Diabetoporosity—diabetes and the bone
Introduction
The coincidence of diabetes mellitus type 2 (T2DM) and osteoporosis in the elderly was overlooked for a long time, whereby the prevalence in both diseases increases with age (1). Only recently, direct links have been revealed for many shared pathophysiological features, risk factors, and consequences such as an increased fracture risk.
The risk for bone fractures is at least two times higher in T2DM patients (2), where conventional bone mineral density (BMD) measurements by dual energy bone densitometry (DXA) might show normal or even increased planar BMD. This coincidence shows a very low awareness of bone fracture risk in T2DM patients, with the need for additional diagnostic procedures to cover an individual early risk detection. The topic is if ascending importance, as the numbers of T2DM patients are constantly rising. The International Diabetes Federation estimates an increase by 55% until 2040, with a current number of 415 million T2DM patients worldwide (3).
However, fracture risk is not restricted to T2DM patients. Bone fractures occur even more frequently in patients with diabetes mellitus type 1 (T1DM), where patients may express an up to 12-fold risk for severe bone fractures and concomitant sequelae including delayed healing and post-surgical complications, such as chronical wound infections (4). In addition, both T1DM and T2DM patients tend to have worse fracture outcomes than patients with normal glucose control. The risk for mortality in both T1DM and T2DM patients after a major fracture (e.g., hip fracture) is nearly 50% increased as compared with hip fracture patients without diabetes including a higher risk of postoperative cardiac events and prolonged hospitalization by 1–4 days (5).
As T1DM shows earlier disease onset in general, higher fracture risk is already seen during childhood and persists later in life, in both women and men. Whereas BMD has been found to be slightly decreased in most T1DM patients, the contrary is the case in most T2DM patients. However, BMD does not always reflect fracture risk in the case of diabetic patients. Other factors have therefore to be addressed to understand the underlying pathophysiological mechanisms. One of the most surprising recent discoveries was the involvement of the microbiome in bone properties. Microbiome-associated inflammatory and autoimmune changes e.g., in diabetes mellitus are regarded as additional factors for low BMD and draw a link between gut microbiota and the bone (6).
In addition, diabetes medication per se has been shown to interfere with bone and glucose metabolism. The most prominent example are thiazolidinediones (TZDs), high-affinity ligands and activators of peroxisome proliferator–activated receptor G, which have been connected to increase bone resorption and bone marrow fat and thus to exalt fracture risk which led to their withdrawal from the market (4). However, other drugs have been demonstrated to be beneficial for bone remodelling, such as metformin via the activation of osteoblast differentiation.
As bone fractures and their sequelae are the central outcome of diabetoporosity, this review will focus on bone metabolism relevant for an increased fracture risk in these patients based on decreased bone mass and properties in T1DM and impaired skeletal properties despite preserved BMD in T2DM, aggravated by comorbidities such as macro- and microvascular alterations including nephropathy, angiopathy and neuropathic changes.
We aim to document current knowledge about the involvement of bone in glucose and energy metabolism, with reference to the articles by Carlo Foresta (“OCN and fertility—male and female aspects”), Valentina Biasin (“Sclerostin, BMP, WNT and the lung”) and Ines Foessl (“miRNAs and bone—future of bone biomarkers”) as well as Maria Carolina Gomez (“Bone and the microbiome—a new dimension”) and Martina Rauner (“OPG and RANKL—osteoimmunology”) in this issue. All the more, these links strengthen the complex relation of glucose and energy metabolism and the bone system, which opens new insights in these important interactions.
Contribution of diabetes mellitus type 1 in bone pathophysiology
Many factors contribute to fracture risk, which are well known and defined: lower BMD, older age, female sex, and the use of glucocorticoids—all of these factors are main predictors for bone fractures in patients with T1DM and T2DM (4). However, there are additional specific factors in diabetes mellitus, which have drawn a lot of attention in recent years.
Mechanisms of reduced bone strength in diabetes mellitus type 1
To date, there is no final picture of factors influencing bone strength and the increased occurrence of bone fractures in T1DM. Contributors for skeletal homeostasis in healthy individuals, such as lifestyle and nutrition, physical activity, as well as genetic and epigenetic factors are relevant in T1DM patients, too (7). Of note, the age at disease manifestation, the overall duration and concomitant diseases, such as macro- and microvascular complications, nephro- and neuropathy as well as hypogonadism or thyroid problems might heavily aggravate the disposition to diabetoporosity and fractures (8).
In children with T1DM, the achievement of peak bone mass might be impaired. DXA-derived BMD has been reported to be 0.5–1.0 standard deviations (SD) lower as compared to healthy children of the same age and Tanner’ development. Bone volume and geometry show smaller dimensions with negative consequences during growth and less resistance during loading and falls (8). These phenomena are likely to be linked to the reduced rate of insulin and a decreased growth hormone/insulin-like growth factor 1 (IGF-1) relation during childhood, impairing bone formation inT1DM prodromes and manifestation. Later in life, deficits in bone size seem to be resolved, but effects in crucial phases in growth—the “vulnerable phase”—might be translated in later fracture risk (4).
In adult patients with T1DM, DXA-derived BMD measurements show a 0.5–1.0 SD lower z-score as compared with healthy adults. However, this moderate BMD decrease might not be entirely responsible for the increased fracture risk. Aspects of bone quality including complex interactions with glucose and bone cell homeostasis via growth factors, hormones, accumulation of advanced glycation end products (AGEs) in bone collagen, vitamin D and calcium deficits as well as nutritional problems via microvascular impairment have to be taken into account.
Trabecular bone score (TBS) based on DXA and imaging data derived from high resolution peripheral quantitative computed tomography (HR-pQCT) will be discussed in further detail below—they indicate a structural deficit in bone microarchitecture in T1DM.
Bone cells and tissue in diabetes mellitus
In vitro experiments showed several significant alterations of bone formation in hyperglycaemia. Sclerostin expression in osteocytes was found to be increased and osteoclast activity was decreased by AGEs via the reduction of RANKL expression. These changes may cause low bone turnover in T1DM (and T2DM). High glucose and AGEs are therefore strong candidates to cause osteocyte apoptosis and the impairment of cortical bone (9). Pre-treatment with parathyroid hormone (PTH) reversed these effects and improved osteocyte function.
Glucose has been shown as the main nutrient of osteoblasts. The expression of the glucose transporter GLUT1, key factor of glucose uptake in osteoblasts, precedes the expression of runt-related transcription factor 2 [RUNX2, also known as core-binding factor subunit alpha-1 (CBF-alpha-1)], which is the earliest marker of osteoblast differentiation (10). In in-vitro experiments, there was almost no osteoblast differentiation via Runx2 in the absence of physiological glucose levels, whereas hyperglycaemia restored collagen synthesis in Runx2-null osteoblasts. Interestingly, Runx2 favours Glut1 expression in a feed-forward loop and therefore determines the onset of osteoblast differentiation during development and the extent of bone formation throughout life, again closely linking bone and glucose metabolism (10).
In animal models of T1DM, such as the commonly used NOD mice, BioBreeding diabetes prone rats and streptozotocin-treated rats and mice (4), there is a well-known reduction in trabecular and cortical bone mass, bone formation rate, and bone turnover as compared to controls or insulin-treated animals. Again, AGEs have been found to be accumulated in bone collagen, which might explain impaired bone quality.
In humans, histomorphometric studies of bone biopsies are scarce. In T1DM patients with prevalent fractures, similar structural changes in bone have been found in dynamic histomorphometry. Again, AGEs like pentosidine were accumulated in the bone tissue. Higher levels of mineralization were associated with a reduced modulus of elasticity and less flexibility of bone matrix (4). A generally lower level of bone formation markers and frequent vitamin D deficits will be discussed below (11). However, there were no such differences between healthy controls and T1DM patients without microvascular complications, shifting the focus to vascular influences as very important factors for bone damage during unfavourable glucose control (12).
Bone vessels in diabetes mellitus type 1
Vasculature involvement in bone is not only important for bone growth, but also for the constant remodelling, and fracture healing, as bone tissue and bone marrow receive up to 10% of cardiac output (13). Haemodynamic and oxygen changes, energy metabolism, the exchange of e.g., osteoblast progenitors and stem cells (pericytes) and the removal of metabolic waste are closely linked to bone formation and resorption via a complex system of sinusoid and classic capillaries. T1DM, as well as T2DM, can impair vasodilation and angiogenesis, and favour vascular calcification in bone tissue. Even there are no human studies available to date to proof it, a reduction in bone and bone marrow blood flow and a reduction in vascularisation is thought to be detrimental to osteoblast progenitor niches and bone remodelling activity therefore counting responsible for decreased bone properties and fracture healing.
As the hip is a particular target site of fractures in T1DM, this may be related to the specific vascularisation at the femoral head and macrovascular deficits. Magnetic resonance imaging (MRI) studies in female T1DM patients with an onset of the disease in childhood revealed microvasculopathy and deteriorated trabecular microarchitecture at the tibia (14). In turn, microvascular complications might not only be directly important for bone tissue growth and regeneration, but also indirectly for retinopathy and neuropathological changes, provoking gait abnormalities and falls in patients with diabetes mellitus, thus leading to an even higher fracture risk.
Multifactorial background of bone alterations in diabetes mellitus type 2
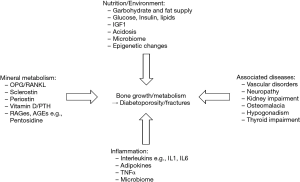
Besides the well-known risk factors for osteoporosis, patients with T2DM and fractures present with a longer T2DM duration and an unfavourable glucose profile (4). In a USA T2DM cohort, patients with a baseline HbA1c >8% had a 1.63 (95% CI, 1.09–2.44) higher risk for any fracture than patients with better glycaemic control. As discussed above, microvascular complications, stroke and cardiovascular disease are additional risk factors for fracture, but study data are sparse (15). Further secondary complications including nephropathy, neuropathy, and retinopathy aggravate the problem (16) (Figure 1).
Falls are a main factor for the majority of fractures in older adults, often after a relatively modest trauma. In patients with T2DM, fall risk was higher [hazard ratio (HR) 1.19; 95% CI, 1.08–1.31] than in controls—interestingly, the fall rate was even higher in insulin-treated T2DM patients. However, the higher risk for falling alone does not fully account for the increased fracture risk in T2DM patients (2).
Hyperinsulinemia and hyperglycaemia have been shown to affect bone remodelling directly and indirectly. Via insulin receptors in all bone cells, interaction of insulin signaling and bone metabolism has been described in many studies, whereby increased insulin signaling enhances bone turnover, but insulin resistance lowers bone remodelling. The term of “insulin resistance of bone” with its manifestation in decreased bone metabolism has been widely discussed (17).
In close relation to impaired microvascular and neurotrophic functions, the concept of chronic low-grade inflammation in T2DM is well established (18). Furthermore, a number of immune mediated inflammatory processes is well known during the development of T1DM (19). As a potential impact of chronic inflammation and abnormal glucose homeostasis on peak bone mass has been documented and, later in life, an influence of chronic inflammation via T2DM and metabolic sequelae is frequently present, the large emerging field of osteoimmunology is discussed in this issue by Martina Rauner (“OPG and RANKL—osteoimmunology”).
The special relationship of bone and fat tissue in diabetes mellitus type 2
A special relationship has been detected quite recently between bone and adipose tissue, with direct consequences on glucose and lipid metabolism and insulin sensitivity. While increased body weight per se has been seen as beneficial for bone properties via mechanical loading (the impact of weight on bone cells and material) and potential hormonal interferences, adipose tissue may negatively influence bone health (20). However, its associations with bone tissue depend on age, sex, menopausal status, adipose depot, and bone compartment.
In T2DM, an increase in visceral adipose tissue is associated with pro-inflammatory and metabolic changes and a negative effect on bone structure and quality. Animal models of T2DM developed more bone marrow fat which correlated positively with fracture risk. As marrow adipose tissue (MAT) accumulates in long bones and vertebrae forming up to 10% of total body fat, volumetric changes may contribute to frail bones. Interestingly, MAT shares some characteristics with beige and white fat with the potential of the generation of favourable anabolic factors, but these properties are also attenuated in T2DM (21).
Indirectly, fat infiltration in muscles and a blunted motoric performance together with retinal and neurological impairment might additionally account for fall risk in elderly T2DM patients (4).
Spectrum of diagnostic procedures of bone in patients with diabetes mellitus
Imaging is the initial diagnostic procedure in bone-related diseases in most cases. Whereas X-ray images of fractures have been done for almost a century and have been the only approach to bone diagnostics for decades, specific techniques have been developed and focussed on bone mineral density (BMD) and structure, as follows.
Dual energy X-ray absorptiometry (DXA)
Though having become a routine method to address BMD almost everywhere, increased fracture risk in T2DM patients is not adequately addressed by DXA due to preserved or even increased BMD. High Z-scores are common in these patients, as reported by a recent meta-analysis showing mean z-scores of 0.41 at the spine and 0.27 at the hip, mostly associated with obesity (22).
While the influence of an increased body mass index (BMI) on bone properties via mechanical loading is evident, bone loss over time might be increased, at least at the hip and at least in postmenopausal women (2). However, obesity is not only important as a weight-bearing component, but also in terms of tissue interaction, discussed below.
Patients with T1DM tend to have lower BMD values than healthy persons of the same age, starting very early during the disease and deteriorating both peak bone mass and bone mass later in life (1).
Due to the lack of diagnostic accuracy for diabetoporosity and concomitant fracture risk, additional BMD-associated techniques have been developed.
The use of risk assessment scores like the “Fracture Risk Assessment Tool” (FRAX) is limited due to the lack of large datasets and potential parameters to be included in the algorithm. Since all of these parameters do not fully capture the underlying pathophysiologic conditions, these scores might systematically underestimate the risk of fractures in T2DM and should therefore be used with prudence.
Trabecular bone score (TBS)
This score is a measure of gray-level bone texture extracted from planar lumbar DXA images correlating with bone microarchitecture, as proposed in 2008. Contrarily to BMD, TBS measurements show lower values in individual T2DM patients and might therefore be a useful tool to mark patients at risk for fractures due to diabetoporosity in clinical practice (23). This is of particular interest, since glycaemic indices have been shown to be associated with TBS, with worse outcomes in patients with poor glycaemic control.
As a BMD-independent predictor of fractures, TBS has shown an equal prediction in patients with and without diabetes (HR 1.27; 95% CI, 1.10–1.46 and HR 1.31; 95% CI, 1.24–1.38, respectively). Therefore, the discriminatory power might be less than expected and T2DM itself remains an independent risk factor for fractures even after adjustment for BMD and TBS (23). The value and cut-offs for fracture prediction are currently investigated in larger cohorts in view of new DXA devices with better resolution properties. As a possible solution, it has been suggested to apply a combination of low lumber TBS and low BMD at the femoral neck to be of interest for vertebral fracture prediction, but this remains probably restricted to T2DM patients with sufficient glucose control (24).
In T1DM patients, TBS values have shown to be lower in a subgroup of patients with prevalent fractures. As a high-risk group for additional fractures, this might reflect severely damaged bone structures. Again, specific TBS cut-offs for the prediction of fracture risk in T1DM have to be determined prospectively in larger groups of unselected T1DM patients (25).
High resolution peripheral HRpQCT
Three-dimensional (3D) techniques such as magnetic resonance imaging (MRI) using high-resolution 3D images with a quantification of bone structure correlating to computed tomography data have been proposed (14). However, the development and access to these techniques is limited; alternatives include 3D computed tomography: HRpQCT is the peripheral application of quantitative computed tomography (pQCT), used for three-dimensional measurements, i.e., volumetric BMD in arms and/or legs and several additional bone parameters such as geometry and structure of the bone (26).
By measuring HRpQCT parameters, patients with T1DM and excellent glycaemic control were not significantly different from healthy controls of the same age, but T1DM patients with retinopathy—as a frequent microvascular complication—tended to have decreased total and trabecular volumetric BMD and a number of microarchitectural abnormalities, such as lower trabecular thickness and calculated bone strength and larger trabecular separation as well as increased network inhomogeneities (27).
A decrease of bone quality and strength in T2DM has been reported by several studies (2), demonstrating a markedly increased cortical porosity compared to healthy controls. Using HRpQCT, severe deficits in cortical bone, favouring diabetoporosity and fractures have been reported. Intracortical pore volume and relative porosity have been shown to be increased in elderly T2DM f women with fractures by 95.4%, as compared to women with T2DM alone, relative porosity was +87.9%, and endocortical bone surface +11.5%. At the distal radius, these patients had an about 5-fold greater relative porosity than non-fracture T2DM patients. Severe deficits in stiffness, loading parameters and cortical load fraction, not assessed by DXA, support the increased fracture risk in T2DM (28). Interestingly, skeletal hypertrophy found in T2DM was present in both genders, but may be accelerated at the tibial cortex in women (29). Why a low bone turnover should be cause or consequence of cortical porosity, remains to be elucidated.
Bone biopsies and histomorphometry
Static and dynamic quantitative histomorphometry, being the gold standard of bone turnover quantification is based on bone biopsies at the iliac crest, best after tetracycline double labelling of bone mineralisation. There are a few studies with limited patients numbers showing decreased bone turnover rates [bone formation rate divided by the surface referent (BFR/BS)] by 70–80% at all bone surfaces (30). Low bone formation as measured by mineral apposition and formation rate and mineralization lag time has been found in several of these studies, whereas results for bone resorption are less clear. However, the surface erosion and the osteoclast number per surface unit was not different. However, bone biopsies are restricted to special clinical centres and patients and less useful as routine methods, even though best representing bone dynamics.
Microindentation
In addition to bone density and microarchitecture, material properties are major factors of bone quality. As a research tool, “microindentation” of bone cortex is measured by a device, creating microscopical indents at the tibial bone following local anaesthesia and measuring in vivo bone material strength index (BMSi) (31). Postmenopausal women with T2DM had significantly reduced BMSi in comparison to healthy controls. Furthermore, BMSi was inversely correlated with HbA1c levels, again relating glycaemic control to bone properties, probably via altered mineralisation or collagen damage.
Laboratory measurements
Bone turnover markers (BTM)
Over the past years, there is an increasing use of biochemical parameters of bone metabolism for diagnostic and monitoring purposes (32). In almost all studies on diabetes mellitus and BTMs, decreased bone formation has been reflected by low formation markers, both in T2DM and T1DM. Important BTMs for bone formation are procollagen type I N-terminal propeptide (PINP) and osteocalcin (OC), for bone resorption c-telopeptide (CTX) and tartrate-resistant acid phosphatase 5b (TRAcP5b) are widely used. Others, like bone-specific alkaline phosphatase (BALP) and N-terminal telopeptide (NTX) in urine may be found within the upper part of the reference range (33). There is especially OC to be consistently decreased in T2DM patients (34), which might show further interactions of bone and other tissues and could be added to a bone-specific monitoring in T2DM management (35).
OC is inversely related to glycaemic control, fasting insulin concentrations and HbA1c. Vice-versa, OC might stimulate insulin secretion, which could be interpreted as a positive effect of OC on glucose metabolism. Higher concentrations of OC were also associated with improved glucose tolerance (35). On the bone side, a decrease in OC has been associated with a higher number of vertebral fractures (11). Independently of BMD values, a decreased ratio of OC to BALP has been shown to indicate higher fracture risk. Several subforms of OC, such as the uncarboxylated OC have been associated with diabetes mellitus and might have different effects in the regulation of energy homeostasis and bone metabolism (36). Undercarboxylated OC (ucOC) has been shown to act as an active hormone mediating glucose control in rodent experiments (37). Supported by G protein-coupled receptors, e.g., GPRC6A (class C, group 6, member A), ucOC is bound at the cell surface receptor, preferably in muscles where exercise may be involved in metabolic control via ucOC. While ucOC increases the uptake of glucose in muscles in mice and in vitro, exercise increases ucOC and insulin sensitivity in vivo. In addition, ucOC increases muscle mass and muscle cell proliferation in aged rodents. Data from human cohorts have been conflicting, involving also carboxylated OC (cOC) (38) in metabolic association and a potentially gender-associated physiology (39-41). However, OC and especially its subforms and several other new markers are not only potential biomarker candidates for osteoporosis and diabetes in future but might also have some therapeutic implications and might change again our view of bone tissue as an active endocrine organ.
The large individual variations in T2DM studies have not favoured the use of BTMs in diabetes and bone monitoring in general. Another limitation is a reduction in enzymatic cross-links found in animal compared to human studies that may lead to an underestimation of bone resorption (e.g., CTX) in diabetes patients (42). In turn, alterations of enzymatic and probably non-enzymatic crosslinking in the bone matrix might add to the increased fracture risk in these patients.
New bone biomarkers with relevance to diabetes mellitus
Several new protein markers have been proposed to support diagnosis and fracture prediction in diabetes patients. For research purposes, protein content of bone tissue has revealed several candidate markers. Pentosidine, the most abundant AGE in tissue was higher in non-diabetic patients with hip fracture and has been associated with bone vertebral strength independently of BMD (43).
In serum, pentosidine, other AGEs, and soluble receptors for advanced glycation end products (sRAGE) have been associated with an increased risk of vertebral fracture in T2DM patients (44), while urinary pentosidine was associated with another fracture risk pattern. By contrast, higher systemic levels of endogenous secretory RAGE (esRAGE) were associated with a lower risk of vertebral fractures, independently of BMD (45).
Osteoprotegerin (OPG) has been mentioned as an independent risk factor for cardiovascular events in the STRAMBO study in older men with and without T2DM, and higher systemic OPG levels have been shown as additional independent fracture risk predictors after adjustment for T2DM (46). The clinical validity of OPG measurements, however, has been reported to be limited (35).
Serum sclerostin, an inhibitor of the Wnt/β-catenin pathway and thus bone formation inhibitor, was found to be significantly increased in T2DM patients (47) with a positive association to bone fractures. However, the role of sclerostin in mediating impaired bone formation in T2DM remains to be established. Sclerostin levels have also been related to fracture risk in T1DM, again, patients with high sclerostin had an 81% increased fracture risk compared to the lowest tertile (48). However, high sclerostin levels are also associated with physical activity and age, it is therefore an open question, if a systemic increase in sclerostin might reflect either bone cell dysfunction and/or vascular problems, common in long-standing diabetes mellitus (28). It remains unclear to date, whether an increasing hyperinsulinemia e.g., in pre-diabetes and T2DM can affect the regulation of systemic sclerostin values (49). On the other hand, both hyperglycaemia and increased AGEs might each directly stimulate sclerostin production and release in osteocytes. In T2DM patients with atherosclerotic lesions, circulating sclerostin has been reported to be elevated, which may point to sclerostin as an important mediator in Wnt signalling in vascular remodelling (50).
A number of cartilage markers in serum have been evaluated over the past decades. Whether they contribute to the manifestation of diabetoporosity in T1DM or T2DM remains to be explored in larger clinical studies (51).
miRNAs as new biomarkers of bone metabolism
MicroRNAs (miRNAs) are small RNAs, secreted into blood and found in the circulation in exosomes, but also bound to proteins and free (52). Their origin is thought to parallel tissue changes and diseases. As a number of them have been shown to be important in T2DM and T1DM, the question arose, if they might play a role in bone metabolism in diabetes patients at risk for bone fractures.
In fact, several miRNAs have been found to be altered in diabetoporosity with complex links to bone growth and adipogenesis with the following examples: miR-550a-5p, miR-188-3p, and miR-382-3p (53). Based on in-vitro experiments, miR-382-3p increased osteogenic cell differentiation, with a competitive inhibition of bone growth by miR-550a-5p, while none of these candidate miRNAs was significantly altered in cell proliferation in general. miR-550a-5p, and miR-382-3p impaired adipogenic differentiation, but there was no effect of miR-188-3p on adipogenesis (54).
In further studies on prospective hip fractures in elderly patients with and without T2DM, we were able to identify a number of new small, non-coding RNAs. Many of them have links to glucose metabolism, already known from in-vitro studies. Replication studies and further identification of target miRNAs and their involvement in bone and energy metabolism is ongoing.
Therapy options in osteoporosis and diabetes mellitus
Effects of diabetes medication on bone
Traditional antidiabetic medication includes insulin in T1DM and a number of therapy options such as biguanides, glucagon-like peptide 1 (GLP-1) analogs (55), dipeptidyl peptidase 4 (DPP4) inhibitors (56) and more recently sodium-glucose cotransporter 2 inhibitors (SGLT2) in T2DM (57). As bone is involved in energy metabolism it can therefore be a target for certain antidiabetic medications. To date, there is no evidence for negative effects on bone of these currently used antidiabetic therapies; in fact, some of these therapies may even be protective against fractures (57). For example, metformin has been shown to activate osteoblast differentiation via Runx2 and the AMPK/USF-1/SHP pathway resulting in a neutral to protective effect on diabetoporosity (58). Furthermore, Glucose-like-peptide 1 (GLP-1) receptor agonists such as exenatide or liraglutide have also been shown to have positive effects on the skeleton, e.g., bone quality and strength (55). While their mechanisms are not entirely clear, an increase of blood supply to bone via bone vessels has been postulated.
In the past, TZDs, high-affinity ligands and activators of peroxisome proliferator-activated receptor G, that target hematopoietic and mesenchymal cells in the bone marrow provoked an increased bone resorption while bone formation remained low with a significant bone loss and consecutive increase of MAT in the bones (58). The negative effect of TZDs was mediated via decreased activity of osteoblast-specific transcription factors (e.g., Runx2, Dlx5, osterix) and changed signaling pathways (e.g., Wnt, TGF-β/BMP, IGF-1) (57). The resulting 2-fold increased fracture risk in women (not in men), along with prolonged treatment duration led to the withdrawal of rosiglitazone or pioglitazone for most of the T2DM patients.
These experiences were the reason for a very careful observation and monitoring of the novel SGLT2 inhibitors by international authorities. Recent studies have described potential alterations in calcium and phosphate homeostasis (e.g., secondary hyperparathyroidism by increased phosphate reabsorption in the kidneys) or more direct effects on bone remodelling with a potential increase in fracture risk (57). By contrast, very recent studies for bone changes during empagliflozin therapy did not show an increased risk of fractures in a pooled analysis of more than 12,000 patients over 4 years (59).
Vice versa, there are only few data about the effects of osteoporosis medication, such as bisphosphonates, denosumab, teriparatide, and others, on glucose control in patients with T2DM and even more scarce data on such effects in healthy patients. Apparently, most of the studies show a neutral interaction of bone and glucose metabolism or in the case of bisphosphonates a potential decrease in new-onset diabetes mellitus (58).
Prophylaxis of bone fractures in patients with diabetes mellitus
There is a broad range of nutritional and lifestyle interventions to reduce fracture risk in diabetic patients. The first goal is stable glycaemic control, as recommended by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) consensus guidelines, suggesting a general HbA1c target of <7% (58), with a personalized approach to therapeutic target control. However, patients with T2DM and osteoporosis with longer duration of T2DM, with clinically recorded cardiovascular complications, or frequent hypoglycaemic phases, should accept a higher HbA1c target of 7.5% to 8% to avoid hypoglycaemia episodes and falls not to further increase their risk of fractures. In these patients, blood pressure, vision and neuropathy have to be checked and controlled constantly.
In addition, an appropriate supply of calcium and vitamin D has to be provided in all diabetic patients with selected supplementations in case of either vitamin D or calcium deficiency (58). While there was a discussion on calcium supplements contributing to vascular calcification in recent years, there was no evidence of increased calcified plaques or atherosclerosis in T2DM (60).
Weight-bearing exercise starting early in childhood in T1DM patients and physical activity in elderly T2DM patients are appropriate. This might also prevent weight-loss associated bone loss via preserved muscle mass.
Fracture risk can also be reduced via the control of diabetes-associated consequences such as diabetic microvascular complications—e.g., neuropathy, nephropathy, and retinopathy. The preservation of good glycaemic control with HbA1c values <8% has been shown to reduce fracture and complication risk. Of note, there was no difference in fracture incidence between intensive glycaemic control (HbA1c 6.4%) versus standard control (HbA1c 7.5%) and also no difference in intensive versus standard blood pressure control in randomized trials (2). However, significant hypoglycaemia has been associated with increased fracture risk in T1DM and T2DM, possibly related to increased fall rates in the elderly population (4).
Based on new evidence of an involvement of gut microbiota in bone quality and strength (61), animal experiments have recently demonstrated that germ-free mice show a protection from osteoporosis-like bone decrease in estrogen deficiency. Short-term colonization of these mice with gut microbiota led to an increase of gut permeability and an increase in pro-inflammatory cytokines in gut and bone, thus activating bone resorption (6). Intervention studies with probiotic supplementation in humans are scarce, with rather small sample sizes. Despite rather short time periods of intervention, they already showed a decrease in markers of bone resorption and pro-inflammatory cytokines. In a recent study with longer duration, 90 elderly women with low BMD were treated with a probiotic containing Lactobacillus reuteri strains. Bone loss (total vertebral BMD) was significantly reduced without major adverse effects over 12 months compared to placebo. Whether probiotic intervention could also be applied in patients with diabetoporosity remains to be explored (62).
Bone-specific medication in diabetes mellitus
Low bone turnover has raised discussions about antiresorptive medication in T1DM and T2DM patients at elevated risk for fractures. Registry and clinical trial data have shown that bisphosphonates are effective in fracture protection in these patients (43). In the absence of strong evidence against them, bisphosphonates such as alendronate [e.g., in the Fracture Intervention Trial (FIT)] may remain the first treatment option. Another antiresorptive, denosumab may be a preferred choice in elderly patients with a declining renal function.
Other medications, such as raloxifene also reduced the risk of (mainly) vertebral fractures in T2DM patients at the same proportion as in non-diabetics [92].
However, the use and potential benefit of anti-resorptive drugs in T2DM patients remains unproven and of potential concern. In this context, teriparatide (a bioactive part of PTH) as an osteoanabolic agent, and in the future potentially abaloparatide [a PHT-related protein (PTHrP) analogue] or romosozumab (an anti-sclerostin antibody), both currently not available for routine care, present a potential interest as they have been proven very successful in animal studies.
Conclusions and outlook
Patients with T1DM and T2DM show decreased bone turnover and a number of pathophysiological conditions arising from interactions of bone and energy metabolism. They are at increased risk of bone fractures. But the pathophysiology of impaired bone in these patients is not entirely clear and has multifactorial sources.
The need of future diagnostic tools, biomarkers and targeted medication is of utmost interest to several areas of medical care, since several hundred million patients worldwide have to be considered and prevented from bone fractures. In turn, there is an increasing body of scientific knowledge on the complex pathophysiology of diabetoporosity which might help to understand interactions beyond the links of bone and energy metabolism—to estimate fracture risk, and to set effective strategies for the reduction of fracture risk in patients with diabetes.
Acknowledgments
This work was partly supported by Center for Biomarker Research in Medicine (CBmed), funded by the Austrian Federal Government within the COMET K1 Centre Program, Land Steiermark and Land Wien.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases”. The article has undergone external peer review.
Conflicts of Interest: The series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases” was commissioned by the editorial office without any funding or sponsorship. Barbara Obermayer-Pietsch served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hygum K, Starup-Linde J, Harsløf T, et al. Diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol 2017;176:R137-57. [Crossref] [PubMed]
- Schwartz AV. Epidemiology of fractures in type 2 diabetes. Bone 2016;82:2-8. [Crossref] [PubMed]
- IOF CSA Bone T. Patient Information. IOF Patient Inf. Available online: http://share.iofbonehealth.org/WOD/2016/fact-sheets/Diabetes-2016-EN.pdf
- Sellmeyer DE, Civitelli R, Hofbauer LC, et al. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes 2016;65:1757-66. [Crossref] [PubMed]
- Hu F, Jiang C, Shen J, et al. Preoperative predictors for mortality following hip fracture surgery: A systematic review and meta-analysis. Injury 2012;43:676-85. [Crossref] [PubMed]
- Ohlsson C, Sjögren K. Osteomicrobiology: A New Cross-Disciplinary Research Field. Calcif Tissue Int 2018;102:426-32. [Crossref] [PubMed]
- Hough FS, Pierroz D. Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol 2016;174:R127-38. [Crossref] [PubMed]
- Hofbauer LC, Brueck CC, Singh SK, et al. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 2007;22:1317-28. [Crossref] [PubMed]
- Tanaka K, Yamaguchi T, Kanazawa I, et al. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun 2015;461:193-9. [Crossref] [PubMed]
- Wei J, Shimazu J, Makinistoglu MP, et al. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell 2015;161:1576-91. [Crossref] [PubMed]
- Starup-Linde J, Lykkeboe S, Gregersen S, et al. Differences in biochemical bone markers by diabetes type and the impact of glucose. Bone 2016;83:149-55. [Crossref] [PubMed]
- Armas LAG, Akhter MP, Drincic A, et al. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone 2012;50:91-6. [Crossref] [PubMed]
- Lafage-Proust M-H, Roche B, Langer M, et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep 2015;4:662. [Crossref] [PubMed]
- Abdalrahaman N, McComb C, Foster JE, et al. Deficits in Trabecular Bone Microarchitecture in Young Women with Type 1 Diabetes Mellitus. J Bone Miner Res 2015;30:1386-93. [Crossref] [PubMed]
- Kotwal A, Drake MT. Our Evolving Understanding of the Relationship Between Diabetes and Bone. Am J Med Sci 2017;354:333-4. [Crossref] [PubMed]
- Heilmeier U, Patsch JM. Diabetes and Bone. Semin Musculoskeletal Radiol 2016;20:300-4. [Crossref] [PubMed]
- Lecka-Czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia 2017;60:1163-9. [Crossref] [PubMed]
- Zhou J, Zhang Z, Qian G. Neuropathy and inflammation in diabetic bone marrow. Diab Metab Res Rev 2018;e3083:1-13.
- Dalmas E. Innate immune priming of insulin secretion. Curr Opin Immunol 2018;56:44-9. [Crossref] [PubMed]
- Dolan E, Swinton PA, Sale C, et al. Influence of adipose tissue mass on bone mass in an overweight or obese population: systematic review and meta-analysis. Nutr Rev 2017;75:858-70. [Crossref] [PubMed]
- Krings A, Rahman S, Huang S, et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone 2012;50:546-52. [Crossref] [PubMed]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes - A meta-analysis. Osteoporos Int 2007;18:427-44. [Crossref] [PubMed]
- Leslie WD, Aubry-Rozier B, Lamy O, et al. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 2013;98:602-9. [Crossref] [PubMed]
- Zhukouskaya VV, Ellen-Vainicher C, Gaudio A, et al. The utility of lumbar spine trabecular bone score and femoral neck bone mineral density for identifying asymptomatic vertebral fractures in well-compensated type 2 diabetic patients. Osteoporos Int 2016;27:421. [Crossref] [PubMed]
- Neumann T, Lodes S, Kästner B, et al. Trabecular bone score in type 1 diabetes—a cross-sectional study. Osteoporos Int 2016;27:127-33. [Crossref] [PubMed]
- Starup-Linde J, Lykkeboe S, Gregersen SH, et al. Bone Structure and Predictors of Fracture in Type 1 and Type 2 Diabetes. J Clin Endocrinol Metab 2016;101:928-36. [Crossref] [PubMed]
- Shanbhogue VV, Hansen S, Frost M, et al. Bone Geometry, Volumetric Density, Microarchitecture, and Estimated Bone Strength Assessed by HR-pQCT in Adult Patients with Type 1 Diabetes Mellitus. J Bone Miner Res 2015;30:2188-99. [Crossref] [PubMed]
- Patsch JM, Burghardt AJ, Yap SP, et al. Women with Fragility Fractures. J Bone Miner Res 2013;28:313-24. [Crossref] [PubMed]
- Patsch JM, Rasul S, Huber FA, et al. Similarities in trabecular hypertrophy with site-specific differences in cortical morphology between men and women with type 2 diabetes mellitus. PLoS One 2017;12:e0174664 [Crossref] [PubMed]
- Manavalan JS, Cremers S, Dempster DW, et al. Circulating Osteogenic Precursor Cells in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab 2012;97:3240-50. [Crossref] [PubMed]
- Farr JN, Drake MT, Amin S, et al. in Vivo Assessment of Bone Quality in Postmenopausal Women with Type 2 Diabetes. J Bone Miner Res 2014;29:787-95. [Crossref] [PubMed]
- Morris HA, Eastell R, Jorgensen NR, Cavalier E, et al. Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin Chim Acta 2017;467:34-41. [Crossref] [PubMed]
- Bonjour JP, Kohrt W, Levasseur R, et al. Biochemical markers for assessment of calcium economy and bone metabolism: Application in clinical trials from pharmaceutical agents to nutritional products. Nutrition Research Reviews 2014;27:252-67. [Crossref] [PubMed]
- Chew CK, Clarke BL. Biochemical Testing Relevant to Bone. Endocrinol Metab Clin North Am 2017;46:649-67. [Crossref] [PubMed]
- Vlot MC, den Heijer M, de Jongh RT, et al. Clinical utility of bone markers in various diseases. Bone 2018;114:215-25. [Crossref] [PubMed]
- Oei L, Rivadeneira F, Zillikens MC, et al. Diabetes, Diabetic Complications, and Fracture Risk. Curr Osteoporos Rep 2015;13:106-15. [Crossref] [PubMed]
- Lin X, Brennan-Speranza T, Levinger I, et al. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018;10:847-67. [Crossref] [PubMed]
- Tubic B, Magnusson P, Mårild S, et al. Different osteocalcin forms, markers of metabolic syndrome and anthropometric measures in children within the IDEFICS cohort. Bone 2016;84:230-6. [Crossref] [PubMed]
- Buday B, Pach FP, Literati-Nagy B, et al. Serum osteocalcin is associated with improved metabolic state via adiponectin in females versus testosterone in males. Gender specific nature of the bone-energy homeostasis axis. Bone 2013;57:98-104. [Crossref] [PubMed]
- Prats-Puig A, Osiniri I, Soriano-Rodríguez P, et al. Undercarboxylated osteocalcin relates to cardiovascular risk markers in offspring of families with metabolic syndrome. Atherosclerosis 2014;233:272-7. [Crossref] [PubMed]
- Schwetz V, Lerchbaum E, Schweighofer N, et al. Osteocalcin levels on oral glucose load in women being investigated for polycystic ovary syndrome. Endocr Pract 2014;20:5-14. [Crossref] [PubMed]
- Saito M, Fujii K, Mori Y, et al. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 2006;17:1514-23. [Crossref] [PubMed]
- Ferrari SL, Abrahamsen B, Napoli N, et al. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Yamamoto M, Yamaguchi T, Yamauchi M, et al. Serum Pentosidine Levels Are Positively Associated with the Presence of Vertebral Fractures in Postmenopausal Women with Type 2 Diabetes. J Clin Endocrinol Metab 2008;93:1013-9. [Crossref] [PubMed]
- Yamamoto M, Yamaguchi T YM et al. Low Serum Level of the Endogenous Secretory Receptor for Advanced Glycation End Products (esRAGE) Is a Risk Factor for of Bone Mineral Density in Patients With Type 2 Diabetes. Diabetes Care 2009;32:0-5.
- Szulc P, Chapurlat R, Hofbauer LC. Prediction of Fractures and Major Cardiovascular Events in Men Using Serum Osteoprotegerin Levels: The Prospective STRAMBO Study. J Bone Miner Res 2017;32:2288-96. [Crossref] [PubMed]
- Gennari L, Merlotti D, Valenti R, et al. Circulating Sclerostin Levels and Bone Turnover in Type 1 and Type 2 Diabetes. J Clin Endocrinol Metab 2012;97:1737-44. [Crossref] [PubMed]
- Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - A systematic review. Bone 2016;82:69-78. [Crossref] [PubMed]
- Drake MT, Khosla S. Hormonal and systemic regulation of sclerostin. Bone 2017;96:8-17. [Crossref] [PubMed]
- Morales-Santana S, García-Fontana B, García-Martín A, et al. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013;36:1667-74. [Crossref] [PubMed]
- Woitge HW, Seibel JM. Markers Bone Cartilage. DM Exp Endo Diab 2017;125:454-64.
- Sun M, Zhou X, Chen L, et al. The Regulatory Roles of MicroRNAs in Bone Remodeling and Perspectives as Biomarkers in Osteoporosis. BioMed Research International 2016;2016:1652417 [Crossref] [PubMed]
- Heilmeier U, Hackl M, Skalicky S, et al. Serum miRNA Signatures Are Indicative of Skeletal Fractures in Postmenopausal Women With and Without Type 2 Diabetes and Influence Osteogenic and Adipogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro. J Bone Miner Res 2016;31:2173-92. [Crossref] [PubMed]
- Ulbing M, Obermayer-Pietsch B. MicroRNAs in bone overview and clinical significance. Osteologie 2015;24.
- Mabilleau G, Pereira M, Chenu C. Novel skeletal effects of glucagon-like peptide-1 (GLP-1) receptor agonists. J Endocrinol 2018;236:R29-42. [Crossref] [PubMed]
- Glorie L, D’Haese PC, Verhulst A. Boning up on DPP4, DPP4 substrates, and DPP4-adipokine interactions: Logical reasoning and known facts about bone related effects of DPP4 inhibitors. Bone 2016;92:37-49. [Crossref] [PubMed]
- Meier C, Schwartz AV, Egger A, et al. Effects of diabetes drugs on the skeleton. Bone 2016;82:93-100. [Crossref] [PubMed]
- Paschou SA, Dede AD, Anagnostis PG, et al. Type 2 diabetes and osteoporosis: A guide to optimal management. J Clin Endocrinol Metab 2017;102:3621-34. [Crossref] [PubMed]
- Salsali A, Zeller C. Analysis of Fractures in Patients With Type 2 Diabetes Treated With Empagli fl ozin in Pooled Data From Placebo-Controlled Trials and a Head-to-Head Study Versus Glimepiride. Diabetes Care 2018;1-8.
- Raffield LM, Agarwal S, Cox AJ, et al. Cross-sectional analysis of calcium intake for associations with vascular calcification and mortality in individuals with type 2 diabetes from the Diabetes Heart Study. Am J Clin Nutr 2014;100:1029-35. [Crossref] [PubMed]
- Hernandez CJ, Guss JD, Luna M, et al. Links Between the Microbiome and Bone. J Bone Miner Res 2016;31:1638-46. [Crossref] [PubMed]
- Nilsson AG, Sundh D, Bäckhed F, et al. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Obermayer-Pietsch B, Francic V, Haudum C, Borzan V, Schweighofer N, Ascani A, Foessl I. Diabetoporosity—diabetes and the bone. J Lab Precis Med 2018;3:98.