
GFR measured by iohexol: the best choice from a laboratory perspective
Introduction
Glomerular filtration rate (GFR) is the best overall measure of the integrated filtering capacity of the nephron (1). It is therefore considered the key parameter in the clinical assessment of kidney function and the classification of chronic kidney disease, in pre-nephrectomy assessment for living kidney donation, as well as in the evaluation of renal function decline over time in kidney disease. Knowledge of GFR is also central to monitoring treatment effect on renal function and for dosing potentially toxic medications with a narrow therapeutic index (1,2).
The rapid development of therapeutic principles in nephrology has led to increasing demands for precise measurements of GFR. The rigorous assessment of GFR requires measuring the clearance of an exogenous marker that fulfils the criteria for an ideal filtration substance (3,4). In the absence of methods for directly measuring single-nephron GFR, especially in clinical trials and when assessing the suitability of living donors, overall GFR is measured indirectly, but accurately, based on the clearance of exogenous filtration markers.
Historically, the renal clearance of inulin was the first ‘gold standard’ procedure for GFR determination (5,6). However, the inulin clearance method cannot be considered practical for routine clinical use because it requires continuous intravenous injection and multiple, timed urine collections. In addition, inulin measurement is expensive, cumbersome and difficult to perform, due to possible endogenous interferences. Furthermore, inulin is not easily available as a ready-to-inject solution for human use.
Given all of these drawbacks, alternatives to standard inulin clearance were developed. To overcome the need for constant infusion and urine collection, a single-injection method for GFR measurement based only on the total area under the curve of plasma marker concentrations versus time has been proposed (7). Radioactive markers, such as ethylenediaminetetraacetic acid, 51Cr-EDTA, diethylene triamine pentaacetic acid, 99mTc-DTPA, and 125I-iothalamate have been shown to be reliable for accurately determining GFR, with values that are comparable with standard inulin clearance (8-11). However, these methods require radiolabeled tracers, which complicate the procedure (special licensing, complicated handling, storage and disposal of waste) and exclude certain patients, such as pregnant women, from the investigation (12).
Thus, non-isotopic approaches using the plasma clearance of contrast media, which fulfil the criteria of ideal markers—such as iohexol, iothalamate, iopamidol, and iopromide—have been proposed as alternatives for measuring GFR.
Iohexol is the most widely used marker for measuring GFR, at least in Europe, and is increasingly being used in other countries, while iothalamate is traditionally the most widely used marker in the Unites States. The use of other contrast media, despite sharing the characteristics of both iohexol and iothalamate, have been reported only occasionally (13,14).
The role of the laboratory is crucial in addressing the choice of a proper marker: a compound that can be easily and precisely quantified in plasma specimens can contribute significantly to setting up an accurate procedure for measuring GFR, which may help the nephrologist to obtain a reliable and reproducible determination of a patient’s renal function.
Iohexol is undoubtedly the most extensively studied type of contrast media currently used for GFR measurement, and is the best choice for laboratories, clinicians and, more importantly, for the patient.
This review will focus on some analytical and practical aspects of the plasma clearance of iohexol as a tool for measuring GFR.
GFR measurement by using iohexol as an exogenous marker
Iohexol is a tri-iodinated benzene-derivative, non-ionic, low osmolality, non-radioactive X-ray contrast medium, with a molecular weight of 821.1 Da, developed in the early 1980s.
First studies in humans documenting biochemical properties and pharmacokinetics parameters (15-18) showed that iohexol is excreted unchanged in urine and its renal clearance is almost identical to 51Cr-EDTA clearance.
The reliability of GFR determination by iohexol plasma clearance was demonstrated a few years later by Krutzén et al. (19). In 42 patients with normal to moderately impaired renal function, a 5 mL iohexol solution and 4 Mbq of 51Cr-EDTA were injected simultaneously. Blood samples were drawn up to 240 min after injection, and GFR was determined using a two-compartment model approach. The results showed an excellent correlation between the clearances of the two markers (correlation coefficient 0.98).
In that seminal paper, they first demonstrated the validity of the Bröchner-Mortensen equation, originally developed for 51Cr-EDTA (20), for the correction of iohexol plasma clearance calculated according to a one-compartment model, i.e., considering only blood samples taken from 2 to 4 hours.
After the appearance of that paper, a vast number of researchers investigated the usefulness of iohexol as a marker for GFR measurement.
In a recent publication, Delanaye et al. (21,22) reviewed the use of iohexol plasma clearance for GFR measurement in both clinical and research settings in depth. Comparisons between iohexol and inulin clearance, as well as with other reference markers, such as iothalamate and radiolabeled tracers, have been discussed exhaustively.
Iohexol plasma clearance is the most convenient method for measuring GFR in almost all clinical settings. However, in particular situations, where the extracellular volume is increased (ascites, oedema, in intensive care units) the evaluation of urinary clearance may be considered a more reliable procedure (21).
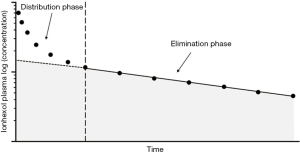
After intravenous injection of 5–10 mL of the pharmacological solution, iohexol disappears from the blood, according to a two-compartment open-model system. Thus, the measurement of plasma clearance requires multiple blood samples (in particular early after marker administration) to correctly estimate both the early phase (fast component), which reflects the distribution between the intra- and extra-vascular volumes, and a late (slow component) one corresponding to the clearance of the compound by the kidney.
The plasma concentrations profile can be analysed through a nonlinear regression iterative pharmacokinetic programme, and clearance (GFR) is calculated by dividing the injected dose by the area under the plasma-concentration time-curve (extrapolated to infinity), AUC.
Abbreviated kinetic profiles for calculating GFR only from the late exponential part of the disappearance curve overcome the disadvantage of multiple blood samplings has become the work-horse for renal function determination.
Plasma concentrations during the late phase can be interpolated either through a kinetic iterative programme or an Excel macro that is easy to implement in a spreadsheet (slope-intercept method). Since the area under the final exponential phase (Figure 1, shaded area) is always lower than the AUC of the complete (biexponential) plasma curve, a correction factor should be used to compensate for the missing early compartment AUC. Different algorithms have been proposed for correcting AUC (23-25): the most widely used (at least in Europe), remains the Bröchner-Mortensen equation (20):
GFRBM =0.990778× Cl1 −0.001218× Cl12, where Cl1 is the plasma clearance calculated according to the one-compartment model.
One-compartment plasma clearance of iohexol can be determined by using different sampling protocols in the slow compartment phase. Though it is generally recommended that one avoid sampling earlier than 2 hours after iohexol injection (to allow completion of the distribution phase) there is no consensus either on the number of blood drawings or on the timing of the last sample. For subjects with normal or slightly decreased renal function, a last sample taken 4 hours after injection of the marker can assure reliable GFR determination. Conversely, as general rule, in patients with reduced renal function, the lower the expected GFR the later the last sample should be taken (at 5, 6, 8 or even 24 hours) (21).
Reducing sampling to one or two blood draws can further simplify the measurement of plasma clearance. It has been reported that the single sample approach, with the calculation of GFR using the Jacobsson formula (26), is more commonly used due to its practical advantages (21,27), and is an attractive procedure in large epidemiological studies when a convenient technique with limited costs is required.
However, caution should be used when adopting such limited sampling protocols: some reports have indicated that single or two-point GFR results are almost superimposable with multiple specimen protocols, except in patients with reduced renal function (21,27,28). Thus, in some specific situations and in particular when decreased GFR is expected, a multiple sample procedure could be more accurate.
From an analytical point of view, the single sample protocols may have some drawbacks. Potential (random) errors associated with a single sample (errors in blood drawing, dilution or contamination of the sample, analytical errors in iohexol concentration measurement, for instance) may play a major role and may result in incorrect GFR determination. Provided that the analytical method does not have a systematic bias, a higher number of sampling points that are fitted by a nonlinear regression programme according to a one-compartment model, allow for a ‘smoothing’ of these possible random errors, resulting in a better fitting and thus in a more reliable GFR determination.
The choice of the procedure selected for GFR measurement may sometimes be driven mainly by specific centre expertise/experience, logistical issues, the availability of personnel, training of the nurses, and costs. In clinical settings, for instance, the impossibility of drawing multiple samples for a relatively extended time after bolus injection of the marker, may direct the nephrologists toward a single-point rather than a multiple-point procedure for measuring GFR. Both clinicians and laboratorians must fully evaluate the impact of their choice and be aware of all the factors affecting GFR determination (21,22).
Analytical methodologies for iohexol determination
The methodology for iohexol measurement can be retrieved from a vast number of papers, either focused on the set-up of the analytical method or describing the procedure for measuring GFR. Thus, the choice of the analytical method can easily be tailored according to the available instrumentation, as well to the personnel’s expertise.
High-performance liquid chromatography with ultraviolet detection (HPLC-UV) is the most commonly used method for determining iohexol (19,29,30). Other validated assays include methodologies such as X-ray fluorescence (XRF) (31) and, liquid chromatography-tandem mass spectrometry (LC-MS/MS) (32-35). Alternative methods, such as UPLC (ultra-performance liquid chromatography), UPLC-MS/MS (UPLC combined with mass spectrometry) (36), capillary electrophoresis (CE) (37-39), neutron-activation analysis (40) as well as an ELISA kit (41) are available, but some of these have not yet been fully validated.
HPLC-UV methods have sufficient sensitivity/specificity and usually require reversed-phase columns (C18, 7.5–25 cm length) and eluent with a low percentage of organic solvent (acetonitrile or methanol): in these conditions (either in an isocratic or in a gradient run) iohexol elutes from the chromatographic column as two peaks, reflecting the isomers present in the pharmacological preparation. These two rotational isomers (endo- and exo-isoforms) are usually resolved by the reversed-phase columns and quantification can be accomplished through the integration of either of the two peaks (area and height of the peak works similarly), even though calculations performed using the higher (second eluting peak) are usually preferred.
The relationship between the two peaks is constant, regardless of the pH variation in the mobile phase (19).
Either plasma (lithium heparin or K-EDTA) or serum specimens (usually 50 to 200 microliters) could be collected to measure GFR: no systematic accuracy problems have been reported to be caused by the choice of biological matrix (42). Light to moderate hemolysis of blood samples is not detrimental in HPLC analysis: iohexol in the blood is quantitatively distributed to the plasma compartment and no additional interfering peaks that could prevent correct quantification of the compound usually appear in the chromatogram.
Iohexol working solution to prepare calibrators can be obtained easily through the dilution of the ready-to-inject solution, either in water or in phosphate buffer. Care should be taken performing the dilution steps, due to the viscosity of the solution: it has been suggested that a weighing procedure should be adopted to ensure calibrators preparation is improved (42).
Calibration curves should be prepared to virtually encompass all the expected concentrations to be measured in patient specimens.
Some HPLC-UV methods for plasma/serum analysis of iohexol have been documented as being linear up to about 1,300–1,500 mg/L (29,43,44). Other analytical methodologies reported a similar performance: by using an LC-MS/MS Vicente et al. (32) found that the assay was linear up to 2,000 mg/L in serum samples and Annesley (36) reported a level of 1,500 mg/L with UPLC-MS/MS.
However, methods implemented to accurately measure iohexol concentrations up to 300–400 mg/L (iohexol concentrations after 5–10 mL of solution injection usually do not exceed these levels), without the need to dilute the sample, could be deemed adequate for GFR measurement even in patients with severely reduced renal function.
All analytical methods published so far require sample preparation prior to the quantification step by chromatographic procedure, except in some CE assays, where direct injection of the biological matrix has been recorded (38). Acid precipitation (by perchloric acid addition) is the first procedure described (19) and, notably, is still used today due to its simplicity and accuracy in removing potentially interfering substances (29,45,46). After vortex mixing and centrifuging, the supernatant is ready for analysis without any other purification or filtering step. Other procedures requiring protein removal by means of zinc sulphate (33,36), of organic solvents such as acetonitrile or methanol (32,37,44), or molecular weight ultrafiltration (34), also work.
Iohexol is quite a stable molecule. It is stable at room temperature, at −20 or −80 °C (31,47). The high stability of iohexol allows GFR measurements to be performed in virtually all medical settings: collected samples can be shipped without any particular notes of precaution to a central laboratory for analysis.
Iohexol stability has been demonstrated even after three freeze-thaw cycles: this guarantees that samples can safely be re-analysed when needed without any detrimental effect on the overall method performance (33,48).
Iohexol proficiency testing programme
Laboratories performing analyses of exogenous compounds for GFR determination must ensure accurate measurement of the compound in plasma and urine: participation in a quality scheme programme is highly recommended. Iohexol is not currently covered by the CAP (49) proficiency testing programme. However, an international programme for iohexol was set up in Europe in 2010 by Equalis AB (Uppsala, Sweden). At the very beginning of the proficiency programme, 22 (Scandinavian) laboratories participated and a single plasma sample was distributed. All but one laboratory determined iohexol concentration by HPLC (116±3.41 mg/L, CV 2.9%). Participants were also asked to calculate GFR using a single-point procedure [most laboratories probably adopted the Jacobsson formula (26)]. Normalized GFR, calculated by 18 laboratories, was 18.4 mL/min with a CV of 6.2%.
Since September 2014, two plasma specimens (pooled plasma with addition of iohexol or taken from patients given iohexol) have been distributed by regular mail four times per year without any temperature control, and there are currently (May 2018) over 30 participant laboratories: of these, about two-thirds used HPLC-UV to quantify iohexol. Data are also provided to allow participants to calculate GFR by means of a single- or two-point approach. The quality goal for iohexol measurement is ±8%.
A report for each quality control round is published online and each laboratory can compare its own results to those of other participating laboratories. This allows laboratories to evaluate and ensure the accuracy of their iohexol measurement.
In the most recent rounds, the CV for the two different measured levels of iohexol was about 4%. Overall, the results indicate that the different methods for measuring iohexol performed similarly. However, a slightly higher CV was observed in the small group of laboratories using UPLC/MS/MS for iohexol determination. A comment on the mean values of GFR determined using both methodologies is also provided.
Participation in a quality control programme not only allows laboratories to evaluate and ensure the accuracy of their iohexol measurements, but also enables calibration between laboratories. Discrepancies between iohexol measurements result in different evaluations of plasma iohexol disappearance, and thus in different GFR measurements. The calibration of iohexol measurements may enable the calculation of comparable GFR results, and this is of paramount importance, since this would make it possible for multicenter studies to be performed even without the need to centralize iohexol measurement in a single reference laboratory.
Recently, Seegmiller et al. (50) reported the effect of the adjustment of iohexol measurements for the samples provided by Equalis in a multicenter comparison study involving four laboratories: the bias between laboratories was reduced to 1–2%, irrespective of the level of iohexol concentration. The authors demonstrated that adopting a stringent calibration practice and/or participating in a quality control survey ensured accurate and reliable iohexol quantification in patients’ plasma or serum.
Furthermore, interlaboratory exchanges of blinded patient plasma/serum samples for method comparison with a well-established analytical assay have been reported as another useful tool for checking the performance of the procedure set up for iohexol determination (32,46).
This pragmatic approach may help to assess whether the analytical method used for iohexol analysis has some effect on measured concentrations. For instance, HPLC-UV- and LC-MS/MS-measured iohexol levels have been reported as having either systematic differences up to 10% (51) or being almost perfectly superimposable (32).
In some specific conditions, such as in patients with oedema or ascites, urinary rather than plasma clearance of iohexol should be determined.
Urinary iohexol concentrations may vary greatly, depending on the renal function of the patient, and may be much higher than the levels determined in plasma.
Validated methods have been published (29,30,38); in any case, a proficiency test for urinary iohexol does not exist, and laboratories must consider that additional investigations should be performed to guarantee the accuracy and reliability of the results in this biological matrix.
Simplified GFR determination by iohexol: dried blood spot testing (DBS)
GFR can be quantified conveniently by determining plasma clearance. However, the pre-analytical steps of these procedures can be slightly cumbersome and time-consuming meaning that the methods are essentially available only in specialized laboratories or in research centres. DBS has been shown to be a valid alternative approach to simplifying the measurement of diverse analytes in biological matrices.
Several reports demonstrate the reliability of iohexol analysis, both in capillary blood samples (52) and in dried blood samples (53). The plasma concentrations were extrapolated from blood levels after correction by hematocrit (iohexol is distributed quantitatively in the plasma compartment).
In a review, Bjornstad et al. (54) showed that GFR determined by the DBS is a promising approach and a practical and superior alternative to GFR estimation. However, in some instances, the findings showed low accuracy and precision of the DBS compared to standard analysis (53,55,56).
Luis-Lima et al., in a recent paper, compared GFR values determined by DBS with the reference multiple-point plasma analysis (57). A robust statistical approach was adopted [evaluation of concordance coefficient of correlation (CCC), total deviation index, TDI, and the coverage probability] (58), to assess the agreement between values. An acceptable agreement was defined as TDI <10%.
The simplified developed procedure required the deposition of a fixed volume of blood, as low as 10 microliters on filter paper, and an overnight drying step at room temperature. The analysis of DBS specimens was accomplished after minor modifications of the conventional HPLC-UV method used for iohexol analysis in plasma. DBS samples were treated with perchloric acid, ultrasonicated and, after centrifuging, iohexol was quantitatively recovered in the clear supernatant. The addition of iopamidol as an internal standard markedly improved the overall method performance. A CCC of 0.996 and a TDI of 9.5 demonstrated that 90% of the 203 GFR evaluated in patients with different degrees of renal function (from advanced renal disease to hyperfiltration) was determined using the DBS approach, with a margin of error ranging from −9.5% to 9.5%, compared to the reference plasma method. A rigorous evaluation of all the possible technical problems related to the DBS procedure, and in particular the use of a fixed blood volume for iohexol analysis allowed the authors to rule out any technical drawbacks, thus confirming the reliability of the assay.
The procedure has several advantages over the conventional plasma method, in particular for the patient, as finger prick sampling is more comfortable that venipuncture. The reduced volume of blood required allows for reliable and safe GFR determination, both in pediatric subjects and in patients with difficult venous access. Furthermore, the simplified pre- and post-analytical steps result in cost reductions for the procedure: no phlebotomy material is needed, samples can be stored at room temperature and eventually shipped to a central laboratory for iohexol analysis by regular mail.
Taken together, these findings definitely demonstrate that renal function can be measured using DBS without sacrificing accuracy and precision, making the procedure a valid and promising alternative to the conventional analysis of iohexol in plasma, since these approaches are interchangeable. However, the lack of a proficiency test for direct measurement of iohexol in whole blood (DBS) may be a possible drawback for this simplified procedure.
Iohexol safety
Radiocontrast media are mainly used for medical imaging in diagnostic and interventional procedures (59). The introduction of non-ionic contrast agents, like iohexol, has lowered the risk of immediate adverse reactions. However, the dose of iohexol (and thus of iodine content) required for CT scans ranges between 80 and 180 mL and between 130–300 mL for coronary interventions (21,59).
The dose currently infused in patients for GFR measurements is generally much lower (usually 5–10 mL of the injectable solution or less) and for this reason the risk of immediate adverse events in renal function evaluations should be limited or negligible.
An old, brief summary of the Lund (Sweden) experience regarding 8,000 GFR procedures (60), recorded no complications except for two patients, who reported transient malaise and vomiting between 1–3 hours after a very low intravenous dose of iohexol (2 to 5 mL).
More recently, a more systematic report on the safety of 5 mL iohexol administration for GFR measurement was published. All the immediate adverse reactions that could be related to iohexol administration were reviewed in 2,891 patients (age: 14 to 87 years) who underwent a total of 15,147 GFR measurements (45). The survey considered patients enrolled in 37 different clinical trials over a period between 1992 and 2016. Patients with chronic kidney disease, diabetic kidney disease, autosomal polycystic kidney disease, end stage renal disease on peritoneal dialysis, as well kidney transplant recipients and living kidney donors, were considered. Measured GFR ranged from 7.3 to 173.7 mL/min/1.73 m2 and, overall, the median number of GFR determination per patient was 4. The report recorded a single event of moderate intensity (flushing, urticaria, and itching) in a diabetic patient a few minutes after iohexol injection. The patient recovered after intravenous corticosteroid injection. In summary, independent of disease conditions and renal function level, the overall rate of iohexol-related events was as low as 0.0066%. These findings definitely rule out any concerns regarding the safety of iohexol as a marker of GFR measurement.
Concluding remarks
A perfect marker of GFR probably does not exist. Inulin has historically been considered the best exogenous compound, since it fulfils all the requirements of an ideal GFR marker and is considered the first ‘gold standard’. However, even for inulin, extrarenal clearance has been documented at least in patients with reduced GFR (<30 mL/min/1.73 m2) (61).
The reliability of GFR measurement with alternative compounds, such as iohexol, iothalamate and radiolabeled tracers, has been compared to inulin results in almost all instances (GFR level, patient pathologies). In a recent systematic review, Soveri et al. showed that iohexol and 51Cr-EDTA plasma clearance (as well as iothalamate and 51Cr-EDTA renal clearance) have sufficient accuracy for measuring GFR (7).
Measuring GFR by means of iohexol is thus a well-established and safe procedure: it has been described intensively in both clinical and research settings and for the measurement of plasma (and renal) clearance a vast number of sampling protocols have been studied in depth, allowing clinicians and laboratorians to choose the most appropriate approach to adopt in their centres and to tailor it to their specific needs.
Of note, due to the reliability of the procedure, iohexol plasma clearance has also gained the role of a reference standard for development of innovative approaches. Wang et al. (62) in pigs and dogs measured GFR by a portable fiberoptic fluorescence analyzer and validated the accuracy of the procedure by comparing their results with a 6 hours iohexol plasma clearance. More recently, Rizk et al. (63) described a GFR measurement procedure in humans that requires the intravenous injection of two fluorescent dextran conjugates of different molecular weight. Subject with different degree of renal function were enrolled in the study, and results showed a close linear correlation with GFR values obtained by iohexol plasma.
The main task for the laboratory is to develop an accurate quantitative analytical method for obtaining reliable concentrations of the marker in plasma/serum and urine. The very large number of papers published in the last 30–35 years is a great help for selecting or developing an ad hoc procedure that can easily be implemented in laboratory facilities, and which fulfils clinicians’ needs.
Iohexol is currently undoubtedly the ‘first choice’ of a safe marker of renal function, due to the ease of quantification with a number of different analytical methodologies, sample stability and, more importantly, the existence of a proficiency test programme. Furthermore, the cost of a 20 mL bottle of injectable iohexol solution is very low in Europe (around 10 euros) and it is available worldwide (Table 1). This could be the first step toward the standardization of the procedure for GFR measurement (21).
Table 1
| Reliability and accuracy of renal function determination |
| Availability of well documented protocols for plasma (multiple or single point sampling) or renal clearance |
| Availability of dried spot blood testing approach to reduce blood volume drawing |
| Easy quantification with sensitive, reproducible analytical assays using different methodologies (HPLC-UV, LC-MS/MS, UPLC-MS/MS, UPLC) |
| Implementation of analytical method(s) in any laboratory facilities without any particular effort |
| Easy procedures for handling and storage of the samples |
| Stability of the specimens |
| Existence of an international proficiency testing program |
| Low cost availability of pharmacological preparation worldwide |
| Proven safety of the low dose injection (5–10 mL) required for the procedure |
GFR, glomerular filtration rate; HPLC-UV, high-performance liquid chromatography with ultraviolet detection; LC-MS/MS, liquid chromatography-tandem mass spectrometry; UPLC-MS/MS, ultra performance liquid chromatography combined with mass spectrometry; UPLC, ultra performance liquid chromatography.
Laboratorians and clinicians should work jointly: choosing the most appropriate GFR procedure in terms of both measurement protocol and analytical method is a clinical decision based on the reliability and accuracy of the results, and convenience for the patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Pierre Delanaye) for the series “Nephrology and clinical chemistry” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.09.07). The series “Nephrology and clinical chemistry” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vart P, Grams ME. Measuring and Assessing Kidney Function. Semin Nephrol 2016;36:262-72. [Crossref] [PubMed]
- Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162133.pdf
- Price M. Comparison of creatinine clearance to inulin clearance in the determination of glomerular filtration rate. J Urol 1972;107:339-40. [Crossref] [PubMed]
- Schulster V, Seldin D. Renal clearance. In: Seldin DW, Giebish G. editors. The Kidney: Physiology and Pathophysiology. New York: Raven Press, 1985.
- Smith HW. The Kidney: Structure and Function in Health and Disease. New York: Oxford University Press Inc., 1951.
- Shannon JA, Smith HW. The excretion of inulin, xylose and urea by normal and phlorizinized man. J Clin Invest 1935;14:393-401. [Crossref] [PubMed]
- Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014;64:411-24. [Crossref] [PubMed]
- Bröchner-Mortensen J, Giese J, Rossing N. Renal inulin clearance versus total plasma clearance of 51Cr-EDTA. Scand J Clin Lab Invest 1969;23:301-5. [Crossref] [PubMed]
- Nosslin B. Determination of clearance and distribution volume with a single injection technique. Acta Medica Scandinavica 1965;442:5.
- Cohen ML. Radionuclide clearance techniques. Semin Nucl Med 1974;4:23-38. [Crossref] [PubMed]
- Chantler C, Garnett ES, Parsons V, et al. Glomerular filtration rate measurement in man by the single injection methods using 51Cr-EDTA. Clin Sci 1969;37:169-80. [PubMed]
- World Health Organization: Report No. 563, 1975.
- Hackstein N, Wiegand C, Langheinrich AC, et al. Measurement of glomerular filtration rate by low-dose iopromide plasma clearance. Acta Radiol 2003;44:162-5. [Crossref] [PubMed]
- Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol 1995;6:257-63. [PubMed]
- Mutzel W, Siefert HM, Speck U. Biochemical-pharmacologic properties of iohexol. Acta Radiol Suppl 1980;362:111-5. [PubMed]
- Aakhus T, Sommerfelt SC, Stormorken H, et al. Tolerance and excretion of iohexol after intravenous injection in healthy volunteers. Preliminary report. Acta Radiol Suppl 1980;362:131-4. [PubMed]
- Aakhus T, Dahlstrom K, Shaw DD, et al. Human pharmacologic trials with iohexol. Acta Radiol Suppl 1983;366:20-2. [PubMed]
- Olsson B, Aulie A, Sveen K, et al. Human pharmacokinetics of iohexol. A new nonionic contrast medium. Invest Radiol 1983;18:177-82. [Crossref] [PubMed]
- Krutzén E, Bäck SE, Nilsson-Ehle I, et al. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med 1984;104:955-61. [PubMed]
- Bröchner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 1972;30:271-4. [Crossref] [PubMed]
- Delanaye P, Ebert N, Melsom T, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 2016;9:682-99. [Crossref] [PubMed]
- Delanaye P, Melsom T, Ebert N, et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016;9:700-4. [Crossref] [PubMed]
- Jodal L, Brochner-Mortensen J. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand J Clin Lab Invest 2009;69:305-13. [Crossref] [PubMed]
- Bröchner-Mortensen J, Jodal L. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part II: Empirically determined relationships between total and one-pool clearance. Scand J Clin Lab Invest 2009;69:314-22. [Crossref] [PubMed]
- Ng DKS, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int 2011;80:423-30. [Crossref] [PubMed]
- Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983;3:297-305. [Crossref] [PubMed]
- Delanaye P, Flamant M, Dubourg L, et al. Single- versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Gaspari F, Guerini E, Perico N, et al. Glomerular filtration rate determined from a single plasma sample after intravenous iohexol injection: is it reliable? J Am Soc Nephrol 1996;7:2689-93. [PubMed]
- Cavalier E, Rozet E, Dubois N, et al. Performance of iohexol determination in serum and urine by HPLC: validation, risk and uncertainty assessment. Clin Chim Acta 2008;396:80-5. [Crossref] [PubMed]
- Farthing D, Sica DA, Fakhry I, et al. Simple HPLC-UV method for determination of iohexol, iothalamate, p-aminohippuric acid and n-acetyl-p-aminohippuric acid in human plasma and urine with ERPF, GFR and ERPF/GFR ratio determination using colorimetric analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2005;826:267-72. [Crossref] [PubMed]
- O'Reilly PH, Brooman PJ, Martin PJ, et al. Accuracy and reproducibility of a new contrast clearance method for the determination of glomerular filtration rate. Br Med J (Clin Res Ed) 1986;293:234-6. [Crossref] [PubMed]
- Vicente FB, Vespa GK, Carrara F, et al. Determination of iohexol in human serum by a semi-automated liquid chromatography tandem mass spectrometry method. Clin Biochem 2015;48:679-85. [Crossref] [PubMed]
- Lee SY, Chun MR, Kim DJ, et al. Determination of iohexol clearance by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci 2006;839:124-9. [Crossref] [PubMed]
- Denis MC, Venne K, Lesiege D, et al. Development and evaluation of a liquid chromatography-mass spectrometry assay and its application for the assessment of renal function. J Chromatogr A 2008;1189:410-6. [Crossref] [PubMed]
- Nyssen L, Delanaye P, Le Goff C, et al. A simple LC-MS method for the determination of iohexol and iothalamate in serum, using ioversol as an internal standard. Clin Chim Acta 2016;463:96-102. [Crossref] [PubMed]
- Annesley TM, Clayton LT. Ultraperformance liquid chromatography-tandem mass spectrometry assay for iohexol in human serum. Clin Chem 2009;55:1196-202. [Crossref] [PubMed]
- Shihabi ZK, Constantinescu MS. Iohexol in serum determined by capillary electrophoresis. Clin Chem 1992;38:2117-20. [PubMed]
- Van Houcke SK, Seaux L, Cavalier E, et al. Determination of iohexol and iothalamate in serum and urine by capillary electrophoresis. Electrophoresis 2016;37:2363-7. [Crossref] [PubMed]
- Kitahashi T, Furuta I. Method development for determining the iohexol in human serum by micellar electrokinetic capillary chromatography. J Pharm Biomed Anal 2004;34:153-8. [Crossref] [PubMed]
- Albert DA, Cohen AJ, Mandelbrot DA, et al. Neutron-activation analysis: a novel method for the assay of iohexol. J Lab Clin Med 2003;141:106-9. [Crossref] [PubMed]
- Iohexol GFR Kits. 2018. Available online: http://www.biopal.com/FIT-GFR.htm
- Schwartz GJ, Wang H, Erway B, et al. Multicenter Laboratory Comparison of Iohexol Measurement. J Appl Lab Med 2018;711-24. [Crossref]
- James TJ, Lewis AV, Tan GD, et al. Validity of simplified protocols to estimate glomerular filtration rate using iohexol clearance. Ann Clin Biochem 2007;44:369-76. [Crossref] [PubMed]
- Schwertner HA, Weld KJ. High-Performance Liquid-Chromatographic Analysis of Plasma Iohexol Concentrations. J Chromatogr Sci 2015;53:1475-80. [Crossref] [PubMed]
- Gaspari F, Thakar S, Carrara F, et al. Safety of Iohexol Administration to Measure Glomerular Filtration Rate in Different Patient Populations: A 25-Year Experience. Nephron 2018;140:1-8. [Crossref] [PubMed]
- Luis-Lima S, Gaspari F, Porrini E, et al. Measurement of glomerular filtration rate: internal and external validations of the iohexol plasma clearance technique by HPLC. Clin Chim Acta 2014;430:84-5. [Crossref] [PubMed]
- Seegmiller JC, Burns BE, Schinstock CA, et al. Discordance Between Iothalamate and Iohexol Urinary Clearances. Am J Kidney Dis 2016;67:49-55. [Crossref] [PubMed]
- Soman RS, Zahir H, Akhlaghi F. Development and validation of an HPLC-UV method for determination of iohexol in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2005;816:339-43. [Crossref] [PubMed]
-
Catalogs Ordering - College of American Pathologists 2018 . Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/2018-surveys-catalog.pdf - Seegmiller JC, Eckfeldt JH, Lieske JC. Challenges in Measuring Glomerular Filtration Rate: A Clinical Laboratory Perspective. Adv Chronic Kidney Dis 2018;25:84-92. [Crossref] [PubMed]
- Delanaye P, Jouret F, Le Goff C, et al. Concordance Between Iothalamate and Iohexol Plasma Clearance. Am J Kidney Dis 2016;68:329-30. [Crossref] [PubMed]
- Krutzen E, Back SE, Nilsson-Ehle P. Determination of glomerular filtration rate using iohexol clearance and capillary sampling. Scand J Clin Lab Invest 1990;50:279-83. [Crossref] [PubMed]
- Niculescu-Duvaz I, D'Mello L, Maan Z, et al. Development of an outpatient finger-prick glomerular filtration rate procedure suitable for epidemiological studies. Kidney Int 2006;69:1272-5. [Crossref] [PubMed]
- Bjornstad P, Karger AB, Maahs DM. Measured GFR in Routine Clinical Practice-The Promise of Dried Blood Spots. Adv Chronic Kidney Dis 2018;25:76-83. [Crossref] [PubMed]
- Salvador CL, Tondel C, Morkrid L, et al. Glomerular filtration rate measured by iohexol clearance: A comparison of venous samples and capillary blood spots. Scand J Clin Lab Invest 2015;75:710-6. [PubMed]
- Maahs DM, Bushman L, Kerr B, et al. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications 2014;28:667-73. [Crossref] [PubMed]
- Luis-Lima S, Gaspari F, Negrin-Mena N, et al. Iohexol plasma clearance simplified by dried blood spot testing. Nephrol Dial Transplant 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Lin L, Hedayat A, Wu W. Statistical tools for measuring agreement. New York: Springer Science & Business Media, 2012.
- Christiansen C. X-ray contrast media--an overview. Toxicology 2005;209:185-7. [Crossref] [PubMed]
- Nilsson-Ehle P. Iohexol clearance for the determination of glomerular filtration rate: 15 years' experience in clinical practice. eJIFCC 2001;13:
- Frennby B, Sterner G, Almen T, et al. The use of iohexol clearance to determine GFR in patients with severe chronic renal failure--a comparison between different clearance techniques. Clin Nephrol 1995;43:35-46. [PubMed]
- Wang E, Meier DJ, Sandoval RM, et al. A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int 2012;81:112-7. [Crossref] [PubMed]
- Rizk DV, Meier D, Sandoval RM, et al. A Novel Method for Rapid Bedside Measurement of GFR. J Am Soc Nephrol 2018;29:1609-13. [Crossref] [PubMed]
Cite this article as: Carrara F, Gaspari F. GFR measured by iohexol: the best choice from a laboratory perspective. J Lab Precis Med 2018;3:77.


