
The role of fecal calprotectin in investigating digestive disorders
Introduction
Calprotectin is a 36-kDa calcium and zinc binding protein found in human neutrophils, monocytes, and macrophages (1,2). It is a heterodimer of two calcium binding proteins—S100A8 and S100A9, and the name was derived from both its calcium binding properties (cal) and antimicrobial activity (protect) in vitro (3,4). Calprotectin was first isolated from human granulocytes in 1980 by Fagerhol et al. (2). Calprotectin constitutes between 30–60% of neutrophil cytosolic proteins (5). It is released from activated neutrophils during periods of active inflammation, as well as upon neutrophil damage or death (6,7). Although calprotectin may be isolated and measured in multiple body fluids including plasma, urine, cerebrospinal fluid, synovial fluid, and pleural fluid, it is primarily clinically useful to gastroenterologists through measurement of fecal concentrations as a direct marker of mucosal inflammation (8). Fecal calprotectin elevation can be seen in both multiple primary gastrointestinal as well as extra-intestinal disease processes (9). Fecal calprotectin can be measured via enzyme-linked immunosorbent assay (ELISA) testing in stool samples to detect intestinal inflammation of any etiology (10). It has a reported stability at room temperature in stool samples for up to one week, although at seven days there has been a noted significant variance in intra-sample concentrations (11). Analysis of a single stool sample is adequate for measurement of fecal calprotectin as good correlation has been seen between one time sampling values and those obtained from 24 hours collections (12). The presence of an elevated fecal calprotectin level, although sensitive for mucosal inflammation, is nonspecific and there are a number of infectious, inflammatory, and neoplastic processes that may lead to an elevation in the fecal calprotectin concentration. Past studies established normal values in healthy persons of 2 mg/L of fecal calprotectin, with a recommended cut-off test of 10 mg/L. A fecal calprotectin cutoff value of 10 mg/L was shown to be 89% sensitive and 79% in identifying organic intestinal disease (13). Utilizing newer assays, current recommendations for an upper limit of normal have increased to 50 µg/g, with even better diagnostic precision for active inflammatory bowel disease (IBD) seen at levels of greater than 100 µg/g (9,14). Unfortunately, there are differences in assay calibration between manufacturers leading to variations in inter-assay agreement, which can affect the specificity of identifying active disease if the same cut-offs are used regardless of the specific assay (15). Patients and providers would both benefit from better co-calibration of fecal calprotectin assays.
Fecal calprotectin and IBD diagnosis
Most often in today’s clinical practice, fecal calprotectin is used in the initial investigation and subsequent monitoring during therapy of patients with IBD. One benefit for patients of using fecal calprotectin to assess disease activity is that it is a noninvasive marker, not requiring phlebotomy, exposure to radiation (such as with cross sectional imaging), or the risks inherent with endoscopy (including exposure to sedation and the risks of bleeding, perforation, etc.), to provide objective data regarding the presence or activity of luminal inflammation. A large 2010 meta-analysis found measurement of fecal calprotectin had a sensitivity of 93% and specificity of 96% for detecting inflammation in adult patients, which could be used to help stratify which patients would benefit from more urgent endoscopic evaluation of their gastrointestinal symptoms (16). A strategy of initial screening with fecal calprotectin and referral for colonoscopy versus colonoscopy avoidance using predefined cut-off levels has been shown to be effective both in reducing health care costs and number of invasive procedures (17). Fecal calprotectin plays an important role both in helping gastroenterologists differentiate IBD from functional or visceral hypersensitivity disorders such as irritable bowel syndrome (IBS), and subsequently in assessing the degree of inflammation in patients with IBD to help tailor or optimize medical management. Neither the American College of Gastroenterology (ACG) nor the European Crohn’s and Colitis Organisation recommend sole reliance on fecal calprotectin when making a diagnosis of IBD, although it is recommended as “an excellent way to confirm intestinal inflammation” by the ACG (10,18).
Fecal calprotectin and IBD management
Fecal calprotectin is not only effective in differentiating IBD from IBS, but also in differentiating active IBD from inactive IBD (19). This kind of objective symptom evaluation is often useful because even patients with a known diagnosis of IBD may have gastrointestinal complaints due to other coexistent processes that may not represent inadequate control or a true flare of their disease (Figure 1). An elevated fecal calprotectin value may be even more accurate than an elevated C-reactive protein (CRP) level for predicting active, endoscopically visual mucosal inflammation (19). Fecal calprotectin is also an inherently gut-specific test compared to the CRP, which can be elevated due to any etiology of systemic inflammation. In patients with known IBD, fecal calprotectin levels correlate with mucosal healing (14,20). There may be a role going forward for monitoring fecal calprotectin in asymptomatic patients, as there is evidence that persistent elevations portend a future relapse, whereas normal levels suggest sustained remission (21). Clinical use of fecal calprotectin measurements will likely continue to increase as objective markers of inflammation, especially those that correlate with the degree of mucosal and/or histologic disease activity, are becoming a more desired treatment endpoint over patient reported symptom assessments in IBD medication and outcomes research.
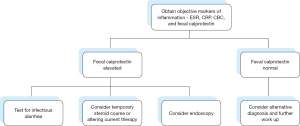
Fecal calprotectin and ulcerative colitis (UC)
Fecal calprotectin levels have been shown to correlate well with clinical disease activity indices, endoscopic indices, and other biomarkers, and normalization of levels are highly predictive of complete mucosal healing in UC patients (22). One study demonstrated that a fecal calprotectin level ≤60 µg/g could predict deep remission from an endoscopic, histological, and Patient Reported Outcome (PRO2 score) standpoint with >85% sensitivity and specificity in UC patients (23). Fecal calprotectin can also be utilized in patients in remission to predict future episodes of relapse, with one study showing values of ≥170 µg/g being >75% sensitive and specific for relapse in the next year (24). Other data has shown an even higher sensitivity (90%) for predicting relapse in CD and UC patients at fecal calprotectin values ≥50 mg/L (25).
Fecal calprotectin and Crohn’s disease (CD)
Past evidence, including a large meta-analysis, raised the concern that fecal calprotectin may be more accurate in screening and assessment for inflammation in ulcerative colitis patients versus patients with CD (14). However, fecal calprotectin has also shown to be highly sensitive in predicting active small bowel inflammation on capsule endoscopy in patients with CD and normal colonoscopy (26). This finding demonstrates another beneficial use of fecal calprotectin—the ability to assess for the presence of small bowel inflammation in patients in a noninvasive manner in a location that would not be visualized on histopathology obtained during ileocolonoscopy, the gold standard in IBD diagnosis and surveillance for disease activity. An elevated fecal calprotectin also seems to correlate well with computed tomography enterography (CTE) findings of active inflammation in small bowel CD (27).
Fecal calprotectin and the postoperative IBD patient
Fecal calprotectin is also useful in postoperative management of IBD patients. In UC patients who have undergone proctocolectomy, one study demonstrated significantly higher fecal calprotectin levels in patients with active endoscopic and histologic findings of pouchitis (28). A small, postoperative prospective study demonstrated a normalization of fecal calprotectin values in CD patients two months following ileocecectomy, and good correlation between subsequent levels >100 g/g and clinically active disease (29). Fecal calprotectin has also been shown to significantly correlate with the Rutgeerts score, an endoscopic scoring system used to monitor for postoperative recurrence of CD, and its measurement may be able to decrease colonoscopy usage in patients with normal fecal calprotectin values (30). Measurement of fecal calprotectin in IBD patients with significantly altered gastrointestinal anatomy due to a history of small bowel or colonic resections can be especially useful, as there may be many other potential etiologies for abdominal discomfort or loose stools (such as bile acid malabsorption, visceral hypersensitivity, bacterial overgrowth, postoperative stricturing, etc.) in these patients that are not due to recurrent or uncontrolled IBD that would require alternative management and could obviate the need for endoscopy.
Fecal calprotectin and infectious diarrhea
As fecal calprotectin is a nonspecific marker of intestinal inflammation, it is also elevated in patients with infectious diarrhea. Fecal calprotectin has been demonstrated to be highly sensitive in assessing for infectious enterocolitis (9). Higher values are more suggestive of bacterial, rather than viral enteritis (9,31). Fecal calprotectin is also elevated in patients with Clostridium difficile infection (CDI), with some data demonstrating the degree of fecal calprotectin elevation correlating with disease severity, which is an important factor in determining treatment recommendations (32,33). Fecal calprotectin levels also may be higher in CDI patients infected with the hypervirulent ribotype 027 strain (33).
Fecal calprotectin and colorectal cancer
Given an ongoing interest in identifying noninvasive markers for population based colorectal cancer screening, research has been conducted to evaluate the role of fecal calprotectin in the diagnosis and subsequent monitoring of patients with colorectal cancer. Past studies have shown higher levels of calprotectin present in the stool of patients with colorectal cancer, however most of these studies have looked at levels retrospectively in patients with known diagnoses of colorectal cancer, and the low specificity of fecal calprotectin for cancer screening specifically make it unlikely to be used for widespread screening purposes going forward (11). Prospective data has also demonstrated that the fecal immunochemical test (FIT) detects a higher proportion of colorectal cancers and high risk adenomas compared with fecal calprotectin among outpatients undergoing screening for intestinal pathology by their primary care providers (34).
Fecal calprotectin and celiac disease
There has been conflicting data regarding whether elevated levels of fecal calprotectin are found in patients with unmanaged celiac disease. There has been some evidence demonstrating an elevated fecal calprotectin in patients with untreated celiac disease, with normalization once a patient was adherent to a gluten free diet (35). One study of 50 patients with recently diagnosed celiac disease found no correlation between a fecal calprotectin level of 75 µg/g or greater with patient symptoms, tissue transglutaminase levels, or histologic severity (36). The general consensus is that there is currently no role for fecal calprotectin in the diagnosis or management of patients with celiac disease.
Fecal calprotectin and other conditions
Abnormal levels of fecal calprotectin can also be found in several other gastrointestinal conditions, although its specificity for any particular disease process will be limited in this setting. In patients with microscopic colitis, for example, patients may have a mildly elevated fecal calprotectin level compared to control patients, but the absolute level in one study was still relatively low, with a median of 48 g/g (37). Up to 38% of patients with active histologic microscopic colitis may have normal levels of fecal calprotectin, as well, further limiting its sensitivity (38).
Fecal calprotectin can also be elevated in patients taking NSAIDs, likely due to NSAID induced enteropathy (39). Fecal calprotectin elevation is not commonly seen with upper gastrointestinal tract lesions such as Barrett’s esophagus or gastric ulceration, and although some patients may have elevated levels compared to controls, levels in this population are likely to be lower than the common cutoff value of 100 g/g (40,41). Levels of fecal calprotectin also appear to be elevated in patients with cirrhosis compared to matched control patients, demonstrating intestinal inflammation that may play a role in suspected gut lumen bacterial translocation leading to infectious complications in these patients (42). Fecal calprotectin elevation can also be seen in patients with acute diverticulitis, and continued elevations after clinical resolution can be predictive of recurrence (43,44).
Conclusions
Calprotectin is a protein released from neutrophils and other inflammatory cells during an inflammatory response. Use of fecal measurement of calprotectin to assess intestinal inflammation, whether in screening for gastrointestinal mucosal inflammation or monitoring disease activity in patients with known IBD, is a valuable tool for clinicians. In patients presenting to primary care providers and gastroenterologists with nonspecific gastrointestinal symptoms, the presence of an elevated fecal calprotectin can help stratify patients towards earlier endoscopic evaluation, while levels below 50 g/g could stratify patients towards more conservative management. As a significant number of patients present to their general practitioners with undifferentiated gastrointestinal complaints, primary care providers may find utilizing fecal calprotectin to be useful in evaluating for active intestinal inflammation. Its use will likely become more prevalent in the future as alternatives to endoscopy and repeated imaging tests become increasingly important to both patients and providers. As the paradigm of treatment for IBD continues to shift from clinical/patient symptom driven endpoints towards therapy with a goal of obtaining mucosal and histologic healing, it is important to have objective markers of inflammation, such as fecal calprotectin, to help guide clinicians in management decisions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hemant Goyal) for the series “Role of biomarkers in gastrointestinal disorders” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.02.03). The series “Role of biomarkers in gastrointestinal disorders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Poullis A, Foster R, Mendall MA, et al. Emerging role of calprotectin in gastroenterology. J Gastroenterol Hepatol 2003;18:756-62. [Crossref] [PubMed]
- Fagerhol MK, Dale I, Andersson T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull Eur Physiopathol Respir 1980;16:273-82. [PubMed]
- Steinbakk M, Naess-Andresen CF, Lingaas E, et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 1990;336:763-5. [Crossref] [PubMed]
- Vrabie R, Kane S. Noninvasive Markers of Disease Activity in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2014;10:576-84. [PubMed]
- Stríz I, Trebichavský I. Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiol Res 2004;53:245-53. [PubMed]
- Boussac M, Garin J. Calcium-dependent secretion in human neutrophils: a proteomic approach. Electrophoresis 2000;21:665-72. [Crossref] [PubMed]
- Voganatsi A, Panyutich A, Miyasaki KT, et al. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol 2001;70:130-4. [PubMed]
- Johne B, Fagerhol MK, Lyberg T, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol 1997;50:113-23. [Crossref] [PubMed]
- Alibrahim B, Aljasser MI, Salh B. Fecal calprotectin use in inflammatory bowel disease and beyond: A mini-review. Can J Gastroenterol Hepatol 2015;29:157-63. [Crossref] [PubMed]
- Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis 2017;11:3-25. [Crossref] [PubMed]
- Ayling RM. New faecal tests in gastroenterology. Ann Clin Biochem 2012;49:44-54. [Crossref] [PubMed]
- Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992;27:793-8. [Crossref] [PubMed]
- Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002;123:450-60. [Crossref] [PubMed]
- Lin JF, Chen JM, Zuo JH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014;20:1407-15. [Crossref] [PubMed]
- Amcoff K, Stridsberg M, Lampinen M, et al. Clinical implications of assay specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand J Gastroenterol 2017;52:344-50. [Crossref] [PubMed]
- van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [Crossref] [PubMed]
- Mindemark M, Larsson A. Ruling out IBD: estimation of the possible economic effects of pre-endoscopic screening with F-calprotectin. Clin Biochem 2012;45:552-5. [Crossref] [PubMed]
- Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol 2009;104:465-83; quiz 464, 484.
- Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008;103:162-9. [Crossref] [PubMed]
- Theede K, Holck S, Ibsen P, et al. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis 2016;22:1042-8. [Crossref] [PubMed]
- Heida A, Park KT, van Rheenen PF. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm Bowel Dis 2017;23:894-902. [Crossref] [PubMed]
- Lee SH, Kim MJ, Chang K, et al. Fecal calprotectin predicts complete mucosal healing and better correlates with the ulcerative colitis endoscopic index of severity than with the Mayo endoscopic subscore in patients with ulcerative colitis. BMC Gastroenterol 2017;17:110. [Crossref] [PubMed]
- Patel A, Panchal H, Dubinsky MC. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm Bowel Dis 2017;23:1600-4. [Crossref] [PubMed]
- Yamamoto T, Shiraki M, Bamba T, et al. Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int J Colorectal Dis 2014;29:485-91. [Crossref] [PubMed]
- Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15-22. [Crossref] [PubMed]
- Egea-Valenzuela J, Alberca-de-Las-Parras F, Carballo-Álvarez F. Fecal calprotectin as a biomarker of inflammatory lesions of the small bowel seen by videocapsule endoscopy. Rev Esp Enferm Dig 2015;107:211-4. [PubMed]
- Shimoyama T, Yamamoto T, Umegae S, et al. Faecal biomarkers for screening small bowel inflammation in patients with Crohn's disease: a prospective study. Therap Adv Gastroenterol 2017;10:577-87. [Crossref] [PubMed]
- Johnson MW, Maestranzi S, Duffy AM, et al. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol 2008;20:174-9. [Crossref] [PubMed]
- Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn's disease. Br J Surg 2009;96:663-74. [Crossref] [PubMed]
- Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease after surgery. Gastroenterology 2015;148:938-47.e1. [Crossref] [PubMed]
- Shastri YM, Bergis D, Povse N, et al. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med 2008;121:1099-106. [Crossref] [PubMed]
- Kim J, Kim H, Oh HJ, et al. Fecal Calprotectin Level Reflects the Severity of Clostridium difficile Infection. Ann Lab Med 2017;37:53-7. [Crossref] [PubMed]
- Peretz A, Tkhawkho L, Pastukh N, et al. Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect Dis 2016;16:309. [Crossref] [PubMed]
- Högberg C, Söderström L, Lilja M. Faecal immunochemical tests for the diagnosis of symptomatic colorectal cancer in primary care: the benefit of more than one sample. Scand J Prim Health Care 2017;35:369-72. [Crossref] [PubMed]
- Balamtekın N, Baysoy G, Uslu N, et al. Fecal calprotectin concentration is increased in children with celiac disease: relation with histopathological findings. Turk J Gastroenterol 2012;23:503-8. [Crossref] [PubMed]
- Capone P, Rispo A, Imperatore N, et al. Fecal calprotectin in coeliac disease. World J Gastroenterol 2014;20:611-2. [Crossref] [PubMed]
- von Arnim U, Wex T, Ganzert C, et al. Fecal calprotectin: a marker for clinical differentiation of microscopic colitis and irritable bowel syndrome. Clin Exp Gastroenterol 2016;9:97-103. [Crossref] [PubMed]
- Wildt S, Nordgaard-Lassen I, Bendtsen F, et al. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol 2007;19:567-74. [Crossref] [PubMed]
- Tibble JA, Sigthorsson G, Foster R, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut 1999;45:362-6. [Crossref] [PubMed]
- Summerton CB, Longlands MG, Wiener K, et al. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 2002;14:841-5. [Crossref] [PubMed]
- Vincent Z, Hornby S, Ball S, et al. Faecal calprotectin as a marker for oesophago-gastric cancer. Ann Clin Biochem 2015;52:660-4. [Crossref] [PubMed]
- Yagmur E, Schnyder B, Scholten D, et al. Dtsch Med Wochenschr 2006;131:1930-4. [Elevated concentrations of fecal calprotectin in patients with liver cirrhosis]. [Crossref] [PubMed]
- Tursi A, Elisei W, Picchio M, et al. Increased faecal calprotectin predicts recurrence of colonic diverticulitis. Int J Colorectal Dis 2014;29:931-5. [Crossref] [PubMed]
- Tursi A, Elisei W, Giorgetti G, et al. Role of fecal calprotectin in the diagnosis and treatment of segmental colitis associated with diverticulosis. Minerva Gastroenterol Dietol 2011;57:247-55. [PubMed]
Cite this article as: McMahon CW, Chhabra R. The role of fecal calprotectin in investigating digestive disorders. J Lab Precis Med 2018;3:19.


