
Diagnostic accuracy of enhanced liver fibrosis (ELF) test for significant fibrosis in patients with autoimmune hepatitis
Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory disease characterized by interface hepatitis, the presence of autoantibodies in the patient’s serum and polyclonal hypergammaglobulinemia (1). Liver fibrosis is one of the main complications of AIH, and the prognosis for patients with this complication mostly depends on their overall fibrosis level (2). Fibrosis is a pathological condition defined as excess accumulation of extracellular matrix (ECM) components, which causes hepatic dysfunction in the long term. The medical follow-up and treatment of liver fibrosis is the main objective in the management of AIH and all the other chronic liver diseases.
The extent and progression of hepatic fibrosis has to be monitored meticulously during treatment and follow-up for patients with AIH. Even though the liver biopsy is still the gold standard assessment tool for hepatic fibrosis, it is not a suitable method for frequent monitoring of patients during the course of the disease (3). Moreover, according to several studies, liver biopsy does not have the maximum capacity to reflect the level of fibrotic conditions during the course of any chronic liver disease. An optimal liver biopsy material can contain 5 to 11 portal tracts which is approximately 1/50000 of the volume of the whole liver (4,5). Chronic liver disease may result in heterogeneous injury in liver and this may end up with heterogeneous liver fibrosis. Different parts of liver tissue may be fibrotic at different stages at the same time (6). Accordingly, liver biopsy may be missing the fibrotic area if the biopsy needle does not go across fibrotic area. It has been shown that 10–30% missed cases of cirrhoses even though liver biopsy had been performed (7). Furthermore, liver biopsy cannot differentiate early stages of fibrosis from advanced fibrosis properly in some cases and disagreements between pathologists may increase the variability and decrease the validation of liver biopsy (8). In addition, liver biopsy requires hospital stay, rising medical costs (9-11). These issues make it uneasy to say that liver biopsy is an excellent tool to predict prognosis of chronic liver diseases (12). For these reasons, the need has arisen to non-invasive tests for monitoring chronic liver diseases (13). This is especially important for the patients with AIH who have received prolonged immunosuppressive therapy and require an assessment for liver fibrosis (14).
Debate continues about the best strategy for the assessment of liver fibrosis. Historically, a liver biopsy has been recommended as the gold standard method for the evaluation of hepatic fibrosis. However, this approach is invasive and inconvenient for frequent use and has associated complications. In the last 2 decades, there has been an increasing interest in biochemical markers to diagnose and follow-up hepatic fibrosis. Non invasive methods in assessment of hepatic fibrosis is an increasingly important topic in diagnostic medicine. Recent advances/developments in the field of diagnostic methods to identify hepatic fibrosis have led to an emerging interest in non invasive methods. Recently, researchers have shown an increased interest in non invasive assessment of hepatic fibrosis by biochemical markers or imaging methods to replace liver biopsy eventually to be alternative for liver biopsy. Therefore, researchers have turned to the development of noninvasive methods for the evaluation and staging of hepatic fibrosis.
The enhanced liver fibrosis (ELF) test is a biochemical test panel made up of serum markers that are indicators of ECM metabolism (15). The ELF test panel consists of the following three parameters: hyaluronic acid (HA), procollagen III N-terminal propeptide (PIIINP), and tissue inhibitor of matrix metalloprotease-1 (TIMP-1). Serum levels of these parameters are used to calculate the ELF score via a statistically developed algorithm. The ELF test has been studied to assess liver fibrosis in various chronic liver diseases, such as hepatitis caused by hepatitis B virus (HBV) and hepatitis C virus (HCV), non-alcoholic fatty liver disease (NAFLD), primary biliary cholangitis (PBC), etc. (16-19). However, the diagnostic value of the ELF test has not been studied in patients with AIH.
The aim of this study was to compare the diagnostic performance of the ELF test with liver biopsy for predicting hepatic fibrosis stages in an etiologically homogenous liver fibrosis group (AIH patients).
Methods
Patients and study design
Patients who were consecutively admitted to an academic hepatology clinic (Hepatology Unit at Hacettepe University Hospital) were prospectively considered for this study. Inclusion criteria were as follows: 18–75 years of age, confirmed diagnosis of AIH, and no history of drug treatment for AIH. Patients meeting any of the following criteria were ineligible: patients who refuse to undergo liver biopsy, patients who were mentally unable to give consent by their own. Serum samples were obtained and stored at −80 °C for further biochemical analysis before liver biopsy on the same day.
Forty-nine consecutive patients with the diagnosis of AIH were included in this prospective study between May 2013 and October 2013. Because 3 of 49 patients refused to undergo liver biopsy, 46 patients were included in final analysis. Clinical and laboratory data were collected prospectively from clinical databases. This prospective study was cleared by Hacettepe University Ethics Committee for Clinical Studies (date of approval: 09.05.2013; number of approval: 06-03 KA-130018). Informed consent was obtained from all patients enrolled in the study.
Biochemical tests
Total serum bilirubin, alanine aminotransferase (ALT) activity, aspartate aminotransferase (AST) activity, γ-glutamyltransferase (GGT) activity, alkaline phosphatase (ALP) activity, platelet count, prothrombin time and autoantibodies [anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA)] were measured in all patients. Routine clinical chemistry tests were performed using Roche Modular P800 Chemistry analyzer (Roche Diagnostics, Indianapolis, IN, USA). Platelet count was performed with Beckman Coulter UniCel DxH 800 Coulter Cellular Analysis System (Beckman Coulter, Inc, Miami, FL, USA). Autoantibodies were measured by ELISA method (Cusabio Biotech Co., Ltd., Wuhan, China).
Liver histology
Liver tissue samples were obtained via a percutaneous liver biopsy. Biopsy samples >15 mm in length with at least six portal tracts were eligible to be analyzed. Samples were fixed in formalin and embedded in paraffin. Two micrometer sections were stained with hematoxylin-eosin and Masson’s trichrome for histological assessment. An expert pathologist analyzed biopsy specimens independently without knowing the ELF test results or other clinical data. The fibrosis stage for all of the samples was evaluated according to the METAVIR scoring system. METAVIR scoring is classified into the following five stages: F0 (no fibrosis), F1 (portal fibrosis without septa), F2 (portal fibrosis with rare septa), F3 (many septa without cirrhosis), F4 (cirrhosis) (20). Significant fibrosis was defined as ≥F2.
The ELF test panel
Blood samples (10 mL) were drawn from each patient in the morning after an overnight (8 to 12 hours) fast. Serum samples were obtained and stored at −80 °C. All serum samples were analyzed for PIIINP, HA, and TIMP-1 levels via a CE-marked automated clinical immunochemistry analyzer (ADVIA Centaur™ XP, Siemens Healthcare Diagnostics, Tarrytown, NY, USA) that performs magnetic separation enzyme immunoassay tests. One experienced operator performed all of the PIIINP, HA, and TIMP-1 analyses as per the manufacturer’s recommendations under the supervision of a medical biochemistry specialist. The ELF score was calculated using the established algorithm on the analyzer {i.e., [ELF =2.278+0.851 ln (HA) +0.751 ln (PIIINP) +0.394 ln (TIMP-1)]}. According to manufacturer’s data, the ELF score is categorized into the following three stages: <7.7 (no or mild fibrosis), ≥7.7 to <9.8 (moderate fibrosis), and ≥9.8 (severe fibrosis) (17). An expert biochemist analyzed ELF test results independently without knowing the biopsy results or other clinical data.
Statistical analyses
Results were expressed as means with standard deviations. The Mann-Whitney U test was used to compare the general characteristics of the fibrosis groups. The diagnostic value of the ELF test for the evaluation of significant fibrosis was assessed by calculating the area under the receiver operator characteristic (ROC) curves [area under curve (AUC)] and 95% confidence intervals of the ELF score and its components. The cut-off value for the ELF test was determined by optimizing the Youden index. Sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV) were calculated from these data. Positive and negative likelihood ratios were calculated based on the sensitivity and specificity values. Statistical analyses were performed using SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA). Statistical significance for comparisons was defined as a two-sided P<0.05.
Results
Patient characteristics
The distribution according to the METAVIR fibrosis stages was as follows: 1 patient (2.2%) in F0, 8 patients (17.4%) in F1 and F2, 19 patients (41.3%) in F3, and 10 patients (21.7%) in F4.
The fibrosis study groups were defined according to the METAVIR stages. Patients having METAVIR scores <F2 were placed in the “no-mild fibrosis” group. Patients with METAVIR scores ≥F2 were considered to have significant liver fibrosis and placed in the “significant fibrosis” group. No-mild fibrosis and significant fibrosis groups were consisted of 9 (19.6%) and 37 (80.4%) patients, respectively. In no-mild fibrosis group, 77.8% of the patients were female whereas 70.3% of the patients in significant fibrosis group were female. No statistically significant difference was observed in the gender distributions of the two groups. The patient characteristics [age, duration of disease, ALT, AST, ALP, GGT, international normalization ratio (INR), immunoglobulin G (IgG), total bilirubin, albumin] for each the fibrosis groups were compared, and the differences between the fibrosis groups were not statistically significant with exception to the total bilirubin levels and the platelet counts (P<0.05) (Table 1).
Table 1
| Related features | All subjects | No-mild fibrosis | Significant fibrosis | P value |
|---|---|---|---|---|
| Sample size | 46 | 9 | 37 | − |
| Age (years) | 39.6±13.2 | 44.1±12.4 | 38.5±1.35 | 0.217 |
| Gender (M/F) | 13/33 | 2/7 | 11/26 | 0.345 |
| Duration of disease (months) | 48.8±46.5 | 24.5±23.2 | 54.7±49.0 | 0.059 |
| ALT (U/L) | 420.2±404 | 426.4±438 | 418.8±402 | 0.828 |
| AST (U/L) | 432±492 | 368±392 | 445.6±518 | 0.723 |
| Total bilirubin (mg/dL) | 1.25±1.4 | 0.41±0.24 | 1.45±1.5 | <0.050 |
| ALP (U/L) | 299±207 | 237±186.3 | 310.5±218.5 | 0.367 |
| GGT (U/L) | 290.8±321 | 290±317 | 291±326.4 | 0.913 |
| Albumin (g/dL) | 3.81±0.6 | 3.88±0.37 | 3.8±0.6 | 0.913 |
| INR | 1.26±0.2 | 1.1±0.16 | 1.3±0.2 | 0.137 |
| IgG (g/dL) | 2.47±1.1 | 1.96±0.5 | 2.6±1.1 | 0.081 |
| Platelet count (109/L) | 220.4±71.7 | 273.1±73.5 | 207.6±66.0 | <0.050 |
| ELF score | 10.22±1.5 | 8.62±1.1 | 10.60±1.4 | <0.050 |
Data were presented as means and standard deviations. The differences between groups were tested by Mann-Whitney U test. M/F, male/female; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IgG, immunoglobulin G; INR, international normalization ratio; GGT, γ-glutamyltransferase; ELF, enhanced liver fibrosis.
ELF test results
The ELF test panel was used to analyze the serum samples of patients. The mean of the ELF scores for the two fibrosis groups according to the METAVIR stages were 8.62±1.05 and 10.60±1.39 for no-mild and significant fibrosis groups, respectively. The difference between the two fibrosis groups was statistically significant (P<0.05) (Figure 1).
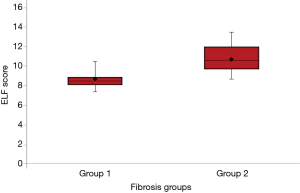
Comparison of the ELF score and liver biopsy
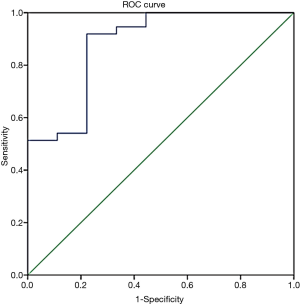
To detect the ability of ELF to appropriately assess liver fibrosis, a ROC was plotted, and the cut-off value was determined (Figure 2). The cut-off value for the ELF score to differentiate significant fibrosis from no-mild fibrosis was determined as 8.84, and AUC was calculated as 0.88 with a 95% confidence interval (0.75–1.00). Sensitivity, specificity, PPV, NPV, and positive and negative likelihood ratios (with 95% confidence intervals) were calculated as 0.92 (0.78–0.98), 0.78 (0.40–0.97), 0.94 (0.81–0.99), 0.70 (0.33–0.94), 4.1 (2.9–5.9), and 10 (2–50), respectively.
Discussion
In recent years, there has been an increasing amount of studies on the usefulness of non-invasive ELF test in chronic liver disease patients. Some studies about ELF test have been carried out with a mixed group of chronic liver disease patients (8,15,21,22). However, no other study has been found in the literature that surveyed the usability of ELF test in AIH patient group.
In our study, the diagnostic value of the ELF test was evaluated by comparing the ELF test results with liver biopsy results. According to the results, the ELF score can discriminate significant fibrosis from mild fibrosis in patients with AIH. Studies on the diagnostic accuracy and performance of the ELF test in assessing liver fibrosis for various chronic liver disease groups have been reported in the literature. Some of these studies involved patients with various chronic liver diseases, while others focused on specific patient populations, such as those suffering from alcoholic liver disease (ALD), NAFLD, chronic HBV hepatitis, chronic HCV hepatitis, chronic hepatitis D virus (HDV) hepatitis, or PBC. This is the first study for applying the ELF test to a group of patients with AIH.
In this study, patients were divided into the following two groups according to their biopsy results and the METAVIR staging system: no-mild fibrosis—F0 to F1 and significant fibrosis—F2 to F4. No-mild fibrosis and significant fibrosis groups were consisted of 19.6% and 80.4% of the patients, respectively. The ELF scores were 8.62±1.05 and 10.60±1.39 for no-mild fibrosis and significant fibrosis groups, respectively, and the difference between the groups was statistically significant (P<0.001). To compare the diagnostic values of the two methods, the ROC curve was plotted. The AUC was calculated as 0.88. The cut-off value to discriminate no-mild fibrosis from significant fibrosis was calculated as 8.84. Moreover, the sensitivity, specificity, PPV, and NPV were calculated as 91.89%, 77.78%, 94.4%, and 70%, respectively. The likelihood ratios were calculated as 4.14 (LR+) and 10 (LR−). These results were similar to the results of some previous studies with chronic liver disease groups (16,18,21).
The first study investigating the diagnostic value of ELF test has been published in 2004. This study was a multi-center project across Europe with participation of 1,021 chronic liver patients. Nine hundred and twenty-one of patients have been undergone liverbiopsy and serum samples were analyzed to calculate ELF scores. ELF scores and liver biopsy results were compared and AUC value was found to be 0.804. It was concluded that ELF test can be an alternative to liver biopsy (15).
In a study with 347 chronic HCV patients, liver biopsy results were compared with ELF test results (cut-off METAVIR ≥F3) and AUC value was calculated as 0.85 (16). Also, the diagnostic value of ELF test and liver biopsy was compared by choosing METAVIR F2, F3 and F4 as cut-off values and AUC values were calculated as 0.901, 0.860 and 0.862, respectively (18). In a 2012 study with 512 chronic HCV patients, the diagnostic value of ELF test was compared with the liver biopsy. To discriminate significant fibrosis from mild fibrosis, METAVIR stage F2 and ELF score 9.0 were selected as cut-off values and AUC value, sensitivity and specificity were calculated as 0.78, 86% and 62% (23). As compared with our results, these values indicate that our study has more sensitive and specific results. In the same study, AUC value was found to be 0.82 when ELF score cut-off was set to be 9.33 and METAVIR stage as F3 and 0.85 when ELF score cut-off as 9.35 and METAVIR stage as F4. The diagnostic value of ELF test had been compared with liver biopsy in a 2012 study with 102 chronic liver disease patients. The AUC value, sensitivity, specificity were found as 0.87, 86% and 70%, respectively, when METAVIR stage F2 and ELF score 8.99 were chosen a cut-off values (21).
This prospective study was designed to determine the diagnostic accuracy of the ELF test in patients with AIH by comparing the diagnostic accuracy of the ELF test to the results of the liver biopsy. Our study has its own importance as it was a uni-centered study carried out with AIH patients which comprise a very small percentage of chronic liver disease group. Even though the diagnostic accuracy of ELF testhad been already determined in various chronic liver diseases, it has been studied primarily and exclusively in this study.
As this study was designed to compare results of ELF test with her biopsy, no healthy individuals were recruited to establish a control group. Having only one patient at F0 grade and two patients with ELF scores below 7.7 led us to select two levels (no-mild fibrosis and significant fibrosis) instead of three levels of fibrosis (no-mild fibrosis/moderate fibrosis/severe fibrosis) to compare grades of fibrosis. ELF scores were calculated from average results of three measurements per each parameter.
The feature of liver biopsy to be open to some errors during practice and evaluation phases despite being the golden standard tool to assess liver fibrosis is an important limitation of this study. ELF scores of 7 patients out of 46 patients have been found to be parallel with their liver biopsy results. This discordance may or may not arise from the incompetence of ELF test to assess liver fibrosis. One of the reasons for these discordant results may be the inability of liver biopsy material to reflect status of the whole liver. ELF test provides evaluation of fibrosis in the liver tissue globally by analysis of serum markers related with ECM metabolism. From this perspective, ELF test may have interpreted those patients’ degree of hepatic fibrosis which have discordance with liver biopsy results.
ELF test components have also some deficiencies in displaying hepatic fibrosis. Firstly, hyaluronic acid is not specific to liver and gives global information about ECM metabolism (24). HA level may increase during the course of rheumatological diseases. In chronic kidney disease, serum HA levels may increase due to the disruption of low molecular weight HA clearance. PIIINP can be found in four different structures in serum. Aside from collagen synthesis, serum levels of PIIINP may also be increased due to degradation of collagen. Clearance of PIIINP is provided via removal from sinusoidal circulation by endocytosis. That is why serum levels of PIIINP are found to be lower in cases of sinusoidal endothelial injury or dysfunction (25). Moreover, PIIINP is not specific to hepatic tissue and reflects global ECM metabolism, like HA. I has been known that serum PIIINP levels may be affected by some drugs with fibrogenic side effects, i.e., methotrexate and it has been offered as a folow-up parameter during treatment with these sort of drugs (26). It has been shown that levels of TIMP-1 increase in hepatic tissue and serum in PSC (primary sclerosing cholangitis), PBC, biliary atresia, AIH patients in accordance with increase in fibrosis (27). Moreover, TIMP-1 has been shown to be not a specific marker for liver tissue and serum levels of TIMP-1 may increase in ALD, hemochromatosis (HC), asthma, colitis and coronary heart disease (28). Each of three parameters are not capable of assessing and evaluating hepatic fibrosis by themselves. In addition, there are not enough studies investigating half-life in circulation, clearance features and affecting conditions of serum levels of HA, PIIINP and TIMP-1. Also, non-hepatic factors affecting serum levels of ELF test components should be furtherly studied.
The limitations of noninvasive methods are lack of clear identification of moderate fibrosis, the inability to reflect other histological features that may have prognostic value. Moreover, there are only very few large-scale validation and reference interval studies for ELF test to be used in routine medical practice. However, having test components in direct relation with ECM metabolism lets ELF test to be superior than other techniques. ELF score is more reliable as its calculation depends on measurement of three parameters and its unique algorithm used in calculation.
Our main limitation in this study was the number of cases included. Prevalence of AIH and conducting this study only in one center has majorly determined our sample size. In the light of all these features, it can be concluded that ELF test can be a potential candidate to detect significant fibrosis diagnosis in patients with AIH. Large-scale, multicentered studies are required for further validation.
Acknowledgments
We are grateful to Siemens Turkey Healthcare, Diagnostics Division for their technical support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hemant Goyal) for the series “Role of biomarkers in gastrointestinal disorders” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The series “Role of biomarkers in gastrointestinal disorders” was commissioned by the editorial office without any funding or sponsorship. This study was presented as a poster at The International Liver Congress (ILC) 2014, 49th annual meeting of the European Association for the Study of Liver (EASL), London, United Kingdom.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This prospective study was cleared by Hacettepe University Ethics Committee for Clinical Studies (date of approval: 09.05.2013; number of approval: 06-03 KA-130018). Informed consent was obtained from all patients enrolled in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahn J, Flamm SL. Autoimmune hepatitis. Curr Treat Options Gastroenterol 2005;8:481-92. [Crossref] [PubMed]
- Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta 2006;364:33-60. [Crossref] [PubMed]
- Fallatah HI. Noninvasive Biomarkers of Liver Fibrosis: An Overview. Adv Hepat 2014;2014:Article ID 357287.
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495-500. [Crossref] [PubMed]
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57. [Crossref] [PubMed]
- Baranova A, Lal P, Birerdinc A, et al. Noninvasive markers for hepatic fibrosis. BMC Gastroenterol 2011;11:91. [Crossref] [PubMed]
- Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol 2006;12:3682-94. [Crossref] [PubMed]
- Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol 2008;5:95-106. [Crossref] [PubMed]
- Pinzani M. Noninvasive methods for the assessment of liver fibrosis: a window open on the future? Hepatology 2011;54:1476-7. [Crossref] [PubMed]
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614-8. [Crossref] [PubMed]
- Standish RA, Cholongitas E, Dhillon A, et al. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006;55:569-78. [Crossref] [PubMed]
- Sebastiani G. Non-invasive assessment of liver fibrosis in chronic liver diseases: implementation in clinical practice and decisional algorithms. World J Gastroenterol 2009;15:2190-203. [Crossref] [PubMed]
- Castera L, Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut 2010;59:861-6. [Crossref] [PubMed]
- Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193-213. [Crossref] [PubMed]
- Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology 2004;127:1704-13. [Crossref] [PubMed]
- Parkes J, Guha IN, Roderick P, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepat 2011;18:23-31. [Crossref] [PubMed]
- Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 2009;136:160-7. [Crossref] [PubMed]
- Kim BK, Kim HS, Park JY, et al. Prospective validation of ELF Test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic Hepatitis B. PloS One 2012;7:e41964 [Crossref] [PubMed]
- Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 2008;48:1549-57. [Crossref] [PubMed]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15-20. [Crossref] [PubMed]
- Wahl K, Rosenberg W, Vaske B, et al. Biopsy-controlled liver fibrosis staging using the enhanced liver fibrosis (ELF) score compared to transient elastography. PloS One 2012;7:e51906 [Crossref] [PubMed]
- Poynard T, Halfon P, Castera L, et al. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem 2007;53:1615-22. [Crossref] [PubMed]
- Guéchot J, Trocmé C, Renversez JC, et al. Independent validation of the Enhanced Liver Fibrosis (ELF) score in the ANRS HC EP 23 Fibrostar cohort of patients with chronic hepatitis C. Clin Chem Lab Med 2012;50:693-9. [Crossref] [PubMed]
- Aguilar Rubido JC. Chapter 8: Liver Function Tests. In: Mahtab MA, Rahman S. editors. Liver: A Complete Book on Hepato-Pancreato-Biliary Diseases. New Delhi: Elsevier India, 2009.
- Dancygier H. editor. Clinical Hepatology Principles and Practice of Hepatobiliary Diseases. New York: Springer, 2010.
- Sherwood AR, Bomford A, Marshall WJ, et al. Chapter 13: The assessment of hepatic function and investigation of jaundice. In: Marshall WJ, Bangert SK. editors. Clinical Biochemistry: Metabolic and Clinical Aspects. Edinburgh: Elsevier, 2014.
- Murawaki Y, Ikuta Y, Kawasaki H. Clinical usefulness of serum tissue inhibitor of metalloproteinases (TIMP)-2 assay in patients with chronic liver disease in comparison with serum TIMP-1. Clin Chim Acta 1999;281:109-120. [Crossref] [PubMed]
- Pereira TN, Lewindon PJ, Smith JL, et al. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol 2004;41:576-83. [Crossref] [PubMed]
Cite this article as: Gungoren MS, Efe C, Kav T, Akbiyik F. Diagnostic accuracy of enhanced liver fibrosis (ELF) test for significant fibrosis in patients with autoimmune hepatitis. J Lab Precis Med 2018;3:21.


