboratory is 100- to 1,000-fold higher than those performed by the radiology. When these two estimates are combined [i.e., (number of errors)/(number of tests)], it can be clearly concluded that errors in laboratory medicine have a frequency approximately 10 times lower than in radiology (9). It can hence be concluded that, indeed, “Houston, we have a (preanalytical) problem”, but it is undeniable that other diagnostic areas actually have even bigger problems than laboratory medicine.
When the total number of errors is classified according to the different phases of the total testing process, it is now undoubtable that the vast majority of these (i.e., up to two-third) tend to occur in the preanalytical phase (7). Basically, a preanalytical error can hence be regarded as any error occurring from test ordering to physical performance of the test, a process including a kaleidoscope of manually-intensive activities that are still needed to collect reliable biological materials for testing (Figure 1).
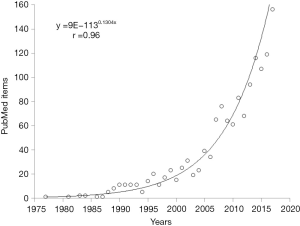
Apparently, the research into the preanalytical phase and its related problems can be seen as a quite long journey, which has started more than 40 years ago. If one enters the search term “preanalytical” in PubMed, the first item that can be retrieved is a review article published by Statland and Winkel in 1977, dealing with within-subject variability of laboratory tests performed in healthy individuals (10), Nevertheless, if one performs a similar digital search using the key word “laboratory error”, the oldest article appearing in PubMed has been published (in Italian) by Mariani in 1954, and actually deals with erroneous data of erythrocytes sedimentation rate (ESR) attributable to problems occurring during collection and preservation of blood (11). Therefore, although the term “preanalytical” has only been officially endorsed in the late 1970s, the issue of the potential impact of preanalytical activities on quality of test results and patient safety is much older. Interestingly, the number of PubMed items retrieved using the keyword “preanalytical” is also shown in Figure 2, and the trend is very closely described by an exponential line, characterized by an extraordinary correlation coefficient (r=0.96; P<0.001). This hence mirrors the exponentially increasing interest in science and medicine for the preanalytical phase and in for its related vulnerability.
The nature of preanalytical errors
Although a thoughtful description of the main sources of preanalytical errors is beside the scope of this article, it is worthwhile giving some hints concerning their relative frequency. Overall, the vast majority of preanalytical errors occur during venipuncture, and are mostly attributable to negligence, suboptimal phlebotomy practice, ignorance of basic preanalytical principles (i.e., inappropriate filling or mixing of blood tubes), contamination with exogenous fluids (12). All these factors then contribute to generate a kaleidoscope of preanalytical problems such as (in decreasing order of frequency) hemolyzed samples, blood collected in wrong tubes, underfilled blood tubes, clotted or misidentified specimens (12,13).
Are we getting better at the preanalytical phase or just better at measuring it?
The answer to this question is quite challenging, if not impossible. It is clear to everybody that whenever certain systems or complex organizations are more strictly observed and monitored, the frequency of failures increases in parallel just because slips, lapses and even errors are more precisely identified and then recorded. This holds true for whatever human activity including industry, finance, information technology (IT) as well as healthcare and (laboratory) medicine (14). The answer to this question gets even more problematic because the number of studies which have systematically monitored (for a sufficiently long period of time) the frequency of errors within the same environment remains limited. One of the best publications that has addressed this issue has been published by Carraro and Plebani, who monitored the type and frequency of laboratory errors, in the same clinical laboratory and using the same monitoring system, over a 10-year period. In the first part of this investigation, published in 1997, the authors reported 0.47% frequency of laboratory errors (15), which had however decreased to 0.31% 10-year afterwards (7). In another study Giménez-Marín et al. performed a prospective study aimed to monitor preanalytical errors in a Spanish clinical laboratory (13), and concluded that the error rate had decreased by approximately 20% over a 5-year period (i.e., between the years 2007 and 2011).
Reliable evidence that the risk of making preanalytical errors can be actually reduced is also supported by a series of interesting interventional studies. As previously discussed, the vast majority of preanalytical errors are attributable to sample collection and, most precisely, to inaccuracies in phlebotomy practice or blood collection systems (12). In a series of interventional studies aimed to optimize the use of blood collection devices in the emergency department we previously showed that both manual aspirations of blood using closed systems (16), as well as specifically-designed blood tube holders (17), can be effective to substantially decrease the rate of spuriously hemolyzed specimens when blood is collected from intravenous catheters. Similar results were obtained by other groups by using low vacuum tubes in association in indwelling catheters (18,19). Regarding blood collection practice, which widely differs among different phlebotomists (20,21), Lillo et al. showed that an educational program targeted for healthcare personnel can substantially reduce the number of sample errors and generate significant improvements of sample quality (22). Similar improvements were recorded by Bölenius et al. after establishing educational intervention programs for phlebotomists (23), and by Ying et al. who applied a training system aimed to improve quality awareness about the preanalytical phase and behaviors of medical staff (24). A higher degree of sample quality could also be ensured by instructing phlebotomists to avoid collecting blood from small and fragile veins (25), by transmission of periodic preanalytical quality reports to phlebotomists and establishment of direct feed-back between laboratory professionals and phlebotomists (26), by strict observance of available blood collection guidelines (27), by design and dissemination of specimen collection modules (28), or by implementation of phlebotomy check-lists (29).
Taken together, these proof-of-evidence studies clearly attest that the correct answer to the crucial question as to whether we are getting better at the preanalytical phase or we are simply systematically and more accurately identifying and monitoring errors, is… both. Indeed, the dissemination of recommendations and guidelines on the best practices for the preanalytical phase, along with the establishment of many international and national working groups on this topic [e.g., the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE) or the International Federation of Clinical Chemistry and Laboratory Medicine Working Group on Laboratory Errors and Patient Safety (WG-LEPS)] (30-32), have greatly contributed to enhance the awareness about the importance of reducing laboratory errors and improving the quality of the preanalytical phase. On the other hand, the increased consciousness on these crucial topics has promoted the development and implementation of a number of quality indicators throughout the total testing process, thus including the preanalytical phases, which are increasingly used by clinical laboratories for monitoring local performance and benchmarking with others (33). Since error recording policies are many and multifaceted (e.g., audit, manual recording processes, incident reporting, laboratory information systems or specific software) (34,35), this process shall entail the development of harmonized means for recording errors and other non-conformities.
Conclusions
The management thinker Peter Drucker, the man who conceived modern business management, is often quoted as saying that “you cannot manage what you cannot measure”. The Italian natural philosopher Galileo Galilei is also quoted as saying that “you should measure what can be measured, and make measurable what cannot be measured”. If we combine these two foremost quotes and translate them into the field of laboratory medicine, what can be concluded is that we should place more efforts for increasing measurement of preanalytical quality indicators and then intervene upstream to correct areas of the total testing process with greater vulnerability. Although we cannot deny that the interest in identifying and recording preanalytical errors has notably increased over time (36), it is also clear that the combination of a greater consciousness about preanalytical issues and technological advancements for assessing sample quality (i.e., automatic measurement of serum indices) and for decreasing the risk of making errors (i.e., specimen labeling devices), have helped decreasing the inherent vulnerability of many preanalytical activities (Table 1) (37). Many other valuable opportunities are in development, including robotic phlebotomy devices and active blood tubes (38).
Table 1
| Education and training of phlebotomists about phlebotomy practice and preanalytical errors |
| Observance of available phlebotomy guidelines |
| Dissemination of specimen collection modules or phlebotomist check-list |
| Certification of phlebotomists |
| Transmission of periodic preanalytical quality reports to phlebotomists |
| Establishment of direct feed-back between laboratory professionals and phlebotomists |
| Use of quality and validated blood collection systems for drawing blood |
| Use harmonized means for recording preanalytical errors |
With diagnostic testing increasingly committed to the cutting-edge personalized medicine (39), but still plagued by many questionable “political” issues (40), best quality throughout the total testing will become even more critical for supporting clinical decision making and safeguarding patient safety.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael Cornes and Jennifer Atherton) for the series “Reducing errors in the pre-analytical phase” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.01.03). The series “Reducing errors in the pre-analytical phase” was commissioned by the editorial office without any funding or sponsorship. Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plebani M, Lippi G. Uncertainty, quality, safety and accreditation in laboratory medicine. J Lab Prec Med 2017;2:80. [Crossref]
- Lundberg GD. Acting on significant laboratory results. JAMA 1981;245:1762-3. [Crossref] [PubMed]
- Plebani M, Lippi G. Closing the brain-to-brain loop in laboratory testing. Clin Chem Lab Med 2011;49:1131-3. [Crossref] [PubMed]
- The Free Dictionary. Laboratory Error. Available online: https://medical-dictionary.thefreedictionary.com/laboratory+error
- Medical laboratories—Reduction of error through risk management and continual improvement. Available online: https://www.iso.org/standard/40918.html
- Lippi G, Cervellin G. From laboratory instrumentation to physician’s brain calibration: the next frontier for improving diagnostic accuracy? J Lab Prec Med 2017;2:74. [Crossref]
- Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem 2007;53:1338-42. [Crossref] [PubMed]
- Center For Chemical Process Safety. Guidelines for Initiating Events and Independent Protection Layers in Layer of Protection Analysis. Hoboken, NJ: John Wiley & Sons, 2015.
- Plebani M, Lippi G. To err is human. To misdiagnose might be deadly. Clin Biochem 2010;43:1-3. [Crossref] [PubMed]
- Statland BE, Winkel P. Effects of preanalytical factors on the intraindividual variation of analytes in the blood of healthy subjects: consideration of preparation of the subject and time of venipuncture. CRC Crit Rev Clin Lab Sci 1977;8:105-44. [Crossref] [PubMed]
- Mariani G. Laboratory errors related to the collection and preservation of blood and technical simplification in the determination of sedimentation rate. Boll Soc Med Chir Cremona 1954;8:47-9. [PubMed]
- Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med 2007;45:720-7. [Crossref] [PubMed]
- Giménez-Marín A, Rivas-Ruiz F, Pérez-Hidalgo Mdel M, et al. Pre-analytical errors management in the clinical laboratory: a five-year study. Biochem Med (Zagreb) 2014;24:248-57. [Crossref] [PubMed]
- Hofmann DA, Frese M. editors. Errors in Organizations. 1st Edition. New York, NY: Routledge, 2011.
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997;43:1348-51. [PubMed]
- Lippi G, Bonelli P, Bonfanti L, et al. The use of S-Monovette is effective to reduce the burden of hemolysis in a large urban emergency department. Biochem Med (Zagreb) 2015;25:69-72. [Crossref] [PubMed]
- Lippi G, Avanzini P, Aloe R, et al. Reduction of gross hemolysis in catheter-drawn blood using Greiner Holdex tube holder. Biochem Med (Zagreb) 2013;23:303-7. [Crossref] [PubMed]
- Heiligers-Duckers C, Peters NA, van Dijck JJ, et al. Low vacuum and discard tubes reduce hemolysis in samples drawn from intravenous catheters. Clin Biochem 2013;46:1142-4. [Crossref] [PubMed]
- Giavarina D. Low volume tubes can be effective to reduce the rate of hemolyzed specimens from the emergency department. Clin Biochem 2014;47:688-9. [Crossref] [PubMed]
- Simundic AM, Cornes M, Grankvist K, et al. Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA). Clin Chem Lab Med 2013;51:1585-93. [PubMed]
- Simundic AM, Church S, Cornes MP, et al. Compliance of blood sampling procedures with the CLSI H3-A6 guidelines: An observational study by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PRE). Clin Chem Lab Med 2015;53:1321-31. [Crossref] [PubMed]
- Lillo R, Salinas M, Lopez-Garrigos M, et al. Reducing preanalytical laboratory sample errors through educational and technological interventions. Clin Lab 2012;58:911-7. [PubMed]
- Bölenius K, Brulin C, Graneheim UH. Personnel's Experiences of Phlebotomy Practices after Participating in an Educational Intervention Programme. Nurs Res Pract 2014;2014:538704 [Crossref] [PubMed]
- Ying Li H, Yang YC, Huang WF, et al. Reduction of preanalytical errors in laboratory by establishment and application of training system. J Evid Based Med 2014;7:258-62. [Crossref] [PubMed]
- Lippi G, Avanzini P, Aloe R, et al. Blood collection from intravenous lines: is one drawing site better than others? Lab Med 2014;45:172-5. [Crossref] [PubMed]
- Salinas M, Lopez-Garrigos M, Flores E, et al. Three years of preanalytical errors: quality specifications and improvement through implementation of statistical process control. Scand J Clin Lab Invest 2009;69:822-6. [Crossref] [PubMed]
- Lima-Oliveira G, Lippi G, Salvagno GL, et al. Impact of the phlebotomy training based on CLSI/NCCLS H03-a6 - procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med (Zagreb) 2012;22:342-51. [Crossref] [PubMed]
- Le RD, Melanson SE, Petrides AK, et al. Significant Reduction in Preanalytical Errors for Nonphlebotomy Blood Draws After Implementation of a Novel Integrated Specimen Collection Module. Am J Clin Pathol 2016;146:456-61. [Crossref] [PubMed]
- Giavarina D, Lippi G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem 2017;50:568-73. [Crossref] [PubMed]
- Cornes MP, Church S, van Dongen-Lases E, et al. The role of European Federation of Clinical Chemistry and Laboratory Medicine Working Group for Preanalytical Phase in standardization and harmonization of the preanalytical phase in Europe. Ann Clin Biochem 2016;53:539-47. [Crossref] [PubMed]
- Lippi G, Simundic AMEuropean Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). The EFLM strategy for harmonization of the preanalytical phase. Clin Chem Lab Med 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Sciacovelli L, Plebani M. The IFCC Working Group on laboratory errors and patient safety. Clin Chim Acta 2009;404:79-85. [Crossref] [PubMed]
- Sciacovelli L, Panteghini M, Lippi G, et al. Defining a roadmap for harmonizing quality indicators in Laboratory Medicine: a consensus statement on behalf of the IFCC Working Group "Laboratory Error and Patient Safety" and EFLM Task and Finish Group "Performance specifications for the extra-analytical phases". Clin Chem Lab Med 2017;55:1478-88. [Crossref] [PubMed]
- West J, Atherton J, Costelloe SJ, et al. Preanalytical errors in medical laboratories: a review of the available methodologies of data collection and analysis. Ann Clin Biochem 2017;54:14-19. [Crossref] [PubMed]
- Lippi G, Sciacovelli L, Simundic AM, et al. Innovative software for recording preanalytical errors in accord with the IFCC quality indicators. Clin Chem Lab Med 2017;55:e51-3. [Crossref] [PubMed]
- Cornes MP, Atherton J, Pourmahram G, et al. Monitoring and reporting of preanalytical errors in laboratory medicine: the UK situation. Ann Clin Biochem 2016;53:279-84. [Crossref] [PubMed]
- Lippi G, Baird GS, Banfi G, et al. Improving quality in the preanalytical phase through innovation, on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med 2017;55:489-500. [Crossref] [PubMed]
- Lippi G, Cadamuro J. Novel opportunities for improving the quality of preanalytical phase. a glimpse to the future? J Med Biochem 2017;36:293-300. [Crossref]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Lippi G, Plebani M. The add value of laboratory diagnostics: the many reasons why decision-makers should actually care. J Lab Precis Med 2017;2:100. [Crossref]
Cite this article as: Lippi G, Mattiuzzi C, Bovo C. Are we getting better at the preanalytical phase or just better at measuring it? J Lab Precis Med 2018;3:11.