
Validation of the analytical performance of Lumipulse G BRAHMS procalcitonin immunoassay on low sample volume
Introduction
Procalcitonin (PCT) is a 116-aminoacid precursor of the mature hormone calcitonin. Albeit PCT is mainly produced by the thyroid C-cells in normal conditions, an extrathyroidal synthesis of PCT is commonplace in patients with severe bacterial infections and bacterial sepsis (1), thus contributing to enhance its blood concentration from <0.1 ng/mL up to 100 ng/mL (2,3). This considerably increase of PCT blood levels is currently considered a cornerstone in the diagnostic approach of patients with severe bacterial infections (especially sepsis), and even for monitoring antimicrobial therapy (3,4).
The clinical significance of measuring PCT in childhood has also been clearly proven by a series of recent meta-analyses (5-7), which were all concordant to conclude that this biomarker may have a valuable role in the diagnostic approach of newborns and children with suspected bacterial sepsis (area under the curve, 0.77–0.87; sensitivity, 0.72–0.85; specificity, 0.54–0.79). Even more importantly, another recent meta-analysis published by Schuetz et al. concluded that PCT-guided antibiotic therapy was effective to significantly reduce antibiotic administration by approximately 50% in patients with acute respiratory infections (8), an important finding which could then be replicated in children populations by Baer et al. (9) and Dai et al. (10).
Insufficient sample volume is one of the major hurdles for performing laboratory analyses (thus including PCT) in the neonatal and childhood ages (11,12). Albeit the modern laboratory instrumentation uses very little amounts of blood, plasma or serum, the feasibility to perform all the tests requested, and which may hence be reasonably necessary to the clinical decision making, is directly dependent on the final volume of blood conveyed to the laboratory (i.e., the larger the volume, the greater the number of tests which could be performed) (13,14). The World Health Organization (WHO) clearly advises that blood sampling for routine or urgent laboratory testing in the childhood and, especially, in the neonatal age may lead to blood volume or hemoglobin depletion (15). Therefore, “minimal risk” limits have been endorsed to safeguard child safety, entailing collection of <5% of total blood volume within 1 day and <10% of total blood volume within 8 weeks, respectively. These limits obviously pose serious challenges and threats to clinical laboratories, since samples with very low volumes of blood may then be unsuitable to perform many of the various tests needed for reaching a final diagnosis of bacterial infection or for monitoring therapeutic effectiveness in patients undergoing antimicrobial treatment. Moreover, an inoculation of low blood volume is a well-known cause of false negative results of blood cultures, which may then lead to underdiagnosing sepsis in neonates or in very young children (16). The problem of insufficient samples extends far beyond the childhood age, since the collection of low volume of blood is quite frequent in patients with difficult veins (17), in critical patients who may need frequent laboratory monitoring and may hence be at risk of iatrogenic anemia (18), as well as for reducing spurious hemolysis when samples are collected from indwelling lines (19). Although performing laboratory analyses on diluted serum or plasma samples is a potential solution to overcome this problem, the functional sensitivity of many techniques used for measuring PCT would be probably unsuitable to generate reliable data.
The new Lumipulse G BRAHMS PCT immunoassay (Fujirebio Diagnostics Inc., Tokyo, Japan) has been recently commercialized. Unlike some other commercial methods, the analytical characteristics of this new technique allow measuring PCT values with a 2- to 20-fold better functional sensitivity (20,21), thus offering an appealing opportunity to analyze PCT in low volume samples by measuring diluted aliquots. Therefore, this study was aimed to investigate the analytical recovery of Lumipulse G BRAHMS PCT immunoassay (Fujirebio Diagnostics Inc., Tokyo, Japan) using diluted lithium-heparin plasma samples.
Methods
A total number of 15 fresh lithium-heparin plasma samples collected in evacuated blood collection tubes (Vacutest Kima, Piove di Sacco, Padova, Italy) and referred over the same working day to the local laboratory (University Hospital of Verona) for routine PCT testing were included in this study. The samples were arbitrarily selected to cover clinically significant measuring ranges of PCT values, i.e., close to the 0.05 ng/mL diagnostic threshold for bacterial infection (“low” PCT values, 0.034–0.085 ng/mL), close to the 0.5 ng/mL diagnostic threshold for severe bacterial infection (“medium” PCT values, 0.092–0.638 ng/mL), and close to the 5–10 ng/mL diagnostic threshold for severe sepsis and septic shock (“high” PCT values, 2.022–9.187 ng/mL) (1). All lithium-heparin plasma samples were diluted with Lumipulse sample diluent, to obtain 5 progressive dilutions (i.e., 1:2; 1:5; 1:10; 1:20 and 1:32). The original samples and each serial dilution were then tested on Lumipulse G BRAHMS PCT immunoassay. The method is a two-step sandwich chemiluminescence enzyme immunoassay (CLEIA) adapted for use on the LUMIPULSE G1200 system (Fujirebio Diagnostics Inc., Tokyo, Japan). The analytical performance of this high-sensitivity PCT immunoassay have been previously described elsewhere (21). Briefly, the limit of blank (LoB), limit of detection (LoD) and functional sensitivity were found to be 0.001, 0.002 and 0.008 ng/mL, respectively, the total analytical imprecision was 2.1%, and the method was found to be linear within a range of PCT concentrations comprised between 0.006 and 75.5 ng/mL. An additional measurement of the original undiluted 15 plasma samples was performed by using the automatic 1:10 dilution set in the analyzer.
All measurements were consecutively performed using the same LUMIPULSE G1200 system analyzer, an identical lot of reagents and within a single analytical session. The PCT results obtained on the dilutions were compared to their theoretical values. The analytical performance on diluted samples was then assessed with linear regression analysis, Pearson’s correlation and percent (%) recovery. The mean absolute bias [and 95% confidence interval (CI)] was calculated with Bland and Altman plot analysis. The statistical analysis was performed with Analyse-it (Analyse-it Software Ltd, Leeds, UK). This study was completely based on pre-existing plasma samples referred for routine PCT testing, so that no patients’ informed consent was needed. The study was carried out in accordance with the Declaration of Helsinki and was approved by the local Ethical Committee (University Hospital of Verona; protocol number: 971CESC, 25 July 2016).
Results
The results of this study are reported in Figure 1, which shows the mean theoretical PCT values of each set of plasma samples (i.e., displaying “low”, “mean” and “high” values) plotted against the mean measured PCT values of the same plasma samples. Overall, the correlation was always excellent (i.e., comprised between 0.999 and 1.000), whilst slopes and intercepts were also satisfactory (Table 1). The mean differences (i.e., recovery) between the theoretical and measured PCT values were comprised between ±14%, ±7% and ±1% for low, mean and high PCT values, respectively (Table 2). The mean recovery of the 1:10 diluted specimens was comprised within ±20% (mean absolute bias, −0.012 ng/mL; 95% CI, −0.022 to −0.001 ng/mL) with manual dilution and within ±21% (mean absolute bias, −0.012 ng/mL; 95% CI, −0.016 to −0.007 ng/mL) with automatic dilution for the low PCT set of plasma samples, within ±5% (mean absolute bias, 0.056 ng/mL; 95% CI, −0.102 to 0.213 ng/mL) with manual dilution and within ±7% (mean absolute bias, −0.023 ng/mL; 95% CI, −0.043 to −0.003 ng/mL) with automatic dilution for the medium PCT set of plasma samples, within ±2% (mean absolute bias, −0.138 ng/mL; 95% CI, −0.743 to 0.467 ng/mL) with manual dilution and within ±1% (mean absolute bias, −0.023 ng/mL; 95% CI, −0.387 to 0.341 ng/mL) with automatic dilution for the high PCT set of plasma samples, respectively.
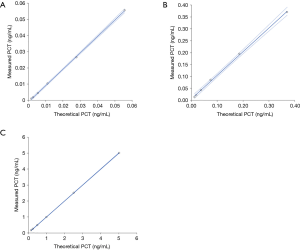
Table 1
| Plasma samples | n | Slope (and 95% CI) | Intercept (and 95% CI) | Correlation |
|---|---|---|---|---|
| “Low” PCT samples | 5 | 1.014 (0.993 to 1.036) | −0.001 (−0.002 to −0.000) | r=1.000 (P<0.001) |
| “Medium” PCT samples | 5 | 0.993 (0.949 to 1.037) | 0.006 (−0.001 to 0.014) | r=0.999 (P<0.001) |
| “High” PCT samples | 5 | 1.001 (0.994 to 1.007) | −0.002 (−0.018 to 0.013) | r=1.000 (P<0.001) |
Table 2
| Dilutions | Sample low | Sample medium | Sample high | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Measured | Mean difference | Theoretical | Measured | Mean difference | Theoretical | Measured | Mean difference | |||
| 1:2 | 0.028 | 0.027 | 0.001 | 0.185 | 0.195 | −0.010 | 2.508 | 2.515 | −0.006 | ||
| 1:5 | 0.011 | 0.010 | 0.001 | 0.074 | 0.085 | −0.011 | 1.003 | 0.992 | 0.012 | ||
| 1:10 | 0.006 | 0.004 | 0.001 | 0.037 | 0.043 | −0.006 | 0.502 | 0.488 | 0.014 | ||
| 1:20 | 0.003 | 0.002 | 0.001 | 0.019 | 0.021 | −0.003 | 0.251 | 0.255 | −0.004 | ||
| 1:32 | 0.002 | 0.001 | 0.001 | 0.012 | 0.014 | −0.002 | 0.157 | 0.164 | −0.007 | ||
Discussion
The clinical significance of measuring PCT for diagnosing both localized and systemic infections, as well as for guiding antimicrobial therapy, is now increasingly recognized (4). The mounting focus on this biomarker has been paralleled by commercialization of a considerable number of semi- or fully-automated immunoassays, which have made its measurement available in the vast majority of clinical laboratories worldwide. Some challenges remain, however. Unlike clinical chemistry tests, for which 2–10 µL may be sufficient for measuring a large number of analytes, the sample volume needed for measuring PCT in serum or plasma ranges between 20 to 200 µL, thus making its assessment rather challenging in low volume samples. Therefore, the availability of analyzers characterized by limited sample aspiration and methods with extraordinarily favorable functional sensitivity would enable the use of dilutions rather than the original samples, thus allowing to measure PCT and contextually saving precious biological materials for additional testing.
The results of our study show that the use of plasma sample dilutions other than the original undiluted plasma specimens is a viable option with Lumipulse G BRAHMS PCT immunoassay for saving serum or plasma volume and contextually producing robust and reliable data. Overall, dilution of samples up to 1:32 was associated with recoveries comprised between ±1% and ±14%, thus fulfilling the conventional 20% imprecision acceptance limit set for most PCT immunoassays (22). In particular, the recovery of the target dilution automatically available in LUMIPULSE G1200 system (i.e., 1:10) yielded data highly comparable to those obtained with external manual dilution of plasma samples (i.e., 1–21% vs. 2–20%). Notably, the sample volume needed for measuring PCT with Lumipulse G BRAHMS PCT immunoassay is 60 µL, so that a 1:10 dilution entails using only 6 µL of plasma or serum. The slightly worse recovery observed for plasma samples with very low PCT values (i.e., comprised between 0.034–0.085 ng/mL) at the 1:10 dilution is not clinically meaningful, since in both cases, entailing manual and automatic dilution, the bias was 0.012 ng/mL, a functional PCT value that is neither measurable with the vast majority of other currently available commercial PCT automated immunoassays (20).
Conclusions
The results of our study suggest that measuring PCT with Lumipulse G BRAHMS PCT immunoassay using diluted (e.g., 1:10) plasma is a viable option to obtain accurate test results and for consuming a much lower volume of plasma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.07.10). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Martina Montagnana serves as the unpaid Associate Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The other authors have no conflicts of interest to declare. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was carried out in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the local Ethical Committee (University Hospital of Verona; protocol number: 971CESC, 25 July 2016). Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meisner M. Procalcitonin - Biochemistry and Clinical Diagnosis. 1st edition. Bremen: UNI-MED, 2010.
- Di Somma S, Magrini L, Travaglino F, et al. Opinion paper on innovative approach of biomarkers for infectious diseases and sepsis management in the emergency department. Clin Chem Lab Med 2013;51:1167-75. [Crossref] [PubMed]
- Neeser O, Mueller B, Schuetz P. Microbiology or host-response markers, or both, for optimal patient management? J Lab Precis Med 2017;2:56. [Crossref]
- Lippi G, Montagnana M, Balboni F, et al. Academy of Emergency Medicine and Care-Society of Clinical Biochemistry and Clinical Molecular Biology consensus recommendations for clinical use of sepsis biomarkers in the emergency department. Emerg Care J 2017;13:6877.
- Yu Z, Liu J, Sun Q, et al. The accuracy of the procalcitonin test for the diagnosis of neonatal sepsis: a meta-analysis. Scand J Infect Dis 2010;42:723-33. [Crossref] [PubMed]
- Vouloumanou EK, Plessa E, Karageorgopoulos DE, et al. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med 2011;37:747-62. [Crossref] [PubMed]
- Pontrelli G, De Crescenzo F, Buzzetti R, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: a meta-analysis. BMC Infect Dis 2017;17:302. [Crossref] [PubMed]
- Schuetz P, Müller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health 2013;8:1297-371. [Crossref] [PubMed]
- Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One 2013;8:e68419 [Crossref] [PubMed]
- Dai BQ, Yuan XT, Liu JM. Value of serum procalcitonin for the guidance of antibiotic therapy in children with lower respiratory tract infection. Zhongguo Dang Dai Er Ke Za Zhi 2015;17:1292-6. [PubMed]
- Schnabl K, Chan MK, Gong Y, et al. Closing the gaps in paediatric reference intervals: the CALIPER initiative. Clin Biochem Rev 2008;29:89-96. [PubMed]
- Dikmen ZG, Pinar A, Akbiyik F. Specimen rejection in laboratory medicine: Necessary for patient safety? Biochem Med (Zagreb) 2015;25:377-85. [Crossref] [PubMed]
- McPherson RA. Blood sample volumes: emerging trends in clinical practice and laboratory medicine. Clin Leadersh Manag Rev 2001;15:3-10. [PubMed]
- Dale JC, Ruby SG. Specimen collection volumes for laboratory tests. Arch Pathol Lab Med 2003;127:162-8. [PubMed]
- Howie SR. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ 2011;89:46-53. [Crossref] [PubMed]
- Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr 2015;61:1-13. [Crossref] [PubMed]
- Lima-Oliveira G, Volanski W, Lippi G, et al. Pre-analytical phase management: a review of the procedures from patient preparation to laboratory analysis. Scand J Clin Lab Invest 2017;77:153-63. [Crossref] [PubMed]
- Woodhouse S. Complications of critical care: lab testing and iatrogenic anemia. MLO Med Lab Obs 2001;33:28-31. [PubMed]
- Giavarina D. Low volume tubes can be effective to reduce the rate of hemolyzed specimens from the emergency department. Clin Biochem 2014;47:688-9. [Crossref] [PubMed]
- Dipalo M, Guido L, Micca G, et al. Multicenter comparison of automated procalcitonin immunoassays. Pract Lab Med 2015;2:22-8. [Crossref] [PubMed]
- Ruzzenente O, Salvagno GL, Gelati M, et al. Analytical evaluation of the novel Lumipulse G BRAHMS procalcitonin immunoassay. Pract Lab Med 2016;6:8-13. [Crossref] [PubMed]
- Carcamo Yañez VA, Göpfert JC, Otto M, et al. Development and Validation of an Ultrasensitive Procalcitonin Sandwich Immunoassay. High Throughput 2017;6:18. [Crossref] [PubMed]
Cite this article as: Salvagno GL, Ruzzenente O, Gelati M, Danese E, Montagnana M, Lippi G. Validation of the analytical performance of Lumipulse G BRAHMS procalcitonin immunoassay on low sample volume. J Lab Precis Med 2018;3:66.


