
International Conference on Laboratory Medicine: celebrating 25 years
The reasons of a name
The inspiration to organize a Conference on Laboratory Medicine was originally suggested by Professor Angelo Burlina’s wife, few weeks after his death, on October 1993. Cecilia Tomè-Burlina inquired me about the opportunity of organizing a meeting devoted to the memory of her eminent husband, for keeping the memory alive of the seminal teaching and work endeavored by Angelo Burlina for improving quality of clinical laboratories in Italy and abroad. The title of the conference, namely its focus on “laboratory medicine”, was a logical consequence of the fact that Angelo Burlina recognized and emphasized, earlier and more straight forwardly than any other scientist, the importance of better identifying this term, reflecting the increasing role of “laboratory” testing in modern medicine. At that time, different terms were used, such as “clinical pathology”, of UK and US heritage, as well as “medical biology”, from France expression and heritage “Biologie Medicale”. In 1982, Angelo Burlina first put forward the importance of using the term “Laboratory Medicine” in the preface of his book “Le Motivazioni”, which is in fact a sensitive introduction to laboratory medicine (1). In 1986, he founded, and became the first president of, the Italian Society of Laboratory Medicine (SIMEL). In 1994 he defined laboratory medicine as “the clinical discipline (branch) which investigates data on nature and gravity of structural and functional changes, in samples obtained from patients or on patient themselves, with chemical, physical and biological tools (methods). These data are processed and converted into information to be used together with clinical signs and symptoms for preventive, diagnostic, therapeutic, monitoring and rehabilitation purposes” (2). In the ensuing years, the term “laboratory Medicine” has received increasing consensus, so that the International Federation of Clinical Chemistry (IFCC) changed its name to “International Federation of Clinical Chemistry and Laboratory Medicine”. Soon afterward the European Federation of Clinical Chemistry also modified its name in European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). The same change was adopted by many other national scientific societies and journals, including “Clinical Chemistry and Laboratory Medicine” (CCLM), which was formerly named “European Journal of Clinical Chemistry and Clinical Biochemistry”. A large body of evidence has then accumulated to show that laboratory medicine has transformed the practice of medicine during the last decades, by providing tests and services for prevention, early diagnosis, prognosis, monitoring and follow-up of disease, and so driving advances in all fields of science and medicine. Among the many barriers for obtaining an accurate and rapid diagnosis in the US, Michael Laposata included the evidence that in his country, but not in some others (especially in European countries), the discipline is still defined as “clinical pathology” and the post-graduate training does not allow students to achieve an appropriate level of knowledge and skills, which seems now unavoidable in modern clinical laboratories, since laboratory medicine is still regarded as a part of pathology. Laposata, therefore, emphasized the need to consistently adopt the term “Laboratory Medicine”, even in the US, thus inherently endorsing the earlier Burlina’s insight (3).
The second reason justifying the name of the International Conference was the willingness to secure international popularity to the meeting, with foreign speakers delivering lecturers in English. From early years, this was eased by Angelo Burlina’s activity, who founded, and was appointed first president of, the International Society of Clinical Enzymology, currently known as International Society of Enzymology (ISE), then followed by the organization of a series of scientific meetings inviting many worldwide speakers.
The beginning
The first edition of the International Conference on Laboratory Medicine was organized on the October 25th, 1994, in the Archivio Antico, a historical venue of the University of Padova. Although the speakers were all eminent scientists, they were essentially friends or relatives of Professor Burlina, thus including both his sons (Alberto and Alessandro), and some of his acolytes, such as Martina Zaninotto, Mario Plebani, Marco Pradella and Paolo Rizzotti. Different topics were discussed, ranging from general themes such as “laboratory medicine in the new era” (Morton Schwartz), the need for integrating clinical and laboratory medicine, (Gaetano Crepaldi), “medical decision-making” (Mario Werner), molecular biology in laboratory medicine (Franco Salvatore), tumor biomarkers (David Goldberg), along with more specific topics such as the contribution of clinical laboratories in inherited metabolic disease (Alberto Burlina), ulcer disease (Mario Plebani), and myocardial damage (Martina Zaninotto).
Albeit some criticisms and concerns were initially raised, especially from the executive board of SIMEL, the meeting was successful and I was hence persuaded to follow up with this event, which some people started to call “Memorial Burlina”. The following year (i.e., 1995), it was hence decided to focus the International Conference on a specific field, that was identified as “biochemical markers of atherosclerosis and myocardial damage”. Some lectures were hence centered on traditional and promising risk factors for cardiovascular disease (Klaus Heuck, Giuseppe Lippi and Alberto Burlina), and others were focused on laboratory strategies for accurate and rapid diagnosis of myocardial damage and acute myocardial infarction (Martina Zaninotto and Mario Plebani). In particular, the lecture delivered by Jack H. Ladenson, the inventor of cardiac troponin I immunoassay (which has later become the reference test in laboratory diagnostics of cardiac diseases), shall be seen as a milestone in the history of the conference (4). The topic of cardiac biomarkers was, and still remains, one of the topics which have been mostly discussed over time in the many editions of the conference. In particular, the title of the 1999 conference (i.e., “Novel Aspects of Enzymes in Human Disease”), which was organized as a satellite meeting of the IFCC-World Lab in Venice, allowed to dedicate an entire session to “cardiac markers: an update”, with participation of eminent scientists such as Fred A. Apple, Johannes Mair, Mauro Panteghini, Alex Katrukha, Mario Plebani and Hugo A. Katus, the last of whom discovered and developed the cardiac troponin T immunoassay (5). The importance of cardiovascular disease and the central role of laboratory medicine not only for diagnosing but also for disease prevention, has then persuaded me to organize a further Conference on this topic in 2015. Its title, “Risk Factors and Personalized Medicine”, recognized the progress of laboratory medicine in the identification of genetic markers of cardiovascular risk (Alessandro Doria) and genetic screening for primary and secondary hypertension (Gian Paolo Rossi), along with biomarkers of endothelial activation (Gian Paolo Fadini). Moreover, some speakers introduced the evolving role of laboratory medicine in pharmacogenetics (Carlo-Federico Zambon), and personalized medicine (Mario Plebani). Notably, the keynote lecture was delivered by Hugo A. Katus, who was invited once again to discuss the emerging role of cardiac troponins measurement in acute coronary disease.
Consolidation
Starting from the year 1996, the conference found its definitive identity, through identification of essential aspects influencing the development of laboratory medicine and its growing importance in modern medicine. In that year, the title of the conference was “Role of Laboratory Medicine in Healthcare”, with lectures describing the evolution from analytical control towards total quality (Callum G. Fraser, Per Hiltoft Petersen and Henk J. Goldshmidt), and importance of certification and accreditation of clinical laboratories (Sharon S. Ehrmeyer, Cheryl Blair, Mario Plebani, Pierangelo Bonini and Jean Claude Libeer). The final session of the Conference was focused on the issues of appropriateness (as a new frontier) and the integration of laboratory medicine in healthcare. Notably, only years later the issue of appropriateness started to be considered a priority in the literature and by the scientific community. The topics of that meeting paved the way to take a step forward for increasing the value of the Conference. In fact, the 1997 title of the Conference (“Laboratory and Clinical Reasoning”) mirrored the need to better understand both the nature and mission of laboratory testing in medicine. Which George D. Lundberg, the father of the seminal concept of the “brain-to-brain loop”, was invited to the Conference and discussed the need for careful and global consideration of all steps of the testing process, starting from appropriateness of test request and ending with the appropriate utilization of laboratory information for diagnostic or therapeutic decision (6). Some years later, this seminal concept was revised by adding two other brains (i.e., the laboratory and patient brain) to the physician brain, thus recognizing the evolution of the relationship between the patient, the clinician and the laboratory (7). Other invited speakers discussed the evolution of clinical laboratories and the feed-back with the clinics (Bernardino Fantini and Cesare Scandellari), as well as the importance of clinical and laboratory reasoning (Giovanni Federspil and Mario Plebani) (8). Claudio Rugarli and Mario Werner described the importance of data communication and the value of medical information
In 1998, the conference made a further step forward, as reflected by its title “Laboratory Medicine in the Year 2000: Opening Our Minds to the Changes”, which merged advancements in automation (Robin Felder, Richard Jones and others) with the increasing role of information technology in modern laboratories (Trevor Steele and Paolo Mocarelli). However, the most innovative session was dedicated to the topic or “errors”, with lectures on errors in medicine (Pierangelo Bonini) and in laboratory medicine (Glen Hortin, Matin Hinkley, Jean Claude Libeer, Mario Plebani and Paolo Carraro). In particular, Plebani reported the results of a seminal study published in Clinical Chemistry in 1997, which completely changed knowledge and perspectives on errors in clinical laboratories, by demonstrating that pre- and post-analytical phases are more vulnerable to errors that the analytical phase (9). This insight not only revolutionized theoretical knowledge, but also translated into new and more practical efforts to put under control both the analytical and extra-analytical phases. This effort also allowed developing a global vision of the testing process, focused on the ultimate quality as a patient-centered concept which is mow unavoidable for assuring patient safety and better quality of care (10).
As reasonable consequence, one year later the conference was entitled “Quality and Accreditation of Medical Laboratories: State-of-the-Art, Harmonization and Projects in the European Union”. This title reflected the growing importance of harmonization, certification and accreditation programs for medical laboratories, avoiding possible confusion and conflicts, and paved the way to the essential goal of developing an internationally-recognized and specific standard for medical laboratories accreditation, which was finally released in 2007 (“ISO 15189: Medical laboratories - Requirements for quality and competence”). In the 2000 conference, several worldwide experts in quality and accreditation, who played an essential role in developing the international standards, were invited to the Conference. In particular, these included David Burnett and Desmond Kenny, who should be praised for their important role in this project, as well as Mario Plebani, Vic Blaton and Des Huisman. One session was devoted to external quality assessment (EQA), an essential tool for monitoring the quality of laboratory services, with lectures delivered by Jean Claude Libeer, David Goldie, Sandra Secchiero, Martina Zaninotto and Laura Sciacovelli.
In 2001, another important piece was added to the mosaic, mirrored by the title “Continuous Education, Duties and Responsibilities of Professionals in Medical Laboratories”. This allowed to recognize the increasing importance of continuous education of laboratory professionals. The evolving role of laboratory medicine, and the dramatic developments and improvements in this field, necessitates new competences and skills. In particular, the role of laboratory professionals as clinical consultants was emphasized by Michael Laposata (11), whilst the duties and responsibilities of laboratory scientists and technologists were described by Eleftherios Diamandis and John Wood, respectively. At that time, the fil rouge linking the various editions of the conference was clearly defined, i.e., that the concept of total quality in laboratory medicine represents the “core” of the meeting, and it finds essential elements in recognizing the central role of accreditation programs to evaluate and improve quality, the need for close interaction between laboratory and clinicians for assuring high quality care and, finally, the need of modifying and improving education of laboratory professionals.
Time after time
In 2002, the title “Appropriateness in Laboratory Medicine” allowed to better investigate the topics of appropriate test selection, interpretation and utilization (Mario Plebani, T. J. Hindmarsh, Massimo Gion, Paolo Simioni and Paolo Carraro), the role of information technology in improving interpretation and utilization of laboratory results (Henk M. J. Goldshmidt), as well as the increasing importance of an evidence-based approach (Tommaso Trenti). The main lecture on “Clinical laboratory consultation—a solution to appropriate laboratory use” was delivered by Desmond Burke, an eminent person in the field of laboratory medicine, who published a prophetic article on the future of laboratory medicine in the 21st century (12). One year later, the topic of the conference was “Quality Specifications: from Theory to Practice”, aimed to emphasize the maturity achieved by research on quality specifications and the need to translate theoretical insights into practice. Eminent speakers and pioneers in that area were invited to deliver lecturers, including James Westgard, Carmen Ricòs, Callum G. Fraser, Mario Plebani, and Sharon S. Ehrmeyer. The application of this theoretical concepts in the areas of hematology (Mauro Buttarello), cardiac biomarkers (Martina Zaninotto), thyroid diseases (Allan H. Wu), point-of-care-testing Sharon Ehrmeyer) and EQA schemes (Laura Sciacovelli) was also discussed. In 2004, the topic of the conference was “Enzymes Meet Proteomics”, aimed to acknowledge the increasing role and application of proteomics in laboratory medicine (Mario Plebani, Daniel Chan, Piero Pucci, Pier Giorgio Righetti, Daniela Basso), as well as the heritage of clinical enzymology, which was one of Professor Burlina’s favorite area of research and expertise. The heritage of clinical enzymology and its role in current laboratory medicine was well recognized, as reflected by the title of the 2006 conference “Enzymes: Old Molecules with New Clinical Applications”. Once again, the main clinical applications of enzymes as biomarkers of cardiac damage was explained by eminent scientists such as Jack H. Ladenson, Allan Jaffe, Giuliana Fortunato, Martina Zaninotto and Geràrd Siest. Other sessions were dedicated to the clinical significance of measuring enzymes for diagnosing gastrointestinal diseases (Cesare Montecucco, Mario Plebani, Daniela Basso, Imerio Angriman) and cancer (Paolo Bernardi, Spiridione Garbisa, Eleftherios P. Diamandis, Catharine Sturgeon and Daniel Chan).
In 2005, the conference was dedicated to the emerging concept of “Clinical Governance in Healthcare and in Laboratory Medicine”, and acknowledged the efforts made for emphasizing that improving effectiveness and quality for patients as leading vocation of medicine and laboratory medicine, rather than being focused on efficiency and cost containment. The lectures were delivered by Andrew Moore, Peter J. Degeling, Maria Laura Chiozza, Danielle Freedman, Michael Deighan, and Myriam Lugon. Additional contributions were focused on the relationship between evidence-based medicine and clinical governance (Tommaso Trenti), the role of accreditation (David Burnett), and, once more, the emerging role of point-of-care-testing (Danielle Freedman).
Back to the past, for looking at the future
In 2007, an already discussed topic was resumed, with the purpose to take a step forward on education of laboratory professionals. The need for improving training in laboratory medicine was discussed by David Bruns, whilst Brian R. Smith analyzed the core curriculum of laboratory professionals according to the recent developments in laboratory diagnostics (13). Gian Cesare Guidi presented a lecture on undergraduate education in laboratory medicine (14), Gianni Casca discussed the issue of education and training of medical technologists, whilst Giorgio Federici’s lecture was focused on post-graduate education in laboratory medicine. Last but not least, Mario Pazzagli described the initiative of the European Register of specialist in laboratory medicine and Mario Plebani provided an overview on the changing landscape in laboratory medicine, especially focused on translational medicine. In 2008, once again the conference was devoted to the issue of errors in medicine, with a direct link with patient safety. Other outstanding speakers were Lucian L. Leape, a universally recognized pioneer of initiatives aimed to reducing the risk of errors in medicine, James Reason, the inventor of the “Swiss cheese” model of human error, and Albert Wu, whore presented an important branch of the World Health Organization (i.e., World Alliance for Patient Safety). Additional lectures were presented by Giuseppe Lippi, on risk management in the pre-analytical phase, by Oswald Sonntag, on analytical interference and analytical quality, Elisa Piva, on interpretative comments and critical values, whilst Mario Plebani, Maurice O’Kane, Giorgio Darin and David Williams discussed different aspects of laboratory errors. In the last session, Laura Sciacovelli, Maria Laura Chiozza and Chiara Signori described different models for minimizing error risk and improving patient safety, such as failure modes and effects analysis (FMEA), process and risk analysis and the model of quality indicators (MQI) (15). The increasing significance of errors in medicine was the driver for dedicating another conference to this issue. Therefore, the title of the 2012 meeting was “Diagnostic Errors and Quality Indicators in Laboratory Medicine”. The key-note lecture was delivered by Mark L. Graber, a widely-recognized leader in the field of diagnostic errors, whilst additional speakers explored the relationship between diagnostic errors and clinical reasoning (Maria Laura Chiozza), and between diagnostic errors and anatomical pathology (Massimo Rugge), radiology (Luigi Pescarini), and clinical laboratories (Maurice O’Kane). Paul Epner, Julian Barth, Laura Sciacovelli and Zhiguo Wang also discussed the use of quality indicators for reducing the error risk in clinical laboratories. Under the chairmanship of Mario Plebani and the umbrella of the IFCC, an MQI was launched, with specific application to the pre-analytical (Ana Maria Simundic), intra-analytical (Martina Zaninotto), and post-analytical (Mario Plebani) phases. The efforts made for developing quality indicators led the way to organizing the 2016 Conference, entitled “Towards Performance Specifications for the Extra-Analytical Phases of Laboratory Testing”. The identification of quality indicators covering all the different phases of laboratory testing allowed defining tentative performance specifications for both analytical and extra-analytical activities. The opening lecture was delivered by Mario Plebani on “Quality indicators and performance specifications for the extra-analytical phases of laboratory testing” (16), and was then followed by other presentations dealing with appropriateness of test request (Mauro Panteghini), patient and sample identification (Giuseppe Lippi), sample collection (Ana-Maria Simundic), sample handling and transportation (Martina Zaninotto), sample acceptance and rejection (Sverre Sandberg), reference values and decision limits (Ferruccio Ceriotti), turnaround time (Paolo Carraro), and critical results (Elisa Piva).
Present and future of clinical laboratories
The development of laboratory medicine and directions for the future were the topics of two conferences, in 2009 “laboratory diagnostics in the Third Millennium: Where, How, and Why” and 2014 “Clinical Laboratories: Navigating Between Commoditization and Clinical Partnership”. The former meeting focused on the increasing importance of “decentralized” testing in pharmacies (Giuseppe Lippi), home testing (Sverre Sandberg), and point-of-care testing (Ann M. Gronowski, Ivo Casagranda, Giuliano Soffiati). Further speakers focused their lectures on consolidation of the “hospital central laboratory” (Martina Zaninotto), laboratory networks (Norbert Blankaert) and the merger of in vitro and in vivo diagnostics (Michael Feldman). The second meeting started with an opening lecture by Mario Plebani on “Clinical laboratories: profit center, production industry or patient-care resource?” (17), aimed to better addressing the changing role of laboratory testing and preventing the risk of its identification with a commodity. The true mission of clinical laboratories and the contribution to clinical decision-making was discussed by Michael Laposata, Sverre Sandberg, Leonardo Fabbri, Pierfranco Conte, whilst Giovanni Barletta presented interesting data on “Quality, volumes and costs in laboratory medicine”.
In 2010, the main topic of the conference was “Beyond Normal Values”, thus dealing with the evolution of the theory of reference values (Gèrard Siest), the search for common reference intervals (Ferruccio Ceriotti), the impact of standardization on the suitability and interpretation of laboratory results (Mauro Panteghini), the concept of reference change value (Callum G. Fraser). A keynote lecture was delivered by George D. Lundberg, entitled “laboratory Information: the Brain-to-Brain Loop 40 Years Later”, whilst Mario Plebani discussed the threats and challenges to the brain-to-brain theory.
In 2013, for celebrating the first 20 years of the International Conference, the meeting was focused on “Harmonization in Laboratory Medicine: the complete picture”. The term “complete picture” well recognizes the topics discussed in previous meetings, along with the need for clinical laboratories to provide comparable results and information, starting from harmonization of test request (Stuart Smellie), pre-analytical processes (Giuseppe Lippi), terms and units (Davide Giavarina), reference values and decision limits (Ferruccio Ceriotti), and critical values notification (Elisa Piva). The relationships between standardization and harmonization were the topic of Mauro Panteghini’s lecture by, whilst Greg W. Miller discussed the roadmap to harmonization and Mario Plebani delivered a keynote lecture on the complete picture of harmonization in laboratory medicine (18). The last edition of the conference, in 2017, was devoted to the topic of “Uncertainty, Quality, Safety and Accreditation in Laboratory Medicine” (19), thus merging several aspects of previous meetings, for better defining the concept of quality in laboratory medicine (Patrick M. M. Bossuyt, Mario Plebani), the debate regarding the approaches to uncertainty versus total error (Wytze Oosterhuis, Andrea Padoan), and compliance with essential requirements of ISO 15189 accreditation (Ada Aita, Giorgia Antonelli, Laura Sciacovelli and Silvia Tramontin).
25 years on: role and value of an International Conference
In agreement with the title of a recent article, quality and future of clinical laboratories should be well represented by the Vico’s whole cyclical theory of the recurring cycles (20). Ahead of a monumental development and enormous advances of laboratory medicine, the gap between laboratory and clinics, the consolidation of analytical activities in focused factories and “mega facilities” is generating a vision of laboratory service as a simple commodity and, even worse, disconnected with care pathways. However, as happened in the past, there are many reasons to predict a restoration of the true nature of laboratory service as an integral part of the diagnostic and therapeutic process. The internal and external drivers have been already described and derive, at least partially, from lessons learnt during the previous International Conferences. The “core principle” of all Conferences is the search for better quality in laboratory medicine (Figure 1). This should be achieved by promoting development of internal and external programs and processes. In particular, the discovery that pre- and post-analytical phases are more vulnerable to errors than intra-analytical processes led us to promote the vision of laboratory testing as described by Lundberg, in the seminal concept of the “brain-to-brain loop”, and focusing on the ultimate goal that is an action on the patient to improve the diagnostic and therapeutic process (6), along with major emphasis on the emerging role of laboratory medicine in identifying risk factors, disease prevention and personalized medicine. The vision of the testing process as a continuum, led us to promote a project on quality indicators covering all steps of the testing process, thus including pre-pre and post-post-analytical steps, and for identifying performance specification which should create a culture of, monitor, and continuously improve, quality (21). In particular, we have highlighted the need to assure and improve patient safety as laboratory tests increasingly impact both clinical decision-making and outcomes. Nevertheless, this would need initiatives aimed to harmonizing and standardizing not only analytical methods, but all procedures and processes, thus finally assuring an accurate and comparable laboratory information. The education and training of laboratory professionals should also be improved, not only considering the need to acquire knowledge and skills in emerging technologies such as mass-spectrometry (MS), genomics, proteomics, metabolomics, but also clinical advice in test request and interpretation, for finally promoting a reliable “laboratory stewardship”. This term well represents the goal of laboratory services, i.e., assuring “the right test for the right patient, at the right time, to generate the accurate, clinically relevant results at the right time to optimally influence clinical care”.
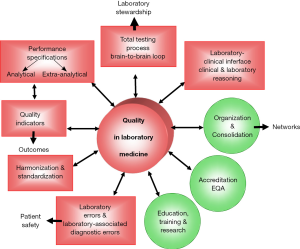
However, understanding the stepwise diagnostic process and effective diagnostic skills lie at the heart of medical education: diagnostic tests have limitations and, when used improperly, can be misleading. The core curricula of students should hence provide higher knowledge on laboratory and diagnostic tests, even because the process of diagnostic decision-making should be increasingly shared (22). Obviously, the promotion of initiatives aimed at improving the laboratory-clinical interface are essential, and this is the reason driving us to accurately revise and improve the knowledge of clinical reasoning. It has been recently emphasized that “although diagnostic tests such as a serum chemistry assay are constantly titrated against standards to avoid error, physician’s diagnostic capabilities are not systematically calibrated” (23). Therefore, the dynamic between laboratory professionals and clinicians shall be improved for developing a real patient-centered care, for reducing the risk of diagnostic errors, and for improving patient safety (24).
Actually, quality is strongly influenced by laboratory organization. Although a reasonable consolidation and networking seems essential to reduce costs, improve efficiency and save resources, it cannot be identified with the focus on reducing cost per-test and achieving larger volumes, since this will increase the risk of inappropriate requests, reduce the surveillance on pre-analytical variables (in particular sample quality and transportation), and will hence isolate the laboratory from the clinics. In the last 25 years, monumental changes have influenced care delivery and laboratory services. The series of International Conference of Laboratory Medicine, the proceedings of which have been published in some qualified Journals such as Clinical Chemistry and Laboratory Medicine, Clinica Chimica Acta, Clinical Biochemistry and, last but not least, Journal of Laboratory and Precision Medicine represents a tool for updating our knowledge and for offering better services to our patients and our stakeholders.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Laboratory Medicine-25 Years on”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.08.02). The series “Laboratory Medicine-25 Years on” was commissioned by the editorial office without any funding or sponsorship. Mario Plebani served as an unpaid Guest Editor of the series. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burlina A. Introduzione alla Medicina di Laboratorio. C. G. Edizioni Scientifiche, Torino, Italy, 1982.
- Burlina A. Elementi di Patologia e Biochimica Clinica per il Corso integrato di medicina di laboratorio. C.G. Edizioni Scientifiche, Torino, Italy, 1994.
- Laposata M. Obtaining a Correct Diagnosis Rapidly in the United States Is Associated With Many Barriers Not Present in Other Countries. Am J Clin Pathol 2018;149:458-60. [Crossref] [PubMed]
- Ladenson JH. A personal history of markers of myocyte injury Clin Chim Acta 2007;381:3-8. [myocardial infarction]. [Crossref] [PubMed]
- Katus HA. Development of the cardiac troponin T immunoassay. Clin Chem 2008;54:1576-7; discussion 1577. [Crossref] [PubMed]
- Lundberg GD. Acting on significant laboratory results. JAMA 1981;245:1762-63. [Crossref] [PubMed]
- Plebani M, Laposata M, Lundberg GD. The brain-to-brain loop concept for laboratory testing 40 years after its introduction. Am J Clin Pathol 2011;136:829-33. [Crossref] [PubMed]
- Plebani M. The clinical importance of laboratory reasoning. Clin Chim Acta 1999;280:35-45. [Crossref] [PubMed]
- Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997;43:1348-51. [PubMed]
- Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta 2009;404:16-23. [Crossref] [PubMed]
- Kratz A, Laposata M. Enhanced clinical consulting--moving toward the core competencies of laboratory professionals. Clin Chim Acta 2002;319:117-25. [Crossref] [PubMed]
- Burke MD. Laboratory medicine in the 21st century. Am J Clin Pathol 2000;114:841-6. [Crossref] [PubMed]
- Smith BR, Aguero-Rosenfeld M, Anastasi J, et al. Academy of Clinical Laboratory Physicians and Scientists. Educating medical students in laboratory medicine: a proposed curriculum. Am J Clin Pathol 2010;133:533-42. [Crossref] [PubMed]
- Guidi GC, Lippi G. Undergraduate education in Laboratory Medicine. Clin Chim Acta 2008;393:9-12. [Crossref] [PubMed]
- Plebani M, Astion ML, Barth JH, et al. Harmonization of quality indicators in laboratory medicine. A preliminary consensus. Clin Chem Lab Med 2014;52:951-8. [Crossref] [PubMed]
- Plebani M. EFLM Task Force on Performance Specifications for the extra-analytical phases. Performance specifications for the extra-analytical phases of laboratory testing: Why and how. Clin Biochem 2017;50:550-4. [Crossref] [PubMed]
- Plebani M. Clinical laboratories: production industry or medical services? Clin Chem Lab Med 2015;53:995-1004. [Crossref] [PubMed]
- Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med 2013;51:741-51. [Crossref] [PubMed]
- Plebani M, Lippi G. Uncertainty, quality, safety and accreditation in laboratory medicine. J Lab Precis Med 2017;2:80. [Crossref]
- Plebani M. Quality and future of clinical laboratories: the Vico's whole cyclical theory of the recurring cycles. Clin Chem Lab Med 2018;56:901-8. [Crossref] [PubMed]
- Plebani M. Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med 2016;54:1881-91. [Crossref] [PubMed]
- Brush JE Jr, Brophy JM. Sharing the Process of Diagnostic Decision Making. JAMA Intern Med 2017;177:1245-6. [Crossref] [PubMed]
- Cifu AS. Diagnostic errors and diagnostic calibration JAMA 2017;318:905-6. [Crossref] [PubMed]
- Lippi G, Cervellin G. From laboratory instrumentation to physician’s brain calibration: the next frontier for improving diagnostic accuracy? J Lab Precis Med 2017;2:74. [Crossref]
Cite this article as: Plebani M. International Conference on Laboratory Medicine: celebrating 25 years. J Lab Precis Med 2018;3:72.


