
How can we deal with an unexpected preoperative prolongation of the activated partial thromboplastin time (APTT)? —a real world experience
Introduction
There is now consolidated evidence that results of laboratory testing are integral to the clinical decision making (1). Although this concept is straightforward and legitimate in virtually all branches of modern medicine, it hires an even greater significance for patients with bleeding disorders, wherein an accurate and discretionary use of laboratory resources accomplishes an unreplaceable diagnostic value (2,3).
Preoperative laboratory testing is conventionally defined as performing a panel of laboratory analyses aimed at predicting the risk of perioperative complications, defining the status of coexisting disorders, and detecting unidentified factors or conditions which may ultimately enhance the risk of adverse events (4). Albeit there is still open debate on the number and type of laboratory analyses that shall be included within preoperative test menus (5), the activated partial thromboplastin time (APTT) is frequently part of these panels (6), since it may help identifying a number of possible underlying hemorrhagic conditions that may expose the patient to a significantly enhanced risk of perioperative bleeding, or may else provide valuable information on the presence of prothrombotic conditions necessitating specific prophylaxis and tailored postsurgical management. Whilst significant APTT prolongations may hence be frequently encountered in patients with congenital or acquired deficiencies of clotting factors of the formerly known “intrinsic pathway” [i.e., prekallikrein, kininogen, factor (F) VIII, IX, XI and XII], or with inherited or acquired inhibitors against clotting factors (e.g., acquired haemophilia), abnormal values may also be found when analyzing unsuitable plasma samples, as well as in patients with lupus anticoagulant (LAC) or in treatment with anticoagulant drugs (i.e., heparin, warfarin or direct oral anticoagulants) (7,8). The evidence that some of these conditions are not clear causes of significant perioperative bleeding (e.g., factor FXII deficiency, LAC), but their association with prolonged APTT encountered during preoperative testing may possibly lead to delaying surgical procedure or, in the worst scenario, to the inappropriate administration of replacement therapy, has contributed to raise doubts as to whether including APTT within preoperative test panels may cause more harms then benefits (9-11).
Test ordering according to the Bayesian principle of pre-test probability (12), thus entailing an accurate collection of preoperative clinical history and physical examination, seems the most reasonable strategy not only for optimizing resources (both economic and human) in the laboratory, but also for preventing harm and inconvenience to the patients (13). This article is hence aimed to validate a tentative diagnostic algorithm for investigating patients with unexpected prolongation of APTT, detected during preoperative laboratory testing, and combining clinical and laboratory data.
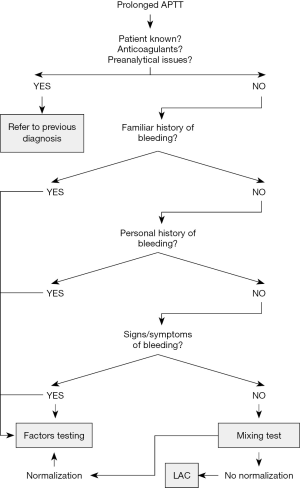
Methods
The study population consisted of all patients undergoing preoperative laboratory testing (thus including APTT) at the Istituto Fiorentino Cura e Assistenza (IFCA, Florence, Italy) before undergoing surgical procedures, during a 3-month period (between March 23rd and June 25th, 2018). In the local laboratory, APTT is performed using HemosIL SynthaSil (Instrumentation Laboratory, Bedford, USA). The local reference range is comprised between 0.89–1.30 in ratio, and between 25.1–36.5 in seconds, respectively. Whenever prolonged APTT results are observed, the abnormal data might then be investigated according to a tentative algorithm. Briefly, APTT results are combined with familiar history of bleeding (yes/no), personal history of bleeding (yes/no) and signs/symptoms of bleeding, eventually followed by results of additional hemostasis tests, including mixing test, LAC and clotting factor assays, as summarized in Figure 1. Regarding follow-up testing, mixing test is performed according to the method described by Rosner et al. (14) using HemosIL SynthaSil (Instrumentation Laboratory, Bedford, USA; normalization defined for values <15%), “intrinsic pathway” clotting factors activity is measured by means of one-stage clotting assays using HemosIL SynthaSil (Instrumentation Laboratory, Bedford, USA) and HemosIL Factor deficient plasmas (Instrumentation Laboratory, Bedford, USA; local reference values: FVIII, 60–150%, FIX, 60–150%, FXI, 65–120%, FXII, 70–130%), whilst LAC testing is carried out with silica APTT (Silica Clotting Time, Instrumentation Laboratory, Bedford, USA; reference values, <1.16 normalized ratio) and dilute Russell viper venom time (dRVVT Screen and dRVVT Confirm, Instrumentation Laboratory, Bedford, USA reference values, <1.16 normalized ratio), as for current recommendations (15). All tests are carried out using ACL TOP 500 (Instrumentation Laboratory, Bedford, USA) and are regularly validated by performance of internal quality control (IQC) procedures and participation to an External Quality Assessment (EQA) scheme. Sensitivity and specificity of the tentative algorithm were calculated according to the final diagnosis (i.e., successful surgery and identification of bleeding or prothrombotic disorders). The statistical analysis was carried out using MedCalc 17.9 (MedCalc Software, Ostend, Belgium).
Results
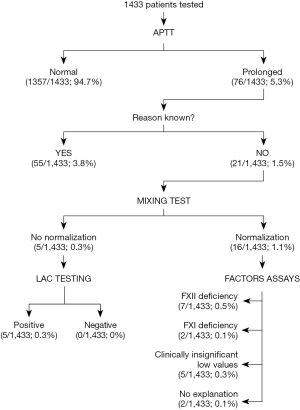
Overall, 1,433 patients underwent preoperative laboratory testing throughout the 3-month study period, 76 (5.3%) of whom were found to have a prolonged APTT value (i.e., >36.5 s). The remaining 1,357 (94.7%) patients underwent successful surgery, without further investigation. According to the algorithm (Figure 1), 55/76 (72.4%) of patients with prolonged APTT were excluded from further laboratory testing because they had already received a previous diagnosis explaining the prolongation or were taking anticoagulant drugs, so that they were considered not worthy enough of additional urgent investigations. In the remaining 21/76 (27.6%) patients, a mixing test was planned, since the prolongation of the APTT could not be otherwise explained. In 5/21 (23.8%) of these patients the mixing test was positive (i.e., no normalization), so that LAC testing was scheduled. In all these five patients the results of LAC testing was positive. In the remaining 16/21 (76.2%) patients, the mixing test was negative (i.e., normalization), so that clotting factors assays were scheduled. The results of these tests are shown in Figure 2. Briefly, 7 patients were finally diagnosed with FXII deficiency (FXII activity comprised between 29% and 68%), two patients with mild FXI deficiency (i.e., FXI activity of 47% and 51%, respectively), whilst 5 patients were found to have clinically insignificant low values of one or more clotting factors and 2 patients had no identifiable explanation for the APTT prolongation. In one patient with FXII deficiency, a LAC positivity of borderline significance could also be detected. Notably, no significant correlation (Spearman’s test) could be observed between residual clotting factor activity in plasma and APTT (r =0.03; P=0.949) in the 9 patients with prolonged APTT due to either FXII or FXI deficiencies. Overall, the sensitivity and specificity of the preoperative algorithm for detecting clinically significant conditions (i.e., FXI deficiencies or LAC) were 1.00 (95% CI, 0.59–1.00) and 0.99 (95% CI, 0.98–0.99), respectively.
Discussion
A prolonged APTT may be caused by a number of clinically significant conditions, such as congenital or acquired clotting factors deficiencies or clotting factors inhibitors, but may also be due to clinically silent conditions, the most frequent of which is indeed FXII deficiency (8). Beside patients presenting with a positive familiar or personal history of bleeding, and for whom second-line testing (i.e., clotting factors assays) is virtually mandatory (16), the investigation of the remaining patients is still a matter of concern.
Performance of mixing test is a cost-effective approach for obtaining rapid and clinically useful information in patients with prolonged APTT (17). Once preanalytical causes of prolongation have been ruled out (18), the normalization of this tests is suggestive for the presence of clotting factor deficiencies, whilst failure to normalize is highly suggestive for the presence of endogenous (i.e., anti-factor antibodies, thus including inhibitors and LAC) or exogenous (i.e., heparin) inhibitors. A protocol (Figure 1) has hence been designed, exactly around this principle, in that the diagnostic strategy will be driven by the familiar or personal clinical history, by the presence of signs/symptoms of bleeding, and by performance of mixing test in patients in whom the APTT prolongation cannot be otherwise explained. Overall, mixing test was hence performed in 21/1,433 (1.5%; i.e., in 27.6% of those with prolonged APTT) (Figure 2). Although this volume of tests will not pose a substantial economic and organization burden on the laboratory, the identification of two patients with modest FXI deficiency (i.e., FXI activity of 47% and 51%) carries important clinical consequences. Patients with FXI values >40% do not usually have enhanced risk of perioperative bleeding, but it has also been demonstrated that some of them may need replacement therapy before undergoing high-risk surgical procedures, especially involving organs with higher fibrinolytic potential (i.e., mouth, nasal cavity or prostate) (19). These patients, who are usually asymptomatic until subjected to procedures at higher risk of haemorrhage, would have not been recognized without performing a preoperative APTT. Interestingly, the lack of correlation observed between APTT and both FXII and FXI values attests that the extent of prolongation of this test may not be a reliable guidance for suspecting either the type or the severity of clotting factor deficiency.
Albeit the clinical usefulness of preoperative APTT for identifying patients at increased risk of perioperative bleeding has hence clearly emerged from our data, the identification of five additional asymptomatic patients with positive LAC testing is another valuable finding. There is now reliable evidence that patients with LAC or antiphospholipids antibodies have a significantly enhanced risk of postoperative thrombosis, especially those undergoing cardiac or vascular surgery (20,21). Specific prophylaxis and tailored postsurgical treatment has hence been advocated in these patients, since this approach may be effective to lower their otherwise increased thromboembolic risk (22).
On the other hand, no clinically significant abnormalities could be detected in the remaining 14 patients undergoing mixing test (Figure 2). Although these may hence be theoretically regarded as “false positive” results, the overall diagnostic sensitivity and specificity of the local algorithm for identifying patients with clinically significant prolongation were both ≥0.99, thus inherently confirming its potential clinical usefulness. Interestingly, in 7 of these patients the final diagnosis was FXII deficiency, which has a well-known impact on APTT prolongation in vitro, but whose clinical significance is most likely meaningless, wherein no significant bleeding can be conventionally observed in patients with even homozygous deficiencies of this factor (23).
Conclusions
Taken together, the results of this study seemingly confirm previous suggestions that a preoperative algorithm including clinical (familial or personal history of bleeding) and laboratory data (results of APTT, mixing test, LAC and clotting factor assays) may be a reasonable and sustainable strategy for identifying patients at enhanced risk of perioperative bleeding or thrombosis (24,25). We also highlight that consideration should be made to include mixing test among the laboratory armamentarium of routine and urgent analyses, since it is a relatively easy and inexpensive test, which may provide rapid and valued clinical information for perioperative patient management.
Acknowledgments
The authors are thankful to the Department of Experimental and Clinical Medicine, University of Florence (Florence, Italy) for providing a kind support for performing lupus anticoagulant and clotting factors testing.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.09.04). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This observational study was entirely based on results of routine analyses performed in the laboratories of the Istituto Fiorentino Cura e Assistenza (IFCA, Florence, Italy), test results did not impact the clinical management and, therefore, ethical approval or patient’s permission to review patient data were unnecessary. The study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and under the terms of all relevant local legislations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Plebani M, Favaloro EJ. The Model List of Essential In Vitro Diagnostics: nuisance or opportunity? Diagnosis (Berl) 2018. [Epub ahead of print].
- Lippi G, Franchini M, Favaloro EJ. Diagnostics of Inherited Bleeding Disorders of Secondary Hemostasis: An Easy Guide for Routine Clinical Laboratories. Semin Thromb Hemost 2016;42:471-7. [Crossref] [PubMed]
- Bonar RA, Lippi G, Favaloro EJ. Overview of Hemostasis and Thrombosis and Contribution of Laboratory Testing to Diagnosis and Management of Hemostasis and Thrombosis Disorders. Methods Mol Biol 2017;1646:3-27. [Crossref] [PubMed]
- Martin SK, Cifu AS. Routine Preoperative Laboratory Tests for Elective Surgery. JAMA 2017;318:567-68. [Crossref] [PubMed]
- Bock M, Fritsch G, Hepner DL. Preoperative Laboratory Testing. Anesthesiol Clin 2016;34:43-58. [Crossref] [PubMed]
- Lippi G, Montagnana M, Mattiuzzi C, et al. Preoperative laboratory testing. Minerva Med 2005;96:397-407. [PubMed]
- Poli G, Castiglioni P, Montagnana M, et al. Troubleshooting an isolate prolongation of activated partial thromboplastin time in a patient with acute myocardial infarction-a paradigmatic case report. Ann Transl Med 2016;4:426. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Activated partial thromboplastin time: new tricks for an old dogma. Semin Thromb Hemost 2008;34:604-11. [Crossref] [PubMed]
- Lippi G, Franchini M, Brazzarola P, et al. Preoperative screening: the rationale of measuring APTT in risk assessment. Haematologica 2001;86:328. [PubMed]
- Dembitzer FR, Suarez Y, Aledort LM. Screening coagulation testing using the APTT: which reagent to choose? Am J Hematol 2010;85:726. [Crossref] [PubMed]
- Levy JH, Szlam F, Wolberg AS, et al. Clinical use of the activated partial thromboplastin time and prothrombin time for screening: a review of the literature and current guidelines for testing. Clin Lab Med 2014;34:453-77. [Crossref] [PubMed]
- Cervellin G, Borghi L, Lippi G. Do clinicians decide relying primarily on Bayesians principles or on Gestalt perception? Some pearls and pitfalls of Gestalt perception in medicine. Intern Emerg Med 2014;9:513-9. [Crossref] [PubMed]
- Lippi G, Cervellin G. From laboratory instrumentation to physician’s brain calibration: the next frontier for improving diagnostic accuracy? J Lab Precis Med 2017;2:74. [Crossref]
- Rosner E, Pauzner R, Lusky A, et al. Detection and quantitative evaluation of lupus circulating anticoagulant activity. Thromb Haemost 1987;57:144-7. [PubMed]
- Favaloro EJ. Variability and diagnostic utility of antiphospholipid antibodies including lupus anticoagulants. Int J Lab Hematol 2013;35:269-74. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med 2018;56:1035-45. [Crossref] [PubMed]
- Kershaw G, Orellana D. Mixing tests: diagnostic aides in the investigation of prolonged prothrombin times and activated partial thromboplastin times. Semin Thromb Hemost 2013;39:283-90. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Preanalytical Issues in Hemostasis and Thrombosis Testing. Methods Mol Biol 2017;1646:29-42. [Crossref] [PubMed]
- Wheeler AP, Gailani D. Why factor XI deficiency is a clinical concern. Expert Rev Hematol 2016;9:629-37. [Crossref] [PubMed]
- Ahn SS, Kalunian K, Rosove M, et al. Postoperative thrombotic complications in patients with lupus anticoagulant: increased risk after vascular procedures. J Vasc Surg 1988;7:749-56. [Crossref] [PubMed]
- Massoudy P, Cetin SM, Thielmann M, et al. Antiphospholipid syndrome in cardiac surgery-an underestimated coagulation disorder? Eur J Cardiothorac Surg 2005;28:133-7. [Crossref] [PubMed]
- Agaba AE, Charaklias N, Babu-Victor A, et al. Antiphospholipid syndrome: a series of surgical emergencies and the current evidence for its management. Ann R Coll Surg Engl 2006;88:370-4. [Crossref] [PubMed]
- Danese E, Montagnana M, Lippi G. Factor XII in Hemostasis and Thrombosis: Active Player or (Innocent) Bystander? Semin Thromb Hemost 2016;42:682-8. [Crossref] [PubMed]
- Capoor MN, Stonemetz JL, Baird JC, et al. Prothrombin Time and Activated Partial Thromboplastin Time Testing: A Comparative Effectiveness Study in a Million-Patient Sample. PLoS One 2015;10:e0133317 [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Hemostasis practice: state-of-the-art. J Lab Precis Med 2018;3:65. [Crossref]
Cite this article as: Balboni F, Lippi G. How can we deal with an unexpected preoperative prolongation of the activated partial thromboplastin time (APTT)? —a real world experience. J Lab Precis Med 2018;3:79.


