
FIT for purpose: enhanced applications for faecal immunochemical tests
Introduction
Colorectal cancer (CRC) is one of the most prevalent cancers worldwide, with approximately 1.4 million new cases diagnosed and 608,000 deaths per year (1). Despite the high likelihood of cure following quality treatment when diagnosed at an early stage, CRC remains the second leading cause of cancer death globally. Screening aims to reduce CRC incidence and mortality through detection and removal of early stage cancers or precursor adenomas. Globally faecal occult blood tests (FOBT) with either guaiac FOBT (gFOBT) or the newer faecal immunochemical test (FIT) for hemoglobin (Hb) (2) are widely used in CRC screening of average risk populations with positive results triggering colonoscopy.
Screening with gFOBT reduces both incidence and mortality from CRC (3-6), through early detection of neoplasms exhibiting a bleeding phenotype. However technical issues associated with the methodology can result in both false negative and false positive results. gFOBT detects the presence of heme using an oxidation method, and is therefore susceptible to interference from either intake of anti-oxidants such as vitamin C (increasing risk of a false negative), or intake of heme in red meat or of peroxidase-containing fruits and vegetables (increasing risk of a false positive) (7). In addition, the test is not specific to colonic bleeding, and therefore detected heme can originate from the upper gastrointestinal tract. Accordingly the test has specificity issues as well as limited sensitivity for both CRC (33.3%) and high risk (advanced) colonic adenomas (6.8%) (8). These limitations have prompted many countries to adopt FIT as the CRC screening test for mass population screening where most targeted are of average-risk for CRC. FIT uses antibodies specific for the globin moiety of human Hb. This methodology is sensitive to low concentrations of globin that originate from the colon and is unaffected by medication and diet (9). It is also a consumer preferred test over the gFOBT (10), with this contributed to by the easier sampling technique and the lack of dietary restriction (11). Head-to-head comparison against gFOBT have shown that sensitivity for commonly used FITs were better for both CRC (73.3–87.5%) and advanced adenomas (22.2–42.6%), compared to gFOBT (CRC: 23.0–33.0%; advanced adenomas 6.8–23.0%) (8,12). Recent studies support the role of this technology in early detection of CRC, as well as reducing incidence and mortality from CRC (13-15).
A FIT positive test result is based on the presence of Hb above a set threshold (typically between 15 and 20 µg Hb/g faeces), with its use as a screening modality to determine who should proceed to colonoscopy. An advantage of the FIT is that some FIT brands are able to provide quantitative Hb results, although most countries using FIT for CRC population screening only report qualitative test results even when using a quantitative test. A quantitative result provides the ability for more sophisticated applications and the faecal Hb concentration correlates with pathology in that cancers bleed more than advanced adenomas which bleed more than those with diminutive adenomas and no evidence of pathology (16).
In this review we will discuss how knowledge of Hb concentrations can extend FIT usage, for example in combination with clinical factors or other biomarkers to improve screening efficiency, as well as allowing for efficient and cost-effective management of colonoscopy resources in screening populations. We will also explore some non-traditional uses of FIT, in symptomatic patients or higher risk subgroups.
Improving FIT screening sensitivity
FIT has a high sensitivity for colonic neoplasia (8), but with a specificity of 94% (17) substantial numbers of colonoscopies on FIT positive individuals do not detect neoplasia. Increasing participation in FIT for CRC screening has resulted in longer waiting lists for diagnostic colonoscopy. Australian screening program data from 2006–2009 revealed only 23% of participants had their diagnostic colonoscopy within 30 days (the recommended benchmark at that time) after a positive FIT (18). Delays to diagnostic colonoscopy not only increases severity of pathology outcomes but also increases patient anxiety. Studies indicate that there is a higher likelihood of being diagnosed with stage II CRC after a 7–9-month delay (OR 1.88) (19), and that a delay of 12 months will increase the incidence of all CRC by 4% compared to more timely procedures (20). These data also suggest that delays increase mortality by up to 16% and decrease programmatic cost effectiveness by 9% (20). From a patient perspective, while a positive FIT result increases anxiety, this decreases after colonoscopy (21). This further supports need for timely diagnostic procedures.Below we discuss use of FIT to triage patients for diagnostic colonoscopy, and how test sensitivity may be improved.
Neoplasia prediction through combining FIT results with clinical data
A number of personal and lifestyle factors are associated with a higher risk of CRC development. It is possible that the knowledge of these could be applied to determine how to prioritise FIT positive individuals for earlier procedures. Stegeman et al. combined a number of risk factors with the FIT result including age, gender, smoking status, family history of CRC and calcium intake and reported sensitivity for advanced neoplasia (CRC or advanced adenoma) was better than with FIT alone (22). Another study found a higher risk for advanced neoplasia in individuals with high faecal Hb concentrations, older age, male gender, smoking and metabolic syndrome (23). Further studies are needed to validate these models for clinical triage purposes.
Neoplasia prediction through combining FIT with other biomarkers
Neoplasia detection by FIT relies on lesions shedding blood into the faeces. However, some lesion are more likely to escape detection if they bleed little or not at all. The degree of bleeding seems to be location and/or histopathology dependent. For instance right sided lesions are less likely to be associated with a positive FIT result compared with left sided lesions (24), due to degradation of Hb during transport along the colon, and serrated adenomas with hypovascularisation and mucous cap also have poor sensitivity with FIT (25). To identify other phenotypes apart from bleeding, there could be value in combining test technologies. There are now data examining outcomes by adding different biomarkers to FIT, either in faeces, or in other body fluid such as blood.
Our group has previously explored the value of blood biomarkers to detect a different pathobiology than that detected by FIT. Circulating tumour DNA (ctDNA) can be detected in the blood stream through assaying for DNA changes specific to CRC such as mutations or methylation. An example of aberrant methylation shown to be related to colorectal neoplasia is in the genes BCAT1 and IKZF1 (26). In a prospective study involving 1381 participants undergoing colonoscopy, and who completed both FIT and the methylated BCAT1/IKZF1 blood test, we found that a blood test for these two genes in combination with the FIT, improved CRC sensitivity to 89% which was greater than either test alone (FIT =79%, blood test =62%) (27). Another study has suggested that combination of a positive FIT result and markers of iron deficient anaemia indicates an increased risk of advanced colorectal neoplasia (28). However including a blood test for iron deficient anaemia did not detect additional cancers compared to FIT alone. A further limitation was that the study did not include menstruating women. Nevertheless this combination of tests might guide triage strategies for those more likely to have advanced neoplasia.
A recent review of different fecal biomarkers assessed in combination with FIT found that the biomarkers based on DNA mutations (p53 and APC), DNA methylation (PHACTR3), and microRNA (miR-106a) expression all improved diagnostic test accuracy for advanced colorectal neoplasia, with sensitivity of 71–81% for the combined test compared to 52% for FIT alone. In contrast biomarkers based on proteins (transferrin, calgranulin C, TIMP-1, peanut agglutinin, calprotectin and M2-PK) did not (29). One combination test that is more advanced with testing is the multitarget fecal DNA test (Cologuard). The DNA test includes assessment of DNA mutation (KRAS), DNA methylation (NDRG4 and BMP3), as well as an immunoassay for Hb (i.e., a FIT). The detection of advanced adenomas and large sessile serrated adenomas was improved in using the combination test (42.4% for both) compared to FIT alone (23.8% and 5.1% respectively) (30). Another study that assessed the combination of FIT with microbiota testing (sequencing the 16S rRNA genes) detected 92% of cancers compared to 75% for FIT alone. In addition the tests each detected distinct small subsets of adenomas (31). However the technical limitations of this work were that the FIT was performed on frozen fecal aliquots which can decrease test sensitivity through reducing fecal Hb concentrations (32). In addition not all samples were collected prior to colonoscopy so it is unclear how practical this approach is at present.
Prior to wide use of the combination options, it is important that they undergo a full evaluation such as that suggested by Pepe (33,34). These include validation of accuracy, and critical evaluation in practice on an intention-to-screen basis. The majority of the novel biomarker tests described have however, only been assessed in single studies, or not in a screening setting. Cost effectiveness is an important consideration with the combination tests as many of these are associated with a reduction in specificity, which will increase the burden on colonoscopy resources. While the multitarget fecal DNA test is now commercially available, its cost effectiveness has been questioned. The point has been made that for the multitarget fecal DNA test to be cost effective it will need to increase screening participation over the current rates in the FIT program alone (35).
Tailoring FIT screening programs to the individual
The technology of many brands of FIT allows Hb concentration to be reported quantitatively. While most diagnostic laboratories follow manufacturers’ recommendations for positivity thresholds, with quantitative results it is possible that thresholds can be adjusted to match the desired sensitivity and specificity of a screening activity (9). Possible options to exploit this are described below.
Personalizing FIT screening based on risk factors
As colonoscopy capacity is limited in many countries, there is a need to ensure feasible access and cost-efficient use of this resource. Limiting CRC screening from 50 to 74 y is one approach to maximize the benefits per unit cost. Within this age range it may be possible to pre-select who would benefit most from screening. Identified risk factors for CRC include familial genetic predisposition, previous medical history and lifestyle factors. Family history can increase an individual’s risk of developing CRC up to four-fold (36,37). Of major impact is the increase in incidence of adenomas and polyps with age (38). Gender is also important (39,40), as men are 1.4 times more likely to be diagnosed with CRC compared to women (38). In addition, smoking, alcohol, BMI, physical activity and nutrition have been shown to play a role in development of CRC (41).
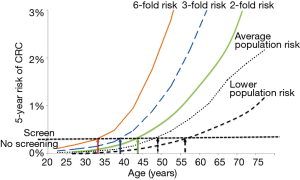
Knowledge of these risk factors could be used for better selection in individuals of the age at which FIT screening should commence. Models for risk prediction have included family history of CRC, aspirin use, smoking, vegetable intake, physical activity, BMI, gender, prior colonoscopies and hormone replacement therapy use or estrogen status (42,43). Individuals at elevated risk for CRC according to such a stratification model may start FIT screening at an earlier age than recommended for general population, while those in lower risk subgroup could commence screening at an older age. While the models being developed are promising, these are still under development but there is the tantalising possibility of incorporating multiple environment risk factors, multigenerational family history for CRC, as well as other cancers (44) to enhance the risk stratification and guide screening recommendations. This is illustrated in Figure 1, which shows how age of screening could be tailored to clinical risk status for CRC. It demonstrates how if we can determine an individual’s risk status for CRC development (compared to the average risk population), we can then recommend the age at which screening should commence (based on setting a threshold for CRC risk at which screening should commence).
Adjusting positivity thresholds to suit the individual
A number of risk factors can also be reflected in the range in FIT positivity reported by different screening programs. We and others have previously shown that faecal Hb concentrations vary by gender, age and deprivation status (24,38,45). Hb concentrations have also been compared between different geographical populations. A large study showed that faecal Hb concentrations were higher in Scotland compared to Taiwan and Italy. This study also demonstrated age and sex differences in faecal Hb (46). Information on the distribution of Hb concentrations within a population may be important to allow screening programs to tailor positivity thresholds. Ignoring the different faecal Hb concentrations between different ages and gender, has implications for the effectiveness of screening in subgroups defined by these demographics. One study has suggested that the FIT positivity threshold can be increased in women under 60 years, which would not affect the cancer detection rate, but would decrease the number of colonoscopies performed by 44.5% (47). By individualizing FIT positivity thresholds, there could also be a reduction in the risk of interval cancers.
Adjusting screening intervals based on Hb concentrations
In addition to the known risk factors that have been discussed above, it appears that the risk of advanced neoplasia at screening may be predicted by FIT results from previous screening episodes. There is a 27% to 35% lower rate of positivity (48-50) if study enrollees have previously undergone screening. This highlights the importance of participation to the success of a population screening program. A higher FIT Hb concentration is also associated with a higher risk of advanced neoplasia (16,51), risk of interval cancer at a future colonoscopy (52), as well as increased mortality for CRC (53). Therefore an individual will have lower risk of advanced neoplasia after previous participation in FIT screening and have a lower fecal Hb concentration.
Screening programs routinely report FIT results in a qualitative fashion, i.e. positive or negative, and around the world, threshold Hb concentrations defined as positive range from 15 to 80 µg Hb/g faeces. Only individuals with a positive FIT test undergo a colonoscopy, whereas participants with a negative FIT result are recommended to repeat screening (in typically 1–2 years), regardless of the actual Hb concentration. Recent studies in large screening cohorts show that values, even if below the positivity threshold, are predictive of future colorectal neoplasia (54-58). Some of these studies are summarised in Table 1.
Table 1
| Country/region | Positivity threshold | Age range (years) | Round 1—no detectable Hb | No. AN at follow-up (%)1 | Round 1—definition of high negative | No. AN at follow-up (%)1 |
|---|---|---|---|---|---|---|
| Taiwan (57) | 20 ìg Hb/g faeces | 40–69 | 0 ìg Hb/g faeces (n=18,995) | 115 (0.6) | 16–19.9 ìg Hb/g faeces (n=508) | 17 (3.3) |
| Italy (54) | 20 ìg Hb/g faeces | 58–69 | 0 ìg Hb/g faeces (n=73,233) | 351 (0.5) | 10–19 ìg Hb/g faeces (n=3,126) | 256 (8.2) |
| Scotland (58) | 80 ìg Hb/g faeces | 50–74 | 0 ìg Hb/g faeces (n=16,621) | 19 (0.1) | 60–79.9 ìg Hb/g faeces (n=125) | 13 (10.4) |
1No. AN (advanced neoplasia) = advanced adenoma + colorectal cancer, with the percentage calculated of the population in round 1. Hb, hemoglobin.
One of the first studies to describe this was performed in Taiwan. They analysed data from 44,324 FIT negative participants (negative value defined as less than 20 µg Hb/g faeces) from the community screening program. They showed that the prevalence of advanced neoplasia was higher in the individuals who had a Hb concentration at 16.0–19.9 µg Hb/g faeces at the previous screening round (just below the positivity threshold of 20 µg Hb/g faeces), compared to those with a lower initial Hb concentration, with an adjusted hazard ratio for advanced neoplasia of 3.41 (relative to participants with 0.2–3.9 µg Hb/g faeces) (57). A second study by the same group following up 54,921 FIT participants showed an association between baseline Hb concentration and risk of neoplasia, with the prediction based on faecal Hb concentration superior to that using a model based on conventional risk factors (56).
Similar findings were reported from a pilot study in Italy with 118,723 participants who had a negative FIT result. The prevalence of advanced neoplasia was highest following a faecal Hb concentration of 10–19 µg Hb/g faeces (where the positivity threshold was 20 µg Hb/g faeces) with an incidence of 8.2% compared to 0.5% following a FIT result of 0 µg Hb/g faeces (54). Consistent with this are data from the Scottish program where a higher FIT positivity threshold is applied (80 µg Hb/g faeces). 37,780 participants with negative results were assessed for outcomes at the next screening round. Of those that were FIT positive in the subsequent round, the odds ratio for advanced neoplasia was 14.3 (95% CI: 8.9–23.1) for those with a baseline FIT concentration of 20–39.9 µg Hb/g faeces, and 38.0 (95% CI: 20.2–71.2) for those with a baseline FIT concentration of 60–79.9 µg Hb/g faeces, relative to those with a baseline of less than 20 µg Hb/g faeces (58).
These findings were also assessed over four rounds of FIT screening in the Dutch screening program and also included CRC diagnosed outside of the program (55). Using data from 7,663 FIT negative (below 10 µg Hb/g faeces) participants, individuals with a baseline Hb concentration of 8–10 µg Hb/g faeces had a higher incidence of advanced neoplasia than the participants with a baseline FIT result of 0 µg Hb/g faeces (hazard ratio of 8.2). Consecutive high concentration negative FITs were also predictive of future advanced neoplasia risk—participants with two consecutive FIT concentrations of 8 µg Hb/g faeces had a 14-fold increased risk compared with those individuals with FIT values of 0 µg Hb/g faeces.
Despite each study using country-specific definitions of a negative FIT result, the data support the contention that faecal Hb is a strong predictor of future risk of neoplasia. The reason these concentrations are associated with increased risk is because early lesions with low levels of bleeding were missed because they were not colonoscoped. At a later time lesions become evident due to progressively advancing neoplasia resulting in higher faecal Hb concentration. This information could be used to improve the cost effectiveness of screening programs by extending the screening intervals for those with a low negative and increasing the frequency for those with a high negative result. Quantitative FIT results could therefore be applied to tailor screening intervals.
Reducing burden on endoscopy resources
Following a positive FIT, participants are advised to have further investigation with colonoscopy. FIT positivity rates thus have a direct impact on colonoscopy workload, as well as increasing demand for surgical, pathology and radiology services. Continued surveillance of FIT positive patients found to have neoplasia or a family history of CRC further stretches the endoscopy workloads and waiting times. In 2005, 44.3% of colonoscopies in the United States were performed for surveillance (59). Similarly the proportion of colonoscopies done for surveillance was 33.9% in the Netherlands (60). A review in 2010 of 17 countries showed that many countries already have difficulties meeting increasing endoscopy demand, without the addition of diagnostic colonoscopy following a positive FIT, which will further increase demand, resulting in increased waiting times for higher-risk and symptomatic patients (61). One approach to optimize colonoscopy utilisation would be to use FIT to triage symptomatic patients or postpone surveillance colonoscopies as detailed below.
Matching positivity rates to colonoscopy capacity
Quantitative FIT in screening programs allow thresholds for a positive and hence colonoscopy workload to be adjusted to match the capacity of the endoscopy services (9). The Netherlands, Scotland and New Zealand are examples of countries where this has been done. Scotland has opted for a higher FIT positivity threshold (80 µg Hb/g faeces) to match the positivity rate (approximately 2.4%) of the gFOBT originally used in the national screening program (62). The Netherlands started screening with a positivity threshold of 15 µg Hb/g faeces, but increased this to 47 µg Hb/g faeces when high colonoscopy demand occurred after the FIT results returned higher than expected positivity (13.4% vs. 6.4%) and participation rates (4.0% vs. 2.7%) (63). Similarly, the New Zealand CRC screening program originally set a FIT positivity threshold of 15 µg Hb/g faeces in a pilot program, but have increased this to 40 µg Hb/g faeces for the full program to manage colonoscopy demand (64). It needs to be recognized that increasing the FIT positivity threshold has the benefit of higher specificity, at the cost of a lower sensitivity, although the improvement in sensitivity with a lower threshold mainly relates to adenoma detection rather than cancer (65,66).
Alternatively, FIT positivity thresholds could be lowered, with the screening interval extended. This has been modelled using data from the Dutch screening program (67), with the standard scenario of a FIT positivity threshold of 10 µg Hb/g faeces and biennial screening compared to a hypothetical scenario with a single round of screening and lower positivity thresholds. It was reported that the diagnostic yield of FIT with advanced neoplasia with the altered scenario was similar to the standard screening strategy, which reduces the number of screening rounds needed. This however was not the case when modelled using data from the Scottish screening program (68), which reported that there would be an overall increase in the incidence of interval cancers. This shows that further studies are needed in this area, and results may be program specific.
Use of FIT to triage symptomatic patients
The longer waiting lists for colonoscopy have become a problem in many countries and have led to an urgent need to effectively prioritize procedures. For symptomatic patients, the UK has now incorporated FIT into the NICE guidelines (69) based on the assumption of a low risk of CRC or inflammatory bowel disease associated with undetectable faecal Hb. As symptoms such as change in bowel habits, abdominal pain, anaemia and weight loss have poor clinical sensitivity for CRC (but are common in non-neoplastic gastrointestinal illnesses), FIT can be used to guide decisions to either support or reject the need for colonoscopy. For example it was shown that symptomatic patients with a faecal Hb concentration <10 µg Hb/g faeces had a negative predictive value of 100% for CRC and 94.4% for advanced adenoma (70) suggesting the test could be used to rule-out colorectal neoplasia. Interestingly faecal Hb concentration did not correlate well with clinical symptoms highlighting the non-specific nature of the latter (70). This was supported by a study that concluded that FIT had a higher diagnostic accuracy for significant colorectal disease compared with the NICE guidelines (71). Another study showed that a combination of FIT result, age and gender, and symptoms combined into a single model ruled out CRC in symptomatic patients with the lowest risk score (72). A further study suggested the use of a positive FIT as a rule-in test, where a positive FIT had a significantly higher sensitivity for CRC than the then current NICE guidelines (73). There is an extensive review on this area provided by Steele and Fraser [2018] (74), with the studies described demonstrating innovative clinical application of FIT in the primary care setting to determine which patients could benefit from colonoscopy.
Reducing the number of surveillance colonoscopies in increased risk individuals
Surveillance is recommended for asymptomatic individuals at higher risk for CRC, with colonoscopy being the recommended method. Family history of CRC as well as a personal history of adenoma can increase an individual’s risk of developing CRC (36,37,75). Colonoscopy at regular intervals (generally every 3–5 years except in familial syndromes) for these patients is generally performed until they reach an age where surveillance might no longer be considered appropriate. The competing strategies to screen for CRC, whether it be in average risk or high risk subgroups, are invasive and expensive tests such as colonoscopy or more frequent but less invasive and cheaper alternatives typified by FIT. The risk benefit and cost outcomes of these approaches remains unclear. It may be possible for FIT to guide optimal surveillance intervals, which needs to be a balance between preventing interval cancers and avoiding unnecessary procedures.
Despite surveillance, interval cancers are reported to occur after 1.5% of such surveillance colonoscopies (76). As FIT is sensitive for bleeding neoplasia, testing with FIT post-colonoscopy might detect missed lesions as well as rapidly developing lesions. One such investigation showed that FIT between surveillance colonoscopies resulted in detection of additional significant pathology, with detection earlier than would otherwise have occurred (77). Quintero et al. (78) conducted a randomised trial to compare the efficacy of annual FIT versus one-time colonoscopy in people with a significant family history of CRC. Performing annual FIT for three years was equivalent to one colonoscopy, but the number of procedures needed to detect one advanced neoplasia was dramatically reduced (4 in the FIT screening group compared to 18 in the colonoscopy group). This agrees with earlier findings that FIT in high risk patients (either family history or personal history of neoplasia) has a high sensitivity for advanced neoplasia with FIT detecting 100% of CRC and potentially avoiding colonoscopy in 84.6% of patients (79).
While colonoscopy can prevent cancer development through the removal of pre-cancerous adenomas, these procedures involve a certain level of risk. A recent audit of colonoscopies associated with positive FITs in the Swedish screening program found complications in 1% of procedures with an incidence of perforation reported to be 0.1% after a diagnostic colonoscopy and 0.25% after polypectomy (80). Reducing the number of low yield procedures is therefore attractive and the acceptable accuracy of FIT in the high risk population suggests a role in this setting to better manage colonoscopy surveillance workloads.
It is probable that multiple rounds of FIT will be needed to ensure high neoplasia detection rates in the high risk population as well as confidence in delay of colonoscopy with repeated negatives or low haemoglobin concentrations. Our own work showed that multiple rounds of FIT sampling aided the detection of advanced neoplasia, with repeated testing resulting in an increase of sensitivity (77). With simulation of multiple screening rounds sensitivity for advanced adenoma could reach 81% after five screening rounds (81). Conversely, risk of advanced neoplasia decreases with multiple rounds of negative FIT. Our preliminary studies have shown three or more negative FITs resulted in a 60–70% lower risk of advanced neoplasia at surveillance colonoscopy when compared to one negative FIT (82). FIT also appears to be cost effective. FIT saved 45% of colonoscopies and was associated with a lower rate of complications compared to 5 yearly colonoscopy (83). Another simulation showed that FIT screening without surveillance reduced mortality from CRC by 50.4% compared to surveillance alone (84). The use of FIT in surveillance for high-risk individuals could therefore reduce the number of colonoscopies, as well as risk to patient, in a cost effective manner. As FIT appears to be as sensitive as colonoscopy for advanced neoplasia, FIT screening should be considered for high risk individuals when colonoscopy capacity is limited.
Conclusions
Optimizing healthcare practices is needed to ensure the best use of resources in the global prevention of CRC. Available data indicate that in those at average risk, the higher the baseline FIT Hb concentration, the greater the risk for a future finding of advanced colorectal neoplasia, even when the FIT result has not triggered colonoscopic intervention. Less frequent screening of individuals at low risk with a very low faecal Hb concentration might be justifiable. Conversely, those with qualitatively negative FIT but whose actual Hb concentration is close to the positivity threshold, might warrant a closer and more intensive follow-up regimen. Further individualization of screening programs and the age at which they commence may also be adjusted according to FIT concentrations but also according to other genetic or lifestyle risk factors. Emerging data also indicate that testing with FIT is likely to provide benefit for people who are above-average risk for CRC, by reducing the frequency of surveillance colonoscopies. The use of innovative applications with FIT is likely to be a key strategy in the tailoring of CRC screening programs, whether targeting average-risk or higher-risk individuals, as well maximizing the most cost-effective and efficient use of limited endoscopy resources.
Acknowledgments
We thank Prof. Mark Jenkins for providing the illustration.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Faecal Testing”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.03.03). The series “Faecal Testing” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- AIHW. National Bowel Cancer Screening Program monitoring report: 2012–13. Canberra: Australian Institute of Health and Welfare Canberra, 2014.
- Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637-49. [Crossref] [PubMed]
- Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. [Crossref] [PubMed]
- Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603-7. [Crossref] [PubMed]
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472-7. [Crossref] [PubMed]
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365-71. [Crossref] [PubMed]
- Young GP, Cole SR. Which fecal occult blood test is best for colorectal cancer screening? Nat Clin Pract Gastroenterol Hepatol 2009;6:140-1. [Crossref] [PubMed]
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013;49:3049-54. [Crossref] [PubMed]
- Young GP, Symonds EL, Allison JE, et al. Advances in Fecal Occult Blood Tests: the FIT revolution. Dig Dis Sci 2015;60:609-22. [Crossref] [PubMed]
- Le Pimpec F, Moutel G, Piette C, et al. Fecal immunological blood test is more appealing than the guaiac-based test for colorectal cancer screening. Dig Liver Dis 2017;49:1267-72. [Crossref] [PubMed]
- Cole SR, Young GP, Esterman A, et al. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen 2003;10:117-22. [Crossref] [PubMed]
- Smith A, Young GP, Cole SR, et al. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006;107:2152-9. [Crossref] [PubMed]
- Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015;121:3221-9. [Crossref] [PubMed]
- Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust 2013;198:327-30. [Crossref] [PubMed]
- Zorzi M, Fedeli U, Schievano E, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 2015;64:784-90. [Crossref] [PubMed]
- Digby J, Fraser CG, Carey FA, et al. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol 2013;66:415-9. [Crossref] [PubMed]
- Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171. [Crossref] [PubMed]
- Bobridge A, Cole S, Schoeman M, et al. The National Bowel Cancer Screening Program--consequences for practice. Aust Fam Physician 2013;42:141-5. [PubMed]
- Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631-41. [Crossref] [PubMed]
- Meester RG, Zauber AG, Doubeni CA, et al. Consequences of Increasing Time to Colonoscopy Examination After Positive Result From Fecal Colorectal Cancer Screening Test. Clin Gastroenterol Hepatol 2016;14:1445-1451.e8. [Crossref] [PubMed]
- Bobridge A, Bampton P, Cole S, et al. The psychological impact of participating in colorectal cancer screening by faecal immuno-chemical testing - the Australian experience. Br J Cancer 2014;111:970-5. [Crossref] [PubMed]
- Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer 2013;133:2408-14. [Crossref] [PubMed]
- Kim NH, Kwon MJ, Kim HY, et al. Fecal hemoglobin concentration is useful for risk stratification of advanced colorectal neoplasia. Dig Liver Dis 2016;48:667-72. [Crossref] [PubMed]
- Symonds EL, Osborne JM, Cole SR, et al. Factors affecting faecal immunochemical test positive rates: demographic, pathological, behavioural and environmental variables. J Med Screen 2015;22:187-93. [Crossref] [PubMed]
- Chang LC, Shun CT, Hsu WF, et al. Fecal Immunochemical Test Detects Sessile Serrated Adenomas and Polyps With a Low Level of Sensitivity. Clin Gastroenterol Hepatol 2017;15:872-879.e1. [Crossref] [PubMed]
- Pedersen SK, Baker RT, McEvoy A, et al. A two-gene blood test for methylated DNA sensitive for colorectal cancer. PLoS One 2015;10:e0125041 [Crossref] [PubMed]
- Symonds EL, Pedersen SK, Baker RT, et al. A Blood Test for Methylated BCAT1 and IKZF1 vs. a Fecal Immunochemical Test for Detection of Colorectal Neoplasia. Clin Transl Gastroenterol 2016;7:e137 [Crossref] [PubMed]
- Kim NH, Lee MY, Park JH, et al. A Combination of Fecal Immunochemical Test Results and Iron Deficiency Anemia for Detection of Advanced Colorectal Neoplasia in Asymptomatic Men. Yonsei Med J 2017;58:910-7. [Crossref] [PubMed]
- Niedermaier T, Weigl K, Hoffmeister M, et al. Fecal Immunochemical Tests Combined With Other Stool Tests for Colorectal Cancer and Advanced Adenoma Detection: A Systematic Review. Clin Transl Gastroenterol 2016;7:e175 [Crossref] [PubMed]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287-97. [Crossref] [PubMed]
- Baxter NT, Ruffin MT 4th, Rogers MA, et al. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med 2016;8:37. [Crossref] [PubMed]
- Symonds EL, Cole SR, Bastin D, et al. Effect of sample storage temperature and buffer formulation on faecal immunochemical test haemoglobin measurements. J Med Screen 2017;24:176-81. [Crossref] [PubMed]
- Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054-61. [Crossref] [PubMed]
- Young GP, Senore C, Mandel JS, et al. Recommendations for a step-wise comparative approach to the evaluation of new screening tests for colorectal cancer. Cancer 2016;122:826-39. [Crossref] [PubMed]
- Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology 2016;151:427-439.e6. [Crossref] [PubMed]
- Ait Ouakrim D, Lockett T, Boussioutas A, et al. Screening participation for people at increased risk of colorectal cancer due to family history: a systematic review and meta-analysis. Fam Cancer 2013;12:459-72. [Crossref] [PubMed]
- Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992-3003. [Crossref] [PubMed]
- AIHW. National Bowel Cancer Screening Program Monitoring report July 2011 - June 2012. Australian Institute of Health and Welfare Canberra, 2013.
- Mansouri D, McMillan DC, Grant Y, et al. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS One 2013;8:e66063 [Crossref] [PubMed]
- Young GP. Population-based screening for colorectal cancer: Australian research and implementation. J Gastroenterol Hepatol 2009;24:S33-42. [Crossref] [PubMed]
- Colditz GA, Atwood KA, Emmons K, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control 2000;11:477-88. [Crossref] [PubMed]
- Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol 2009;27:686-93. [Crossref] [PubMed]
- Park Y, Freedman AN, Gail MH, et al. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol 2009;27:694-8. [Crossref] [PubMed]
- Win AK, Macinnis RJ, Hopper JL, et al. Risk prediction models for colorectal cancer: a review. Cancer Epidemiol Biomarkers Prev 2012;21:398-410. [Crossref] [PubMed]
- van Roon AH, Hol L, van Vuuren AJ, et al. Are fecal immunochemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am J Gastroenterol 2012;107:99-107. [Crossref] [PubMed]
- Fraser CG, Rubeca T, Rapi S, et al. Faecal haemoglobin concentrations vary with sex and age, but data are not transferable across geography for colorectal cancer screening. Clin Chem Lab Med 2014;52:1211-6. [Crossref] [PubMed]
- Alvarez-Urturi C, Andreu M, Hernandez C, et al. Impact of age- and gender-specific cut-off values for the fecal immunochemical test for hemoglobin in colorectal cancer screening. Dig Liver Dis 2016;48:542-51. [Crossref] [PubMed]
- Grazzini G, Zappa M, Ventura L, et al. Authors' Reply. Gut 2011;60:1304. [Crossref]
- Ventura L, Mantellini P, Grazzini G, et al. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis 2014;46:82-6. [Crossref] [PubMed]
- Bujanda L, Sarasqueta C, Castells A, et al. Colorectal cancer in a second round after a negative faecal immunochemical test. Eur J Gastroenterol Hepatol 2015;27:813-8. [Crossref] [PubMed]
- van Doorn SC, Stegeman I, Stroobants AK, et al. Fecal immunochemical testing results and characteristics of colonic lesions. Endoscopy 2015;47:1011-7. [Crossref] [PubMed]
- Chiu SY, Chuang SL, Chen SL, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut 2017;66:293-300. [Crossref] [PubMed]
- Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Senore C, Ochipinti P, Roca R, et al. Haemoglobin level at inital FIT and risk of neoplasia at subsequent screening rounds. Gastroenterol 2016;150:S184. [Crossref]
- Grobbee EJ, Schreuders EH, Hansen BE, et al. Association Between Concentrations of Hemoglobin Determined by Fecal Immunochemical Tests and Long-term Development of Advanced Colorectal Neoplasia. Gastroenterology 2017;153:1251-1259.e2. [Crossref] [PubMed]
- Yen AM, Chen SL, Chiu SY, et al. A new insight into fecal hemoglobin concentration-dependent predictor for colorectal neoplasia. Int J Cancer 2014;135:1203-12. [Crossref] [PubMed]
- Chen LS, Yen AM, Chiu SY, et al. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol 2011;12:551-8. [Crossref] [PubMed]
- Digby J, Fraser CG, Carey FA, et al. Faecal haemoglobin concentration is related to detection of advanced colorectal neoplasia in the next screening round. J Med Screen 2017;24:62-8. [Crossref] [PubMed]
- Lieberman DA, Holub J, Eisen G, et al. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc 2005;62:875-83. [Crossref] [PubMed]
- Terhaar Sive Droste JS, Craanen ME, van der Hulst RW, et al. Colonoscopic yield of colorectal neoplasia in daily clinical practice. World J Gastroenterol 2009;15:1085-92. [Crossref] [PubMed]
- Kanavos P, Schurer W. The dynamics of colorectal cancer management in 17 countries. Eur J Health Econ 2010;10:S115-29. [Crossref] [PubMed]
- Steele RJ, McDonald PJ, Digby J, et al. Clinical outcomes using a faecal immunochemical test for haemoglobin as a first-line test in a national programme constrained by colonoscopy capacity. United European Gastroenterol J 2013;1:198-205. [Crossref] [PubMed]
- van Hees F, Zauber AG, van Veldhuizen H, et al. The value of models in informing resource allocation in colorectal cancer screening: the case of The Netherlands. Gut 2015;64:1985-97. [Crossref] [PubMed]
- Ministry of Health. Age Range and Positivity Threshold for the National Bowel Screening Programme. New Zealand. 2017. Available online: http://www.health.govt.nz/our-work/diseases-and-conditions/cancer-programme/bowel-cancer-programme/national-bowel-screening-programme/key-documents-national-bowel-screening-programme, accessed 10/01/2018.
- Grazzini G, Visioli CB, Zorzi M, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer 2009;100:259-65. [Crossref] [PubMed]
- van Rossum LG, van Rijn AF, Laheij RJ, et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer 2009;101:1274-81. [Crossref] [PubMed]
- Haug U, Grobbee EJ, Lansdorp-Vogelaar I, et al. Immunochemical faecal occult blood testing to screen for colorectal cancer: can the screening interval be extended? Gut 2017;66:1262-7. [Crossref] [PubMed]
- Digby J, Fraser CG, Carey FA, et al. Can the performance of a quantitative FIT-based colorectal cancer screening programme be enhanced by lowering the threshold and increasing the interval? Gut 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Diagnostics guidance DG30. Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. 2017. Available online: https://www.nice.org.uk/guidance/dg30, accessed 04/01/2018.
- McDonald PJ, Digby J, Innes C, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis 2013;15:e151-9. [Crossref] [PubMed]
- Rodríguez-Alonso L, Rodriguez-Moranta F, Ruiz-Cerulla A, et al. An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis 2015;47:797-804. [Crossref] [PubMed]
- Cubiella J, Digby J, Rodriguez-Alonso L, et al. The fecal hemoglobin concentration, age and sex test score: Development and external validation of a simple prediction tool for colorectal cancer detection in symptomatic patients. Int J Cancer 2017;140:2201-11. [Crossref] [PubMed]
- Cubiella J, Salve M, Diaz-Ondina M, et al. Diagnostic accuracy of faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis 2014;16:O273-82. [Crossref] [PubMed]
- Steele RJ, Fraser CG. Faecal Immunochemical Tests (FIT) for Haemoglobin for Timely Assessment of Patients with Symptoms of Colorectal Disease. Timely Diagnosis of Colorectal Cancer.: Springer, Cham; 2018. p. 39-66.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133:1077-85. [Crossref] [PubMed]
- Lane JM, Chow E, Young GP, et al. Interval fecal immunochemical testing in a colonoscopic surveillance program speeds detection of colorectal neoplasia. Gastroenterology 2010;139:1918-26. [Crossref] [PubMed]
- Quintero E, Carrillo M, Gimeno-Garcia AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014;147:1021-30.e1; quiz e16-7.
- Hazazi R, Rozen P, Leshno M, et al. Can patients at high risk for significant colorectal neoplasms and having normal quantitative faecal occult blood test postpone elective colonoscopy? Aliment Pharmacol Ther 2010;31:523-33. [Crossref] [PubMed]
- Saraste D, Martling A, Nilsson PJ, et al. Complications after colonoscopy and surgery in a population-based colorectal cancer screening programme. J Med Screen 2016;23:135-40. [Crossref] [PubMed]
- Terhaar sive Droste JS, van Turenhout ST, Oort FA, et al. Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multi-centre cohort study. BMC Gastroenterol 2012;12:94.
- Symonds EL, Meng R, Heddle R, et al. Risk of advanced neoplasia after consecutive negative FOBT results in a colonoscopy surveillance program. J Gastroenterol Hepatol 2015;30:82. [PubMed]
- Nicolás D, Castilla-Rodríguez I, Salgado-Fernández M, et al. Annual fecal immunochemical testing is as effective as colonoscopy every 5 years for familial colorectal cancer screening. Gastroenterol 2017;152:S542. [Crossref]
- Greuter MJ, de Klerk CM, Meijer GA, et al. Screening for Colorectal Cancer With Fecal Immunochemical Testing With and Without Postpolypectomy Surveillance Colonoscopy: A Cost-Effectiveness Analysis. Ann Intern Med 2017;167:544-54. [Crossref] [PubMed]
Cite this article as: Symonds EL, Fraser RJL, Young GP. FIT for purpose: enhanced applications for faecal immunochemical tests. J Lab Precis Med 2018;3:28.


