Recent developments and innovations in red blood cells diagnostics
Introduction
Anemia, which is currently defined by the World Health Organization (WHO) as a hemoglobin value in whole blood <120 g/L in women and <130 g/L in men, respectively (1), can now be regarded as a worldwide endemic disease, with an estimated prevalence of 1.62 billion people, which approximates 25% of the worldwide population (2). Albeit iron deficiency is the leading cause of anemia (i.e., approximating 50% of cases), this condition is rarely present alone, but may coexist with a kaleidoscope of other causes such as nutritional deficiencies (i.e., folate or vitamin B12 deficiencies), acute or chronic bleeding, hereditary red blood cell (RBC) disorders (i.e., hemoglobinopathies, spherocytosis), infections, renal or liver impairment, cancer and chronic inflammatory conditions (Table 1) (1).
Table 1
| Condition | RDW | MCV |
|---|---|---|
| Nutritional deficiencies | ||
| Iron deficiency | ↑ | ↓ |
| Folic acid deficiency | ↑ | ↑ |
| Vitamin B deficiency | ↑ | ↑ |
| β-thalassemia | ↑ | ↓ |
| Hemolytic anemias | ||
| Immune hemolytic anemia | ↑ | ↑ |
| Hereditary spherocytosis | N/↑ | N/↓ |
| Anemic hemoglobinopathies (i.e., SS, SC) | ↑ | N |
| Sickle cell trait | N | ↓ |
| Chronic disorders | ||
| Chronic diseases anemia | N | ↓ |
| Chronic liver disease | ↑ | N/↑ |
| Hematologic disorders | ||
| Aplastic anemia | N | ↑ |
| Chronic leukemias | N | N |
| Myelodysplastic syndrome | ↑ | ↑ |
| Other thalassemias | N | ↓ |
| Acute hemorrhages | N | N |
↑, increased; N, normal; ↓, decreased. RDW, red blood cell distribution width; MCV, mean corpuscular volume.
Since the clinical signs and symptoms of anemia are often poorly specific, and may also be subtle, especially in patients with chronic forms of anemia, laboratory hematology represents a virtually unavoidable part of both the diagnostic reasoning and clinical decision making, since laboratory tests provide irreplaceable information for screening, diagnosis and monitoring of RBC disorders (3,4). Beside the conventional measurement of whole blood hemoglobin content, which is a necessary precondition for diagnosing anemia, the preliminary classification of anemias is usually based on the values of mean corpuscular volume (MCV) and RBC distribution width (RDW) (5), as summarized in Table 1. This approach is still forthright and valid, but does not enable a definitive etiological characterization, and should hence be complemented with a panel of additional laboratory investigations. Importantly, a number of technological advances occurred over the past few decades have enormously contributed to broadening the diagnostic armamentarium and making more efficient and sustainable the diagnostics of RBC disorders. Some on these important innovations will be summarized in the following parts of this article.
Automation of laboratory hematology
Laboratory automation should be regarded as one of the major advancements occurred in RBC diagnostics. Laboratory automation is conventionally defined as a multi-disciplinary integration of robotics, information technology (IT), sample handling and many other technologies aimed at developing and optimizing both workflow and activities within medical laboratories (6). Briefly, laboratory automation may bring paramount benefits to in vitro diagnostic testing, including easier and more efficient management of workflows, better withstanding the increasing complexity and volume of routine and urgent testing, improved turnaround time (TAT), dismissal of many manual activities, enhanced walk-away, cost savings (i.e., especially those attributable to subsidiary staff and technicians), improved standardization of procedures, lower chance of errors throughout the total testing process, decreased biological risk, along with opportunities to implement automatic reruns or reflex testing (7).
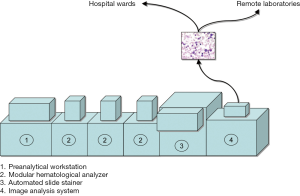
Laboratory automation has been for long limited to clinical chemistry and immunochemistry platforms. The major hurdle encountered in developing models of automation for laboratory hematology is represented by the peculiar sample type, which is whole blood anticoagulated with dipotassium ethylenediaminetetraacetic acid (EDTA) (8). The presence of EDTA in the sample, which irreversibly sequestrates ionized calcium and many other metal ions (9), makes EDTA plasma an unsuitable sample matrix for clinical chemistry and even for coagulation testing, thus generating considerable obstacles for consolidation of laboratory hematology with other branches of laboratory medicine. Nevertheless, a number of technological solutions have been developed for laboratory hematology in recent times. These basically include (I) commercialization of modular, high-throughput and versatile analyzers, which can be easily interconnected by means of sample conveyers, and can fit the organization of small, medium and large facilities, (II) integration of preanalytical workstations, which can be identical to those included in models of total laboratory automation, or can be specifically designed to suit hematological testing, (III) connection with automated slide strainers, which help improving the entire slide making process (i.e., less manual activities and lower biological risk, improved standardization of slide preparation and staining, customization of staining protocols, reduction of TAT) (10), as well as (IV) integration of automated image analysis systems (see the following section of this article). This organization is often referred to as “modular laboratory hematology” (Figure 1).
Digital hematology
Throughout the relatively long history of laboratory hematology, the only reliable means for identifying, enumerating and sizing blood cells has been for long represented by optical microscopy of peripheral blood smear. This practice carries many drawbacks, since it is inevitably time-consuming, is vulnerable to high inter- and intra-observer inaccuracy and imprecision, needs specific education and training of microscopists, and is poorly suited for rapid diagnostics as otherwise needed in patients with many acute hematological disorders (11).
Recent technological advances have led to development and commercialization of innovative automated image analysis systems, which are suited for automation and can hence be directly connected (in series) with hematologic analyzers (Figure 1) (12). These innovative platforms scan the slides (usually at a picture of ×100 objective), and store digitalized images of blood smears at high magnification. The images are analyzed by artificial neural networks based on a preexisting database of blood elements (thus including RBC), which can be locally customized or updated by the users. The images can be transmitted to, and displayed on, computer screens, which can be even placed at long distances from the scanner (i.e., in hospital wards or in remote laboratories) (Figure 1), for analysis and potential reclassification of blood elements. The operator can also increase the size of the images, or expand single sections of the scan, so obtaining a more accurate view. The operator can then accept and conserve the automatic classification, or can move elements from one cell category to another, thus improving the final reclassification. Albeit these automated image analysis systems have been originally developed for analysis of white blood cells (WBC), specific information can also be garnered on erythrocyte morphology, thus including the presence of anysocytosis, hypochromia, microcytosis or macrocytosis, spherocytosis, elliptocytosis, ovalocytosis, stomatocytosis, acanthocytosis, echinocytosis, polychromasia, poikilocytosis and abnormal erythrocytes (i.e., sickle cells and schizocytes, helmet and teardrop cells) (13). Recent data showed that the diagnostic sensitivity of these systems for identifying some critical categories of abnormal erythrocytes (i.e., spherocytes or sickle, target and tear drop cells) is excellent, typically higher than 80% (14), thus making the use of digital image analysis a highly valuable, and probably more accurate and reproducible, alternative to optical microscopy.
Notably, the use of these systems may also enable an efficient recognition of parasitoid infections such as Malaria (15), as well as the reliable identification of intravascular and spurious hemolysis, which would be otherwise undetectable on whole blood specimens (16,17). Interestingly, most of these automated image analysis systems are also capable of optimizing the identification of rare RBC abnormalities, since morphological erythrocyte alterations can be more efficiently visualized on the computer screen (18). Finally, the creation of a large personalized database of images of suggestive RBC abnormalities represents a valuable resource for education and training of students and laboratory professionals (19).
Innovative erythrocyte parameters
Irrespective of the fact that the diagnosis of anemia is relatively simple and straightforward (by measuring total hemoglobin in whole blood), the newer generation of hematologic analyzers is now equipped with many analytical and technical innovations, which enable obtaining other information than that reported with the traditional complete blood cell count (CBC), and which may ultimately provide a substantial improvement for the differential diagnosis of anemias (20). Although a more detailed discussion will be omitted for space constraints, some important aspects deserve a special mention. These innovative parameters most typically include automated reticulocyte and nucleated RBC counts, hemoglobinization of reticulocytes and RBC, reticulocyte hemoglobin content (occasionally defined as CHr and RET-He according to the technology used for its assessment), reticulocyte maturation, automatic analysis and calculation of microcytic and hypochromic RBC (21,22). The various combination of these different parameters not only may be useful to complement clinical history, physical examination and results of more conventional laboratory investigations (i.e., CBC, ferritin, transferrin, iron, haptoglobin, folic acid and vitamin B12, among others) for troubleshooting the underlying cause(s) of anemia (23), but may also be clinically useful for diagnosing, prognosticating and monitoring other non-RBC disorders, as recently shown for patients with sepsis (24), chronic kidney disease (25) and cancer (26). The generation of complex scattergrams (27,28), which is now almost commonplace in the vast majority of hematologic analyzers, is also helpful for more accurately identifying abnormal RBC populations and other atypical elements, as recently shown for diagnosing malaria (29).
Disruptive technologies
Regardless of consolidated laboratory techniques, which have just recently made their way through phenotypic diagnostics of anemia (i.e., capillary electrophoresis) (30), a major innovation has been represented by the application of mass spectrometry and molecular biology in the diagnostics of hemoglobinopathies. The former approach allows a better characterization of hemoglobin variants preliminarily identified by screening techniques such as high-pressure liquid chromatography (HPLC) or capillary electrophoresis (31), whilst molecular diagnostic techniques enable to unravel specific molecular abnormalities characterizing many congenital RBC disorders (32).
Unlike screening tests, the selection of the most appropriate molecular diagnostic approach in patients with inherited hemoglobinopathies or RBC enzymopathies should take into account the prevalence and penetrance of the different mutations in the ethnic populations, across different geographical locations. Therefore, the first step may be represented by polymerase chain reaction (PCR)-based techniques [e.g., restriction-endonuclease PCR (RE-PCR), amplification refractory mutation system (ARMS), resolution melting analysis (HRMA), denaturing gradient gel electrophoresis (DGGE)]. Allele-specific methodologies, such as allele-specific PCR and reverse dot-blot, are especially useful for thalassemia diagnostics in target populations, enable processing a high volume of samples and are relatively inexpensive, permitting to screen some prevalent hemoglobin genes mutations at the same time. Array comparative genomic hybridization (array CGH) can then be used for detecting additional mutations which cannot be identified with first-line DNA analysis. Regarding thalassemias, gap-PCR (gap-PCR) and multiplex ligation-dependent probe amplification (MLPA) are perhaps the best options for screening and also for detecting large deletions or duplications of globin genes, which cannot be identified with conventional DNA sequencing. Sanger or next-generation sequencing (NGS) techniques may then be particularly suited for detecting all known point-mutations, but may also enable indentifying novel or rare mutations, thus helping to uncover new mechanisms of disease. A reliable guidance for the cost-effective integration of these different molecular techniques has recently been published by the European Molecular Genetics Quality Network (EMQN) (33). Notably, emerging evidence also suggests that molecular genetic testing has a pivotal role in patients with diseases characterized by clonal hematopoiesis, thus supporting the diagnostic workout of hematologic malignancies and/or myelodysplastic syndromes (34).
Conclusions
Albeit the laboratory diagnostics of anemia remains a rather simple enterprise, accurate disease characterization has emerged as a mainstay in the era of precision (laboratory) medicine, even for RBC disorders (35). The many technological advances occurred in laboratory medicine over recent times have enabled the introduction of a vast array of innovations (Table 2), which have led the way to a more efficient patient care and a more convenient organization of resources and workflows within the laboratory. In the foreseeable future, the better understanding of phenotypic heterogeneity of RBC disorders, also supported by IT tools such as expert diagnostic systems (36) or artificial neural networks (37), will predictably enable to improve the global management of these disorders at multiple levels. Yet, some additional issues will need to be addressed, on top of it all the current lack of harmonization in laboratory hematology (11).
Table 2
| Automation |
| Modular, high-throughput analyzers |
| Integration of preanalytical workstations |
| Connection with automated slide strainers |
| Integration of automated image analysis systems |
| Digital image analysis |
| High diagnostic sensitivity |
| Improved identification of rare erythrocyte abnormalities |
| Enhanced recognition of parasitoid infections |
| Reliable identification of intravascular and spurious hemolysis |
| Possible use of the digitalized database for education and training |
| Innovative erythrocyte parameters |
| Automated reticulocyte count |
| Nucleated red blood cells count |
| Hemoglobinization of erythrocytes and reticulocytes |
| Reticulocyte hemoglobin content |
| Reticulocyte maturation |
| Microcytic and hypochromic erythrocytes |
| Low hemoglobin density |
| Advanced scattergrams |
| Disruptive technologies |
| Capillary electrophoresis |
| Mass spectrometry |
| Molecular diagnostics |
| RE-PCR |
| ARMS |
| HRMA |
| DGGE |
| Allele-specific PCR and reverse dot-blot |
| Array CGH |
| Gap-PCR |
| MLPA |
| Sanger or NGS |
| IT |
| Expert diagnostic systems |
| Artificial neural networks |
RBC, red blood cell; RE-PCR, restriction-endonuclease polymerase chain reaction; ARMS, amplification refractory mutation system; HRMA, resolution melting analysis; DGGE, denaturing gradient gel electrophoresis; CGH, comparative genomic hybridization; MLPA, multiplex ligation-dependent probe amplification; NGS, next-generation sequencing; IT, information technology.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Laboratory Medicine-25 Years on”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.07.09). The series “Laboratory Medicine-25 Years on” was commissioned by the editorial office without any funding or sponsorship. Giuseppe Lippi served as an unpaid Guest Editor of the series and serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Mario Plebani served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. World Health Organization, Geneva, Switzerland, 2011.
- de Benoist B, McLean E, Egli I, et al. Worldwide prevalence of anaemia 1993-2005. In: WHO Global Database on Anaemia. World Health Organization, Geneva, Switzerland, 2008.
- Coyer SM. Anemia: diagnosis and management. J Pediatr Health Care 2005;19:380-5. [Crossref] [PubMed]
- Guidi GC, Lechi Santonastaso C. Advancements in anemias related to chronic conditions. Clin Chem Lab Med 2010;48:1217-26. [Crossref] [PubMed]
- Bessman JD, Gilmer PR Jr, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol 1983;80:322-6. [Crossref] [PubMed]
- Olsen K. The first 110 years of laboratory automation: technologies, applications, and the creative scientist. J Lab Autom 2012;17:469-80. [Crossref] [PubMed]
- Genzen JR, Burnham CD, Felder RA, et al. Challenges and Opportunities in Implementing Total Laboratory Automation. Clin Chem 2018;64:259-64. [Crossref] [PubMed]
- International Council for Standardization in Haematology. Recommendations of the International Council for Standardization in Haematology for Ethylenediaminetetraacetic Acid Anticoagulation of Blood for Blood Cell Counting and Sizing. International Council for Standardization in Haematology: Expert Panel on Cytometry. Am J Clin Pathol 1993;100:371-2. [Crossref] [PubMed]
- Banfi G, Salvagno GL, Lippi G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med 2007;45:565-76. [Crossref] [PubMed]
- de Bitencourt ED, Voegeli CF, Onzi Gdos S, et al. Validation of the Sysmex sp-1000i automated slide preparer-stainer in a clinical laboratory. Rev Bras Hematol Hemoter 2013;35:404-8. [Crossref] [PubMed]
- Buoro S, Lippi G. Harmonization of laboratory hematology: a long and winding journey. Clin Chem Lab Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Da Costa L. Digital image analysis of blood cells. Clin Lab Med 2015;35:105-22. [Crossref] [PubMed]
- Nakashima MO, Doyle TJ, Phelan-Lewin K, et al. Assessment of semi-quantitative grading of red blood cell abnormalities utilizing images from the CellaVision DM96 compared to manual light microscopy. Int J Lab Hematol 2017;39:e110-2. [Crossref] [PubMed]
- Criel M, Godefroid M, Deckers B, et al. Evaluation of the Red Blood Cell Advanced Software Application on the CellaVision DM96. Int J Lab Hematol 2016;38:366-74. [Crossref] [PubMed]
- Florin L, Maelegheer K, Muyldermans A, et al. Evaluation of the CellaVision DM96 advanced RBC application for screening and follow-up of malaria infection. Diagn Microbiol Infect Dis 2018;90:253-56. [Crossref] [PubMed]
- Lippi G, Pavesi F, Benegiamo A, et al. What do hemolyzed whole-blood specimens look like? Analysis with a CellaVision DM96 automated image analysis system. J Lab Autom 2015;20:60-3. [Crossref] [PubMed]
- Huisjes R, van Solinge WW, Levin MD, et al. Digital microscopy as a screening tool for the diagnosis of hereditary hemolytic anemia. Int J Lab Hematol 2018;40:159-68. [Crossref] [PubMed]
- Horn CL, Mansoor A, Wood B, et al. Performance of the CellaVision(®) DM96 system for detecting red blood cell morphologic abnormalities. J Pathol Inform 2015;6:11. [Crossref] [PubMed]
- Shlebak AA, Bain BJ. Training future haematologists, a privilege or a burden? "A trainer's view Br J Haematol 2017;178:501-7. [Crossref] [PubMed]
- Buttarello M, Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol 2008;130:104-16. [Crossref] [PubMed]
- Urrechaga E, Borque L, Escanero JF. Biomarkers of hypochromia: the contemporary assessment of iron status and erythropoiesis. Biomed Res Int 2013;2013:603786 [Crossref] [PubMed]
- Schoorl M. Innovative haematological parameters in clinical practice. Ned Tijdschr Klin Chem Labgeneesk 2016;41:6-16.
- Schoorl M, Schoorl M, van Pelt J, et al. Application of Innovative Hemocytometric Parameters and Algorithms for Improvement of Microcytic Anemia Discrimination. Hematol Rep 2015;7:5843. [Crossref] [PubMed]
- Buoro S, Manenti B, Seghezzi M, et al. Innovative haematological parameters for early diagnosis of sepsis in adult patients admitted in intensive care unit. J Clin Pathol 2018;71:330-5. [Crossref] [PubMed]
- Garzia M, Di Mario A, Ferraro E, et al. Reticulocyte Hemoglobin Equivalent: an indicator of reduced iron availability in chronic kidney diseases during erythropoietin therapy. Lab Hematol 2007;13:6-11. [Crossref] [PubMed]
- Peerschke E, Pessin MS, Maslak P. Using the hemoglobin content of reticulocytes (RET-He) to evaluate anemia in patients with cancer. Am J Clin Pathol 2014;142:506-12. [Crossref] [PubMed]
- Kakkar N, Makkar M. Red Cell Cytograms Generated by an ADVIA 120 Automated Hematology Analyzer: Characteristic Patterns in Common Hematological Conditions. Lab Med 2009;40:549-55. [Crossref]
- Huh J, Moon H, Chung W. Erroneously elevated immature reticulocyte counts in leukemic patients determined using a Sysmex XE-2100 hematology analyzer. Ann Hematol 2007;86:759-62. [Crossref] [PubMed]
- Buoro S, Manenti B, Seghezzi M, et al. Abnormal scattergrams and cell population data generated by fully automated hematological analyzers: New tools for screening malaria infection? Int J Lab Hematol 2018;40:326-34. [Crossref] [PubMed]
- Lippi G, Plebani M. Capillary electrophoresis for the screening and diagnosis of inherited hemoglobin disorders. Ready for prime time? Clin Chem Lab Med 2016;54:5-6. [Crossref] [PubMed]
- Wild BJ, Green BN, Cooper EK, et al. Rapid identification of hemoglobin variants by electrospray ionization mass spectrometry. Blood Cells Mol Dis 2001;27:691-704. [Crossref] [PubMed]
- Harteveld CL. State of the art and new developments in molecular diagnostics for hemoglobinopathies in multiethnic societies. Int J Lab Hematol 2014;36:1-12. [Crossref] [PubMed]
- Traeger-Synodinos J, Harteveld CL, Old JM, et al. EMQN Best Practice Guidelines for molecular and haematology methods for carrier identification and prenatal diagnosis of the haemoglobinopathies. Eur J Hum Genet 2015;23:560. [Crossref] [PubMed]
- Steensma DP. New challenges in evaluating anemia in older persons in the era of molecular testing. Hematology Am Soc Hematol Educ Program 2016;2016:67-73. [Crossref] [PubMed]
- Lippi G, Bassi A, Bovo C. The future of laboratory medicine in the era of precision medicine. J Lab Precis Med 2016;1:7. [Crossref]
- Nasybullina EI, Nikitaev VG, Pronichev AN, et al. Expert diagnostic system for hemoglobinopathies using the data on blood, erythrocyte, and hemoglobin state. Bull Lebedev Phys Inst 2015;42:206. [Crossref]
- Amendolia SR, Brunetti A, Carta P, et al. A real-time classification system of thalassemic pathologies based on artificial neural networks. Med Decis Making 2002;22:18-26. [Crossref] [PubMed]
Cite this article as: Lippi G, Plebani M. Recent developments and innovations in red blood cells diagnostics. J Lab Precis Med 2018;3:68.