
Precision medicine and cardiac troponin biomarkers: new practice recommendations advance diagnostic precision
A review of the historic use of cardiac biomarkers for evaluation of coronary syndromes creates a twisting network of timelines and advances that illustrate the scientific method of precision medicine. Publication of clinical practice guidelines have established milestones along those timelines and the recent consensus recommendations for use of cardiac troponins (cTn) (1) exists in the context of previous accomplishments and the challenges ahead.
The first milestone was the laboratory medicine practice guideline on cardiac markers by the National Academy of Clinical Biochemistry (NACB) published in 1999 which outlined the use of creatine kinase MB isoenzyme and the emerging biomarkers cTn T and cTn I (2). While the concept of precision medicine was not articulated in 1999, the first guideline defined the identification of patient sub-populations relative to onset of chest pain, ECG changes and serial changes of cardiac biomarkers characteristic of acute myocardial infarction. The second milestone is comprised of the NACB practice guidelines and the Third Universal Definition of Myocardial Infarction that both appeared between 2007 and 2012 and these refined the analytic and clinical applications of cardiac biomarkers with coronary syndromes. Historically, the second milestone emerged as the general concepts of precision medicine were established (3). Precision medicine was described by the National Research Council Committee in 2011 as:
“the tailoring of medical treatment to the individual characteristics of each patient… the ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease, in the biology and/or prognosis of those diseases they may develop, or in their response to a specific treatment.” (3).
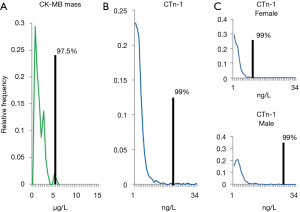
The third and current milestone from the NACB will consist of expert-opinions [published in January 2018 (1)] and evidence-based recommendations (under preparation). This milestone provides constructive suggestions to enable consistent cTn analyses for patients including suggested quality control practice, objective definitions for describing assay performance (e.g., limit of detection, limit of quantitation and limit of blank), recommended reporting units (ng/L without decimal places for patient results) as well as definitions to distinguish contemporary and high sensitivity cTn assays. These recommendations are also intended for clinical trials to improve comparability of studies involving measurement of cTn. The third NACB set of recommendations advances diagnostic precision by advocating high sensitivity cTn assays be reported with sex-specific 99th percentile upper reference limits, rather than a common 99th percentile associated with contemporary cTn assays. Although the clinical utility of sex-specific ranges has yet to be definitively established, this consensus statement encourages their implementation. Laboratories and clinicians have been slow to adopt such ranges for high sensitivity cTn and the NACB has recommended better communications between these groups to promote understanding of pre-analytic and analytic concerns regarding cTn assays. Stat testing in hospital central laboratories commonly aims for a 1-hour turn-around time interval once the specimens have arrived in the laboratory. The current practise recommendations acknowledge this turn-around time goal but encourage efforts to improve service delivery. Although point of care methods provides an appealing alternative testing platform, their implementation has been limited for numerous reasons including clinical specificity and sensitivity as well as standardization and harmonization issues.
The scientific method of precision medicine is exemplified in the series of NACB recommendations or milestones for use of cTn in acute coronary syndromes as each guideline has advanced the tailoring of medical treatments for patients in specific sub-populations (Figure 1). The most recent set of recommendations continues that tradition. It should also be noted that improved measurements of cTn have contributed to resolving the pathobiology of cardiac diseases and establishing new relevant sub-populations that will be the subject of future advances and timelines that continue to be revealed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Executive Editor Dr. Zhi-De Hu (Department of Laboratory Medicine, the Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.04.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu AHB, Christenson RH, Greene DN, et al. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- Wu AH, Apple FS, Gibler WB, et al. National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 1999;45:1104-21. [PubMed]
- National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington (DC): National Academies Press (US), 2011.
- Krintus M, Kozinski M, Boudry P, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657-65. [PubMed]
- Haastrup B, Gill S, Kristensen SR, et al. Biochemical markers of ischaemia for the early identification of acute myocardial infarction without St segment elevation. Cardiology 2000;94:254-61. [Crossref] [PubMed]
Cite this article as: Lyon AW, Lyon ME. Precision medicine and cardiac troponin biomarkers: new practice recommendations advance diagnostic precision. J Lab Precis Med 2018;3:44.


