Laboratory test inappropriateness: lessons revisited and clarified in seven questions
Introduction
The seven questions answered in this article revolve around laboratory test inappropriateness. More specifically, the article discusses the causes behind request inappropriateness, reasons to reduce such inefficiency, strategies to correct it, and a practical pathway to design and establish them to identify and correct the problem and to monitor success after interventions. We include data from the Spanish REDCONLAB working group and discuss insights for the future.
(I) What is the importance of an appropriate test request for the best diagnosis, management and prevention of diseases?
The clinical laboratory plays a crucial role in healthcare (1), since it is involved in 70% of clinical decisions (2). Such a prominent position of the laboratory in patient care should be used by the laboratory professional to improve patient outcome.
In addition to the ‘mission’ of the laboratory—diagnosis, prevention and monitoring of diseases—the ‘vision’ of the laboratory should be to deliver maximum benefit to the patient per euro spent (3). Pathologists should aim for a pathophysiology-centered laboratory (4) according to the individualized patient condition (5). Indeed, the goal of laboratory testing is not the acquisition of information itself, but to improve patient outcome (6) through the promotion of proper laboratory test utilization, namely an appropriate test request and result utilization (7).
The total testing process (TTP) based on the “brain-to-brain loop” concept described by Lundberg (8,9) begins with the clinicians’ clinical question and ends when the test result is interpreted and acted upon, both steps also called pre-pre- and post-post-analytical phases (10,11). Between these two important steps in the TTP, the following additional phases exist: patient identification, sample collection, transport and preparation, and analysis, and report. Laboratory professionals should aim for continuous improvement in laboratory organization through performance measurements (12). Recent studies suggest that the highest incidence in laboratory-related errors occurs in these two phases (pre-pre and post-post) (10,11,13-16). The increase in laboratory automation has led to a reduction in the number of laboratory errors classically regarded as the TTP phases, especially the analysis (17). Traditionally, few resources were allocated to the pre-pre and post-post-analytical phases, to reduce the number of errors in ordering the appropriate diagnostic test or improving laboratory test interpretation (18).
Laboratory tests requests have increased exponentially since 1920 (19). The progressive automation of laboratories (20), the aging population (21) and the limited physician time availability for patient care (22) are among the main causes of this exponential growth in requests for laboratory tests. However, such an increase cannot continue indefinitely, as healthcare costs are also growing dramatically (23). The overall mean rate of inappropriate overutilization is around 20%, however the overall mean rate of inappropriate underutilization is higher, at 44.8% (24). In fact the number of unnecessary tests in the clinical laboratory ranges from 5% to 95% of the total number of laboratory tests (25), with large variations (26). Other authors have indicated that almost two thirds of commonly ordered laboratory tests in an academic internal medicine department could have been avoided because this data did not contribute to improve patient’s management (27). In this scenario, it has been suggested that between 25% and 40% (28,29) of all the requested laboratory tests are questionable and that between 16% and 30% of them (28,29) were inappropriately retested based on criteria such as analyte half-lives and analytical variability.
What is also well known is that the ability of a diagnostic procedure to add value to the diagnosis, prevention and/or follow-up of certain diseases closely depends on how clinicians use it. Indeed, it is a priority to align the request of laboratory tests with the clinical indication or clinical question (4); otherwise, test misuse could result in significant adverse effects when under- or over-requested, regardless of the cost of the test. It is important to study if a laboratory test should or should not be ordered in certain scenarios, and correct tests inadequacies through the utilization of management policies (7). And most importantly, the laboratory professional must take the promotion of the use of the appropriate test as a duty, a task that he has to lead, in consensus with the requesting clinician.
There are already excellent reviews on the topic (4,24,25,30-36). This review intends to provide the pillars to the laboratory professional so the task can be properly conducted.
(II) What is the inappropriateness in laboratory test requesting?
Fryer et al. (4), in an excellent review regarding demand management for laboratory tests, categorizes, defines, and quantifies the inappropriate request: “A request (implying what is ordered by the requestor) that is made outside some form of agreed guidance (including those requested too late)”.
A recent definition of appropriateness is the prescription of “the Right test, using the Right method, at the Right time, to the Right patient, with the Right costs and for producing the Right outcome” (37).
The definition itself implies that test request needs to be in accordance with guidelines. However there is a great variability among laboratories and between requestors, even in identical clinical scenarios of what is appropriate (38-47). Inappropriate requesting includes wrong patient, wrong test, wrong time, and wrong process (29).
Although in some cases the inappropriateness is clear (e.g., prostate-specific antigen in women), in other situations, recommendations or guidelines (evidence-based) are necessary, such as in diabetes mellitus for which the National Institute for Care and Excellence and American Diabetes Association provide guidance on testing intervals (48,49).
It is necessary to establish the test adequacy through consensus with the requesting clinician based on the literature, on sensitivity and specificity of tests, on the cost of investigations, on the recommended repeat testing interval, etc. (50).
(III) What are the causes behind inappropriate test request?
The causes of inappropriate test request are multiple: indiscriminate use of non-agreed-upon tests; routine laboratory test profiles; redundant tests, that provide similar clinical information; lack of awareness of recommended repeat testing intervals; uncertainty in the patient diagnosis, lack of awareness of the cost of testing, etc.
Whiting et al. (51) identified five key interrelated factors that influence a doctor’s decision to order a test for a particular patient: diagnostic, therapeutic and prognostic, patient-related, doctor-related, and policy and organization-related factors. Each one of the factors can affect the test request.
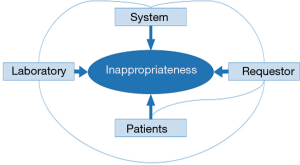
Therefore, the causes of tests inappropriateness (Figure 1) can be attributed to the following factors (4): the laboratory; the requestor; the patient and the system.
Laboratory
There are many reasons for inappropriateness (52): introducing new tests without the evidence that proves their efficacy and effectiveness; not eliminating poor or useless tests from the laboratory repertoire; providing low turnaround time of test results; request forms that include large numbers of tests and profiles; laboratories that perform unrequested tests (algorithms) unrelated to the suspected diagnosis. In all, in most healthcare systems, laboratories are commoditized working as an industry instead of a medical specialty, which gives the impression that testing is easy, because of the automation.
Requesting physician
If requesting physicians knew the basic concepts of testing (sensitivity, specificity, receiver operator characteristic curves, predictive values, biological variability, likelihood ratio, etc.), that would probably significantly reduce inefficient test requests as well as errors in the interpretation of test results. However, as stated more than one century ago, “a medical student often leaves the walls of his alma mater with a false conception of the use of the laboratory in diagnosis” or “there is a danger that laboratory findings may be allowed to take the place of the keen thinking and the educated senses which our professional ancestors used to such good purpose” (53). Currently, this problem not only continues, but is even more serious. For instance, the degree of knowledge shown by physicians regarding the variability of laboratory results is scarce (54). Beyond the importance of the biological and analytical variability, the knowledge of the reference value theory and the clinical sensitivity and specificity of laboratory tests is crucial for an appropriate data interpretation and, hence, for a real laboratory contribution to clinical decision making.
For an accurate clinical use of laboratory data, a correct clinical interpretation of laboratory test results through comparisons with reference values is also required. In fact reference intervals are the most popular decision tool for the interpretation of laboratory reports (55). However, this concept can be misinterpreted in several ways by the requesting physician. In fact, by using the definition of the reference interval, 5% of the healthy people are excluded. In addition, a result within the reference interval does not always signify that the patient is healthy, and a result outside the reference interval does not always indicate sickness.
Requesting physicians often order redundant tests that provide identical information. Inefficient test repetition is more common with hospitalized patients (56); also physicians that routinely request groups of tests regardless the patient’s clinical situation can also influence laboratory demand. Historically, test profiles have been organ-based (liver, kidney, and thyroid profiles), providing a set of tests that offer information for one particular organ. Specific disease profiles (29) try to simplify and standardize the common test requests necessary for the diagnosis or monitoring of a specific pathology. These profiles, that must be established by consensus with the requesting physicians and the laboratory, present more disadvantages than advantages. Even when they are established with the consensus of the laboratory, they are not always based on evidence. The 2011 report of the National Pathology Benchmarking Service, which includes approximately 50 laboratories in the UK, demonstrated that up to 12 different hepatic function profiles were in use (57).
Another factor that results in laboratory test request inefficiency is physicians monitoring the clinical course of a disease with a higher frequency of testing than recommended.
Finally, defensive medicine is increasing (58) and can generate an excessive number of diagnostic tests to rule out extraordinary situations even when a clear diagnosis.
Patient
The pressures on the physician by the patient may increase laboratory demand. The patient perceives that “something is being done”. In fact, the current trend is toward a patient-centered healthcare model, which tries to involve the patient and physician in clinical decision making could increase test over requesting.
Factors regarding the system
The pressure to reduce the hospital average stay may also contribute to the inappropriate requests for laboratory tests; as well as new surgical techniques such as transplantation or special units.
(IV) What are the reasons to reduce inappropriate laboratory tests request?
Our goal as laboratory professionals must be to achieve the maximum information with the fewest number of tests (59). In fact, it is important to reduce over request but also to request every test related to the clinical suspicion, according to evidence or guidelines.
Overall, test requesting is the step where most errors have been reported; many of them are not detected and can cause great harm to the patient. By correcting under requesting we can improve diagnosis as well as monitoring and prevention.
On the other hand, when tests are over-requested, there could be three potential adverse effects, or three main causes of potential adverse effects.
First the cost of the test itself; although usually lab tests are inexpensive, since they are high volume they can generate high expenses. In fact, they belong to the little ticket tests group of diagnostic procedures. Second the consequences of false positive results (60); the patient begins the journey of Ulysses or Imaginary Invalid syndrome (61) that contributes to a great discomfort to the patient, and additional more complex tests, which are usually more expensive. Finally, over request contributes to and increased demand for laboratory tests. Clinical laboratories are at risk of becoming data vending machines. In this situation the test expert, the clinical laboratory professional, could not have time to pay enough attention to provide knowledge instead of data in 70% of clinical decision making, through knowledge management (62). Moreover, irrelevant test results may hide important data for clinical decision making.
In all over test request can generate high cost, and negatively impact clinical decision making and patient safety. However, by correcting over request we can greatly improve costs, clinical decision making and patient safety.
(V) What are the strategies to correct inappropriateness in laboratory test requesting?
As described in the literature, there are strategies designed to be established before, during, and after the test request.
Before
Test catalog review
There is a need to review the tests offered by the laboratory to discard obsolete or add new emerging test. The test catalog of clinical laboratory services should be standardized; however, several factors make this situation very difficult to achieve (29). Possible approaches are to limit the test repertoire, to redesign the request form, the use of disease-specific profiles and the withdrawal of outdated tests. Consequently, in a systematic way, each laboratory needs to constantly review their catalog to match emerging needs (63), eliminate obsolete tests and incorporate new assays.
Educational strategies
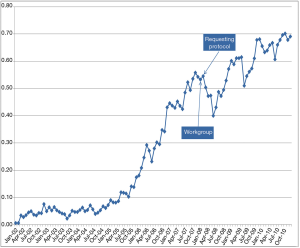
There are many educational interventions, including both verbal and written initiatives (29,31,64,65). In these educational interventions, the laboratory informs the requesting physicians about changes in laboratory repertoire, obsolete tests, recommended repetition intervals, or potential interventions. These sessions or periodic bulletins must be prepared in consensus with requesting clinicians for specific clinical conditions. For example, the laboratory, together with specialists in digestive medicine, could inform general practitioners about the latest recommendations and clinical guidelines for coeliac disease. Although education and communication are crucial (66), the effectiveness of these interventions is variable (33,34). For example, Emergency Department (ED) C reactive protein (CRP) requests decreased after the implementation of a protocol designed in consensus with clinicians and monthly monitoring (Figure 2). However, after a few months, the request for CRP increased (12). Therefore, these strategies require exhaustive monitoring by the laboratory.
Request forms
The design of the request form, either in paper or electronic format, has been used as a strategy to manage laboratory tests demand (33,34,67-70). At present, paper request forms are used less frequently than electronic request forms, offering a wide range of opportunities for managing test demand (71).
Test profiles
Test profiles vary across different laboratories (72). The ideal profile does not exist, and profile differences can cause confusion and affect patient safety (73). Removing tests that provide little information from the profiles is cost-effective and improves patient outcome (74).
Clinical guidelines or protocols
Protocols and clinical guidelines that include laboratory tests request should be established in consensus with requesting clinicians, following, when available, the recommendations of scientific societies and systematic reviews (75).
During the request
Laboratory Information Management Systems (LIMS), patient’s data bases and electronic medical records (EMR) are the pillars for such strategies that depend on the information technology possibilities at each health care center (75). The complexity of laboratory test selection is aggravated by increasingly busy physicians. With information technology we are able to support the clinical decision making by ensuring proper testing and mitigate these challenges (76). Useful software tools can help decrease the number of unnecessary test repetitions (77) via alert messages to the requesting physician. More complex measures have also been described for guiding the requesting physician toward the most appropriate test (78,79).
These software tools can be informative or restrictive. Informative tools inform the requesting physician about a characteristic of the test or patient so they can decide whether to request it or not; these tools can show the cost of the test, generate a comment in case of excessive request frequency, describe tests as redundant, or provide indications of diagnostic utility. Restrictive tools limit the request for the test based on, for example, recommended minimum intervals.
Strategies that are implemented during test request are considered the most powerful (44) because they are maintained over time and do not depend on external factors, such as habits relaxation, staff changes, etc.
After the request
Once a test is requested, strategies are difficult to implement. Clearly inappropriate tests may be rejected (such as repetitions of HLA, karyotype, etc.). At this stage, clinical justification may be required for the request of certain tests with very limited indications, little scientific evidence, or high costs.
(VI) What is the practical pathway to design, establish, and monitor over time test requesting appropriateness strategies? What are the appropriateness indicators to detect the inappropriateness and to monitor after interventions?
Introduction
When establishing new interventions, it is key to detect the inappropriateness and to choose the correct test and population to establish the strategy; and to monitor after the intervention, through process and outcome indicators customized according to the type and stage of strategy.
The laboratory professional must lead each one of the phases of the strategy, as she/he is the one that has the knowledge and experience in laboratory tests.
Indicators
The indicators are the basis on which any strategy should be designed. Shahangian (80) described the desirable attributes of a health measure based on organizations committed to health care quality measurement and improvement, and grouped these criteria for quality indicators into three conceptual areas. First the importance as relevant for clinician and patient, and also potential for improvement; second the scientific soundness as the indicator must be reliable, valid, and understandable for users, and thirdly feasibility of the measure, with a specified numerator and denominator, an understandable and implementable data collection, a data source available and accessible in time, and measurement costs justified by the potential for improvement in care.
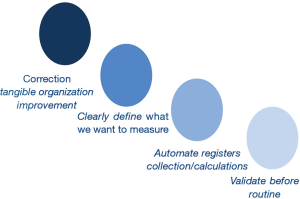
In laboratory daily practice there are four main considerations related to key performance indicators (KPIs). First, it is fundamental to measure KPIs whose correction will result in tangible improvement for the organization. Second, registers to calculate KPIs must be automatically collected; also, calculations must be done in an automatic way. Manual collection could result in the loss of data that does not to occur when data are collected in an automated manner, for example through warehouse programs from LIMS (12). In fact it has been shown that laboratories more focused on detecting and correcting errors could have higher error rates than others that do not pay much attention to it (81). Third, when designing KPIs, it is important to define what we want to measure, and use the latest available technology; for instance, the hemolysis can be measured using the hemolytic index, instead of manual color serum visualization (82). Finally, it is necessary to validate the indicator before establishing it in routine as a tool to improve the organization processes (80,83). If the laboratory does not validate these indicators, the laboratory workers will likely distrust their results and disbelieve the whole laboratory management system (3,84). It is crucial to measure correctly through harmonization of pre-analytical quality indicators (85) (Figure 3).
Table 1 shows the main indicators to be applied in strategies to correct inappropriateness in laboratory test requesting, classified as process and outcome KPIs.
Table 1
| Type | Name | Design | |
|---|---|---|---|
| Numerator | Denominator | ||
| Process: to detect inappropriateness and/or to check if the strategy is working properly | Per inhabitants/admissions | Test request | 1,000 residents/admissions |
| Absolute number of tests added, eliminated or not measured but reported | Test added, eliminated, or not measured but reported | – | |
| Per highly requested test | Test request | Request of a related test | |
| Outcome | Diagnosis | Cases detected | – |
| Costs | Euros saved | – | |
| Cost per case detected | Euros saved | Detected case | |
Process indicators are used to detect test inappropriateness and/or assess progress through regular monitoring after the intervention establishment. Outcome indicators are used to measure the benefit of the strategies in terms of economic savings or patient benefit in diagnosis, treatment, prevention of diseases, or quality of life.
Process indicators
Process indicators are used to detect over or under requested tests and also to monitor after strategy implementation. Test requests per 1,000 inhabitants in a primary care setting (45,86) or per 1,000 patient admissions in an ED (39,44) are very easy to construct. In the former, test-utilization rates are calculated by standardization with the population attended by each laboratory and are very useful in public healthcare models. The latter can be used in any healthcare model. Both indicators indirectly detect test inappropriateness when comparing to other geographical areas (87).
The second type of process appropriateness indicator, ratios of related tests requests, is very useful in detecting test inappropriateness in any setting and can also be used to monitor after strategy implementation. Some even have a set or published goal (88). In this type of indicators, the test whose demand should be corrected figures as the numerator. The third type of indicators “ratio of test demand per a highly requested test” is also useful (89).
Outcome indicators
Outcome indicators are used to measure the benefits that strategy implementation has in the patient, healthcare organizations or society.
There are few laboratory studies that focus on the benefit that laboratory strategies cause in the patient.
Monitoring through outcome indicators is a key: the number of new diagnoses, the cost of each new case detected (90,91), and/or funds saved because of the intervention (92) are very significant end points.
A step-by-step description of strategies to correct inappropriateness in laboratory test requesting
Identify laboratory test inappropriateness
The first step is to identify laboratory test inappropriateness. There are several ways to do it. Traditionally, it has been done through the revision of patient medical records through implicit and explicit criteria. Usually, this is analyzed in prospective studies that take many years before having any answers, and it is rather costly; thus, more practical methods are needed. Listed below are a few ways to identify appropriateness.
Studies of most expensive tests
In countries with public healthcare systems, there are indirect ways to investigate inappropriateness of tests that generate the greatest economic cost.
As an example, a test that generates a great economic cost is 25-OH-vitamin D. In Osakidetza, a region in northern Spain, from 2011 to 2013, the request for 25-OH-vitamin D test has doubled, reaching an annual expense of more than €600,000. As a consequence, the measurement of 25-OH-vitamin D is placed among the 10 standard laboratory parameters that cost most in this region. This fact has led to instructions for the measurement of 25-OH-vitamin D (19), designed through interdepartmental consensus (20). In any case, regardless of being one of the tests that generate more expense, the inadequacy must be confirmed.
Studies on test utilization differences between geographical areas
A second indirect method is to identify laboratory test inappropriateness through studies on test utilization differences between geographical areas. Through those studies, detecting test over- and under-request is possible, and an example is REDCONLAB studies. Every Spanish citizen possesses an individual health care card, which provides access to public health services as a healthcare user throughout the National Health System. The health system in every region is divided into health departments (HDs) that cover a geographic area and its population. It is composed of several primary care centers and usually has a unique hospital. The laboratory located at the hospital attends the needs of every HD inhabitant.
The first study was performed in Valencia region in 2009 with only eight participants. In the first national REDCONLAB study in 2010 (86), a call for data was posted on the REDCONLAB website; via e-mail in the second [2012]. In the 2014 study, the questionnaire was also addressed to the participants of previous current edition, and a LinkedIn group was also created (https://www.linkedin.com/in/REDCONLAB-grupo-a5663bb7).
In every edition, Spanish laboratories willing to participate in the study were invited to fill out an enrollment form and submit their results online. In the four consecutive studies, production statistics (number of tests requested by general practitioners) were obtained. Every patient seen in any primary care center of any of these institutions, regardless of the reason for consultation, sex, or age, was included. Each participating laboratory was required to be able to obtain patient data from local databases and to provide organizational data. In the three national editions of the REDCONLAB study, 38, 76, and 110 laboratories at different hospitals from diverse regions across Spain consecutively participated (39,40,42,44,45,47,86,93-105).
After collecting data, two types of appropriateness indicators were calculated: test requests per 1,000 inhabitants or ratios of related tests requests. Both appropriateness indicators, as shown in Table 1, belong to the category of process indicators.
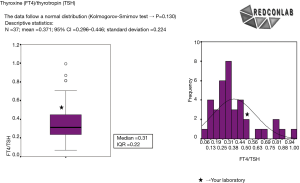
With these data, a frequency histogram and a box plot for each of the indicators were drawn to conform a confidential pre-pre-analytical quality control report that was sent to each participating laboratory indicating their individual results compared to those of others (45). Each report had a sheet for every test ordered per 1,000 inhabitants and also for ratios per related test requests (Figure 4).
Comparison with guidelines or disease prevalence
A third indirect method to detect test inappropriateness is through comparison with guidelines or disease prevalence. Through this type of study, an over-request has been detected in tumor marker request in Italy. Gion et al. (106) developed a model matching the rate of utilization of tumor marker tests with prevalence data as an indirect indicator of laboratory tests inappropriateness. This model is useful when the availability of clear guidelines regarding clinical use of the test and epidemiological data on the disease. This epidemiological-based model does not offer a direct measure of appropriateness—it only shows areas of over-request that might related to inappropriate use, making a deeper study necessary to confirm the inappropriateness or establish interventions to reduce over-request. Also, an epidemiological model was used in the second National REDCONLAB study to identify HbA1c under request. To investigate whether HbA1c was appropriately requested to manage patients with diabetes mellitus, that research compared the theoretically ideal number of HbA1c requests that should have been ordered to the number of real HbA1c requests in a population of 20 million inhabitants in Spain. The former was calculated according to disease prevalence in Spain (6.9%) (107) and to the current guideline recommendations regarding glucose monitoring (HbA1c test at least two times a year) and testing for diabetes mellitus in asymptomatic patients (HbA1c every 3 years in patients older than 45 years) (91). A total of 2,439,729 HbA1c requests would have been necessary to appropriately manage the existing patients with diabetes mellitus. A total of 2,384,408 tests would have been needed to diagnose new patients, according to the current guidelines. Considering the real number of tests performed, a total of 3,280,183 additional tests would have been necessary for both purposes. Not a single HD of the 76 participants reached those theoretical figures.
Retrospective searches in patient’s data base and comparison with guidelines
It is mandatory to take full advantage of the information technologies we have at our disposal. Retrospective searches based on LIMS and patient data bases can inform us about what the real demand patterns are. Through a comparison with guidelines, it is possible to detect laboratory tests under or over request.
Selection of the test and target population
The second step of our approach is the selection of the test and target population for strategy implementation. It is advisable to begin with tests whose demand could be tailored through easy, automatic, and simple strategies. If the only way to diminish 25-OH-vitamin D demand is by manual checking of every patient to determine if the test request relates to the clinical question or diagnosis or suspected diagnosis, a lot of resources would be necessary.
Regarding the test, the simplicity of the potential strategy, the risk of over or under request or consequences of over or under requesting in patient safety, and also financial consequences need to be evaluated (108).
Regarding population, our experience is to begin in primary care or ED, where relatively less effort may positively impact many patients.
Generation of the idea
The third step of our approach is the generation of the idea. Knowledge is not enough for the prominence of the laboratory in clinical decision making. Creative imagination, communication and leadership are also necessary (109). Through their imagination, laboratory professionals should catch any opportunity to help clinicians to request tests appropriately. A crucial condition related to the idea generation, is that the strategy should better rely on automatic processes, based on the LIMS, and patient data base. Once designed through interdepartmental consensus, if established based on information technologies, they will be continuous over time, without additional efforts needed (92).
Pre-design of the strategy
The fourth step, the pre-design of the strategy, is crucial to be done in consensus with requesting physicians and based on LIMS retrospective simulation of the potential strategy results. The benefits that the patient and healthcare organization are going to achieve with the strategy implementation should be investigated and explained in detail.
Strategy final design
The fifth step is the strategy final design. In this step, the procedure must be written down in our standard operating procedure: initiative, objectives, indicators, and goals.
Strategy establishment
The strategy always must be established for a specified period. A specified period of time to monitor, evaluate, and decide whether to continue, stop, or re-design the strategy for better results is necessary.
Monitoring through process indicators
Process indicators (Table 1) show how the strategy is working overtime, in terms of test measurement diminution or increase, depending on whether we are solving test over- or under-requesting (92). Monitoring is always advisable through the use of indicators that measure ratio of the request of related tests. There are a lot of examples such as free thyroxin/thyrotropin or aspartate aminotransferase (AST)/alanine aminotransferase (ALT). This type of indicator has an additional advantage, since some have a set target to be reached, based on guidelines. When this type of indicators is not available, we could always monitor the number of requests in which we are correcting the inappropriateness related to a highly requested test. An example is the ratio of uric acid to glucose request in primary care when solving uric acid over-request (89).
Evaluation through outcome indicators
Laboratory data intervene in 70% of clinical decisions; are we measuring this intervention in terms of patient improvement? This is the main point of any strategy design. From the beginning, and also through consensus, it is necessary to decide what indicators to measure to really study the improvements for the patients, healthcare organizations or society. For example, the improvement in the diagnosis of acute pancreatitis in an ED (110). This requirement is especially important when the strategy is established to correct tests under-request because we are increasing expenses and it is imperative to know if we are detecting occult diseases (90) such as diabetes (91) or primary care hyperparathyroidism patients detected (90), and the cost per detected case. When diminishing uric acid demand, a concomitant decrease in the prescription of allopurinol, and a reduction in the laboratory and overall health system economic costs was obtained (89,92).
Final decision whether to continue or stop the strategy
Through the evaluation of outcome indicators, we could make the right decision whether to continue or to stop strategy in terms of improvement in patient and healthcare organization.
(VII) Main points and insights for the future
Currently, most laboratory errors are generated in the first and last step of TTP. It is a duty of laboratory professionals to correct these TTP errors through interventions based in our knowledge but also in creative imagination, communication and leadership (109), designed in agreement with requesting clinician, and executed and monitored through process and outcome indicators (7).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael Cornes and Jennifer Atherton) for the series “Reducing errors in the pre-analytical phase” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.03.10). The series “Reducing errors in the pre-analytical phase” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Blanckaert N, Bonini P, et al. Causes, consequences, detection, and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med 2009;47:143-53. [Crossref] [PubMed]
- Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem 1996;42:813-6. [PubMed]
- Salinas M, López-Garrigós M, Gutiérrez M, et al. Designing a Balanced Scorecard Management System in a Clinical Laboratory in Spain. Clin Leadersh Manag Rev 2011;25:2-9.
- Fryer AA, Smellie WS. Managing demand for laboratory tests: a laboratory toolkit. J Clin Pathol 2013;66:62-72. [Crossref] [PubMed]
- Burke MD. Clinical laboratory consultation: appropriateness to laboratory medicine. Clin Chim Acta 2003;333:125-9. [Crossref] [PubMed]
- Plebani M. Appropriateness in programs for continuous quality improvement in clinical laboratories. Clin Chim Acta 2003;333:131-9. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Managing inappropriate requests of laboratory tests: from detection to monitoring. Am J Manag Care 2016;22:e311-6. [PubMed]
- Lundberg GD. Acting on significant laboratory results. JAMA 1981;245:1762-3. [Crossref] [PubMed]
- Lundberg GD. How clinicians should use the diagnostic laboratory in a changing medical world. Clin Chim Acta 1999;280:3-11. [Crossref] [PubMed]
- Laposata M, Dighe A. ‘Pre-pre’ and ‘post-post’ analytical error: high-incidence patient safety hazards involving the clinical laboratory. Clin Chem Lab Med 2007;45:712-9. [Crossref] [PubMed]
- Stroobants AK, Goldschmidt HM, Plebani M. Error budget calculations in laboratory medicine: linking the concepts of biological variation and allowable medical errors. Clin Chim Acta 2003;333:169-76. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Gutiérrez M, et al. Achieving continuous improvement in laboratory organization through performance measurements: a seven-year experience. Clin Chem Lab Med 2010;48:57-61. [Crossref] [PubMed]
- Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med 2006;145:488-96. [Crossref] [PubMed]
- Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194-200. [Crossref] [PubMed]
- Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and Delayed Diagnoses in the Emergency Department: A Study of Closed Malpractice Claims From 4 Liability Insurers. Ann Emerg Med 2007;49:196-205. [Crossref] [PubMed]
- Wahls TL, Cram PM. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007;8:32. [Crossref] [PubMed]
- Agarwal R. Measurement of errors in clinical laboratories. Indian J Clin Biochem 2013;28:227-34. [Crossref] [PubMed]
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 2010;47:101-10. [Crossref] [PubMed]
- Wians FH. Clinical Laboratory Tests: Which, Why, and What Do The Results Mean? Lab Med 2009;40:105-13. [Crossref]
- Armbruster DA, Overcash DR, Reyes J. Clinical Chemistry Laboratory Automation in the 21st Century - Amat Victoria curam (Victory loves careful preparation). Clin Biochem Rev 2014;35:143-53. [PubMed]
- Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015;15:415. [Crossref] [PubMed]
- Green ME, Hogg W, Savage C, et al. Assessing methods for measurement of clinical outcomes and quality of care in primary care practices. BMC Health Serv Res 2012;12:214. [Crossref] [PubMed]
- White BA, Baron JM, Dighe AS, et al. Applying Lean methodologies reduces ED laboratory turnaround times. Am J Emerg Med 2015;33:1572-6. [Crossref] [PubMed]
- Zhi M, Ding EL, Theisen-Toupal J, et al. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One 2013;8:e78962 [Crossref] [PubMed]
- van Walraven C, Naylor CD. Do we know what inappropriate laboratory utilization is? A systematic review of laboratory clinical audits. JAMA 1998;280:550-8. [Crossref] [PubMed]
- Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem 2007;53:1338-42. [Crossref] [PubMed]
- Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J 2006;82:823-9. [Crossref] [PubMed]
- Castellví-Boada JM, Castells-Oliveres X. Appropriateness of physicians’ requests of laboratory examinations in primary health care: an over- and under-ulilisation study. Clin Chem Lab Med 1999;37:65-9. [Crossref] [PubMed]
- Fryer AA, Hanna FW. Managing demand for pathology tests: financial imperative or duty of care? Ann Clin Biochem 2009;46:435-7. [Crossref] [PubMed]
- Smellie WS. Appropriateness of test use in pathology: a new era or reinventing the wheel? Ann Clin Biochem 2003;40:585-92. [Crossref] [PubMed]
- Smellie WSA. Demand management and test request rationalization. Ann Clin Biochem 2012;49:323-36. [Crossref] [PubMed]
- Jackson BR. Managing laboratory test use: principles and tools. Clin Lab Med 2007;27:733-48. v. [Crossref] [PubMed]
- Rao GG, Crook M, Tillyer ML. Pathology tests: is the time for demand management ripe at last? J Clin Pathol 2003;56:243-8. [Crossref] [PubMed]
- Janssens PMW. Managing the demand for laboratory testing: Options and opportunities. Clin Chim Acta 2010;411:1596-602. [Crossref] [PubMed]
- Plebani M, Zaninotto M, Faggian D. Utilization management: A European perspective. Clin Chim Acta 2014;427:137-41. [Crossref] [PubMed]
- Hauser RG, Shirts BH. Do we now know what inappropriate laboratory utilization is? An expanded systematic review of laboratory clinical audits. Am J Clin Pathol 2014;141:774-83. [Crossref] [PubMed]
- Lippi G, Bovo C, Ciaccio M. Inappropriateness in laboratory medicine: an elephant in the room? Ann Transl Med 2017;5:82. [Crossref] [PubMed]
- Hampton JR, Harrison MJ, Mitchell JR, et al. Relative contributions of history-taking, physical examination, and laboratory investigation to diagnosis and management of medical outpatients. Br Med J 1975;2:486-9. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Uris J, et al. Variation in laboratory tests ordered for patients treated in hospital emergency departments. Emergencias 2014;26:450-8.
- Salinas M, López-Garrigós M, Flores E, et al. Temporal and regional variability in the request of vitamin D from general practitioners in Spain. Clin Chem Lab Med 2017;55:1754-60. [Crossref] [PubMed]
- Salinas M, Lopez-Garrigos M, Pomares F, et al. An evaluation of hemoglobin A1c test ordering patterns in a primary care setting. Lab Med 2012;43:44-6. [Crossref]
- Salinas M, López-Garrigós M, Flores E, et al. Primary care requests for anaemia chemistry tests in Spain: Potential iron, transferrin and folate over-requesting. J Clin Pathol 2017;70:760-5. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Carratala A, et al. Endocrinol Nutr 2011;58:219-23. [An evaluation of glycosylated hemoglobin requesting patterns in a primary care setting: a pilot experience in the Valencian Community (Spain)]. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Uris J, et al. Differences in laboratory requesting patterns in emergency department in Spain. Ann Clin Biochem 2013;50:353-9. [Crossref] [PubMed]
- Salinas M, López-GARRIGÓS M, Tormo C, et al. Primary care use of laboratory tests in spain: Measurement through appropriateness indicators. Clin Lab 2014;60:483-90. [Crossref] [PubMed]
- Salinas M, Lopez-Garrigos M, Flores E, et al. Evaluating regional variability in the use of the most commonly requested laboratory tests in primary care in Spain: Data from the multi-center national scale REDCONLAB initiative. LaboratoriumsMedizin 2017;41: [Crossref]
- Salinas M, López-Garrigós M, Flores E, et al. Big differences in primary care celiac disease serological markers request in Spain. Biochem Med (Zagreb) 2017;27:231-6. [Crossref] [PubMed]
- National Institute for Health and Clinical Excellence. Type 2 diabetes (CG66). Available online: https://www.nice.org.uk/guidance/CG66
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017;40:S11-24. [Crossref] [PubMed]
- Smellie WSA, Finnigan DI, Wilson D, et al. Methodology for constructing guidance. J Clin Pathol 2005;58:249-53. [Crossref] [PubMed]
- Whiting P, Toerien M, de Salis I, et al. A review identifies and classifies reasons for ordering diagnostic tests. J Clin Epidemiol 2007;60:981-9. [Crossref] [PubMed]
- García Raja A, Caballé Martín I, Giménez Marín A. Uso adecuado del laboratorio clínico: necesidad y tendencias. Rev Lab Clin 2008;1:75-82.
- Reiling J. JAMA 100 years ago: the laboratory in diagnosis. JAMA 2007;297:538. [Crossref]
- Flores E, Leiva M, Leiva-Salinas C, et al. The degree of knowledge shown by physicians in relation to the variability of laboratory test results. Clin Chem Lab Med 2009;47:381-2. [Crossref] [PubMed]
- Jones G, Barker A. Reference intervals. Clin Biochem Rev 2008;29:S93-7. [PubMed]
- van Walraven C, Raymond M. Population-based study of repeat laboratory testing. Clin Chem 2003;49:1997-2005. [Crossref] [PubMed]
- Hindmarsh JT, Lyon AW. Strategies to promote rational clinical chemistry test utilization. Clin Biochem 1996;29:291-9. [Crossref] [PubMed]
- Reynolds RA, Rizzo JA, Gonzalez ML. The cost of medical professional liability. JAMA 1987;257:2776-81. [Crossref] [PubMed]
- The future of clinical chemistry and its role in healthcare: a report of the Athena Society. Clin Chem 1996;42:96-101. [PubMed]
- Salinas M, Lopez-Garrigós M, Flors L, et al. Laboratory false-positive results: A clinician responsibility or a shared responsibility with requesting clinicians? Clin Chem Lab Med 2013;51:e199-200. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Leiva-Salinas C. False positive results: The imaginary invalid syndrome. Aten primaria 2013;45:542. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Uris J. Towards laboratory knowledge, not data, in 70% of clinical decision-making. What "knowledge management" can add to clinical practice? Clin Chem Lab Med 2011;49:1389-90. [Crossref] [PubMed]
- Wu AHB, Lewandrowski K, Gronowski AM, et al. Antiquated tests within the clinical pathology laboratory. Am J Manag Care 2010;16:e220-7. [PubMed]
- Solomon DH, Hashimoto H, Daltroy L, et al. Techniques to improve physicians’ use of diagnostic tests: a new conceptual framework. JAMA 1998;280:2020-7. [Crossref] [PubMed]
- Axt-Adam P, van der Wouden JC, van der Does E. Influencing behavior of physicians ordering laboratory tests: a literature study. Med Care 1993;31:784-94. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Education and communication is the key for the successful management of vitamin D test requesting. Biochem Med (Zagreb) 2015;25:237-41. [Crossref] [PubMed]
- Barth JH, Balen AH, Jennings A. Appropriate design of biochemistry request cards can promote the use of protocols and reduce unnecessary investigations. Ann Clin Biochem 2001;38:714-6. [Crossref] [PubMed]
- Bailey J, Jennings A, Parapia L. Change of pathology request forms can reduce unwanted requests and tests. J Clin Pathol 2005;58:853-5. [Crossref] [PubMed]
- van Wijk MA, van der Lei J, Mosseveld M, et al. Assessment of decision support for blood test ordering in primary care. a randomized trial. Ann Intern Med 2001;134:274-81. [Crossref] [PubMed]
- Salinas La Casta M, Flores Pardo E, Lugo Arocena J, et al. Declining demand for laboratory, following the amendment of the application form. Med Clin (Barc) 2008;131:716. [Crossref] [PubMed]
- Rodriguez-Borja E. Potential of computer physician order entry (CPOE) to improve patient safety related to laboratory test requesting. In: Sonntag O, Plebani M. editors. Laboratory test requesting appropriateness and patient safety. De Gruyter 2016:77-98.
- Broughton PM, Worthington DJ. Laboratories respond differently to the same clinical request. Ann Clin Biochem 1989;26:119-21. [Crossref] [PubMed]
- Smellie WSAAssociation for Clinical Biochemistry’s Clinical Practice Section. Time to harmonise common laboratory test profiles. BMJ 2012;344:e1169 [Crossref] [PubMed]
- Cook S. Experts’ guide to saving money in health. BMJ 2010;340:c1281. [Crossref]
- Venta-Obaya R, Bedini-Chesa JL, Fusté-Ventosa M, et al. Estrategias para la gestión de la demanda analítica en el laboratorio clínico. Consideraciones sobre la implantación de sistemas automatizados, 2013.
- Baron JM, Dighe AS. The role of informatics and decision support in utilization management. Clin Chim Acta 2014;427:196-201. [Crossref] [PubMed]
- Neilson EG, Johnson KB, Rosenbloom ST, et al. The impact of peer management on test-ordering behavior. Ann Intern Med 2004;141:196-204. [Crossref] [PubMed]
- Poley MJ, Edelenbos KI, Mosseveld M, et al. Cost consequences of implementing an electronic decision support system for ordering laboratory tests in primary care: evidence from a controlled prospective study in the Netherlands. Clin Chem 2007;53:213-9. [Crossref] [PubMed]
- Bindels R, Hasman A, van Wersch JW, et al. Evaluation of an automated test ordering and feedback system for general practitioners in daily practice. Int J Med Inform 2004;73:705-12. [Crossref] [PubMed]
- Shahangian S, Snyder SR. Laboratory Medicine Quality Indicators. Am J Clin Pathol 2009;131:418-31. [Crossref] [PubMed]
- Howanitz PJ. Errors in laboratory medicine: practical lessons to improve patient safety. Arch Pathol Lab Med 2005;129:1252-61. [PubMed]
- Plebani M, Lippi G. Hemolysis index: quality indicator or criterion for sample rejection? Clin Chem Lab Med 2009;47:899-902. [Crossref] [PubMed]
- Sciacovelli L, O’Kane M, Skaik YA, et al. Quality Indicators in Laboratory Medicine: from theory to practice. Clin Chem Lab Med 2011;49:835-44. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Gutiérrez M, et al. The financial and learning and growth perspectives of the balanced scorecard in public institutions: Application in the clinical laboratory. Gac Sanit 2012;26:97. [Crossref] [PubMed]
- Plebani M, Sciacovelli L, Aita A, et al. Harmonization of pre-analytical quality indicators. Biochem Med (Zagreb) 2014;24:105-13. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Díaz J, et al. Regional variations in test requiring patterns of general practitioners in Spain. Ups J Med Sci 2011;116:247-51. [Crossref] [PubMed]
- Larsson A. What can we learn from studies on regional differences in the utilization of laboratory tests? Ups J Med Sci 2011;116:225-6. [Crossref] [PubMed]
- Larsson A, Palmer M, Hultén G, et al. Large differences in laboratory utilisation between hospitals in Sweden. Clin Chem Lab Med 2000;38:383-9. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Asencio A, et al. Strategy to improve the request of uric acid in primary care: Preliminary results and evaluation through process and outcome appropriateness indicators. Clin Biochem 2014;47:467-70. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Pomares F, et al. Serum calcium (S-Ca), the forgotten test: Preliminary results of an appropriateness strategy to detect primary hyperparathyroidism (pHPT). Bone 2013;56:73-6. [Crossref] [PubMed]
- Salinas M, Lopez-Garrigos M, Flores E, et al. Automatic laboratory-based strategy to improve the diagnosis of type 2 diabetes in primary care. Biochem Med (Zagreb) 2016;26:121-8. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Asencio A, et al. Laboratory utilization improvement through a computer-aided algorithm developed with general practitioners. Clin Chem Lab Med 2015;53:1391-7. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Uris J, et al. A study of the differences in the request of glycated hemoglobin in primary care in Spain: A global, significant, and potentially dangerous under-request. Clin Biochem 2014;47:1104-7. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Larger differences in utilization of rarely requested tests in primary care in Spain. Biochem Med (Zagreb) 2015;25:410-5. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Request of laboratory liver tests in primary care in Spain: potential savings if appropriateness indicator targets were achieved. Eur J Gastroenterol Hepatol 2015;27:1130-6. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Request of acute phase markers in primary care in Spain. Am J Manag Care 2015;21:e591-6. [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Potential over request in anemia laboratory tests in primary care in Spain. Hematology 2015;20:368-73. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Pomares FJ, et al. Request of thyroid function tests from Primary Care in Spain. Endocrinol Nutr 2016;63:19-26. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Requests of laboratory tests for the diagnosis and management of calcium-phosphate disorders in Spain. Rev Med Chil 2016;144:990-7. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Uncritical request of thyroid laboratory tests may result in a major societal economic burden: Results from a large population study in Spain. Clin Lab 2017;63:1139-45. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Large-Scale Analysis Evaluating Regional Variability in the Request of Laboratory Tests in Primary Care and its Potential Economic Impact. Lab Med 2017;48:271-6. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Urinary albumin: a risk marker under-requested in primary care in Spain. Ann Clin Biochem 2018;55:281-6. [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Glycated hemoglobin: A powerful tool not used enough in primary care. J Clin Lab Anal 2018;32: [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Benchmarking after large-scale, comparative data analysis improves the use of laboratory tests: Lessons from the REDCONLAB initiative. Arch Pathol Lab Med 2017;141:485-6. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Flores E, et al. Procalcitonin in the Emergency Department: A potential expensive over-request that can be modulated through institutional protocols. Am J Emerg Med 2018;36:158-60. [Crossref] [PubMed]
- Gion M, Franceschini R, Rosin C, et al. An epidemiology-based model to estimate the rate of inappropriateness of tumor marker requests. Clin Chem Lab Med 2014;52:889-97. [Crossref] [PubMed]
- Pérez-Hernández B, García-Esquinas E, Graciani A, et al. Social Inequalities in Cardiovascular Risk Factors Among Older Adults in Spain: The Seniors-ENRICA Study. Rev Esp Cardiol (Engl Ed) 2017;70:145-54. [Crossref] [PubMed]
- Salinas M, López-Garrigós M, Rodriguez-Borja E, et al. Laboratory Test Requesting Appropriateness and Patient Safety. Walter de Gruyter GmbH, 2016.
- Maria S. Knowledge is not enough the Prominence of the Laboratory in Clinical Decision Making Through Creative Imagination, Communication and Leadership. J Hematol Thromboembolic Dis 2013;1:e102
- Salinas M, Flores E, López-Garrigós M, et al. Application of a continual improvement approach to selecting diagnostic markers for acute pancreatitis in an emergency department. Emergencias 2017;29:113-6. [PubMed]
Cite this article as: Salinas M, Flores E, López-Garrigós M, Leiva-Salinas C. Laboratory test inappropriateness: lessons revisited and clarified in seven questions. J Lab Precis Med 2018;3:34.