Measurement of vitamin D metabolites by mass spectrometry, an analytical challenge
Introduction
In recent years, vitamin D has gained enormous attention from patients, the medical community, and the public and scientific world. The great interest in vitamin D-related health issues is due to an increased awareness of the high prevalence of vitamin D deficiency, its associations with bone health and multiple non-bone-related diseases. At present, 25-hydroxy vitamin D(25(OH)D) is the recommended test for the assessment of vitamin D status (1). Despite the availability of a reference method (2,3), a reference material (NIST SRM 2972) and a vitamin D standardization program (VDSP) the comparability of 25(OH)D results between laboratories and methods is still problematic (4). The vast majority of laboratories use automated immunoassays for the measurement of 25(OH)D, which harbor a range of intrinsic analytical issues that are difficult to overcome. For example, more than 99% of 25(OH)D in blood is bound to vitamin D binding protein, albumin and other carriers. Releasing vitamin D efficiently from these carriers is difficult for automated immunoassays, as they can’t use strong organic solvents, such as methanol or acetonitrile. Selectivity is another problem. Assays are expected to measure 25(OH)D2 and 25(OH)D3 in an equimolar fashion without detecting any of the other chemically related vitamin D metabolites or steroid hormones that are also present in blood. Last but not least, immunoassays are subject to matrix interferences, such as heterophilic antibodies, which have a prevalence of up to 13% in the general population (5,6).
In general, liquid-chromatography-tandem-mass-spectrometry (LC-MS/MS) does not suffer from the above mentioned limitations and is considered the gold standard for the measurement of 25(OH)D. The impact of analytical variability has recently been shown by a re-standardization of 55,844 previously measured samples from 14 population studies, which confirmed substantial over- or underestimation of 25(OH)D by most immunoassays (7). For this re-standardization the authors used VDSP protocols that require the reanalysis of a subset of 100–175 biobanked samples from each study with a certified LC-MS/MS method, which is traceable to the NIST higher-order reference measurement procedure (RMP) (3,8). The results were used to develop master regression equations for the re-standardization of all data points. However, the principal advantages of LC-MS/MS can only be obtained when the instrument provides sufficient sensitivity, the method is traceable to the NIST SRM 2972 standard and a proper validation of the analytical system has been performed. Moreover, when using LC-MS/MS in population studies, throughput and automation are important aspects to consider.
Despite many advantages, LC-MS/MS methods are not free from analytical challenges. A comparison of three LC-MS/MS methods from independent laboratories demonstrated relevant differences (9). In external quality assurance programs the 25(OH)D results obtained by LC-MS/MS methods can vary for more than 100% (10).
Beside the difficulties related to 25(OH)D, measurement of 24,25(OH)2D and 1,25(OH)2D is also challenging. Both analytes have similar physical and chemical properties as 25(OH)D and share the same analytical difficulties. While 1,25(OH)2D can be analyzed by radioimmunoassay (RIA), automated immunoassays and LC-MS/MS no immunoassays exist for the quantitation of 24,25(OH)2D. Because of the analytical issues related to the measurement of vitamin D, LC-MS/MS is most suited for the quantitation of both analytes. The capability of LC-MS/MS methods to detect multiple analytes simultaneously has also triggered attempts to measure two or more vitamin D metabolites in one analytical run. While an extensive number of original studies and review articles has addressed the performance of 25(OH)D immunoassays, the analytical challenges regarding the measurement of vitamin D metabolites by LC-MS/MS are not widely known. Therefore, the aim of this review is to provide a comprehensive overview about existing LC-MS/MS methods for the measurement of vitamin D metabolites and their analytical performance.
Vitamin D structure and metabolism
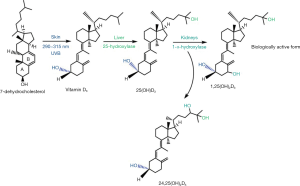
The analytical challenges related to the measurement of vitamin D arise at least partially from the fact that in human blood over 50 vitamin D metabolites circulate with variable activity (11)..Although 25(OH)D and 1,25(OH)2D are the only compounds that are routinely measured in clinical laboratories others, such as 24,25(OH)2D, have gained substantial interest and may add additional clinical information. It is therefore important to understand the chemistry and metabolism of the principal vitamin D metabolites (Figure 1) (12).
The term vitamin D does not refer to a single compound rather than to a group of fat-soluble secosteroids that are derived from cholesterol. Secosteroids are characterized by a broken bond in the B-ring of the four-ring steroid core. Vitamin D3 (cholecalciferol) and D2 (ergocalciferol) are the two principal forms of vitamin D in human blood, which differ in the composition of their side chains. In contrast to vitamin D2, the side chain of vitamin D3 harbors a double-bond between carbons 22 and 23 and a methyl group on carbon 24. Vitamin D3 represents the endogenous form of vitamin D in humans and is synthesized in the skin (13). Vitamin D2, instead, is ingested via the diet, fortified foods or vitamin supplements.
The endogenous synthesis of vitamin D3 in the skin requires 7-dehydrocholesterol, a precursor of cholesterol, as a substrate. As a principal component of the plasma membrane of cells in both the dermis and epidermis, cholesterol is essential for the epidermal barrier function. In addition, cholesterol modulates epidermal differentiation and desquamation. The skin ensures an adequate supply with cholesterol through active local synthesis. Epidermal cholesterol synthesis provides a ready source of 7-dehydrocholesterol (provitamin D). Vitamin D synthesis in the skin starts with the opening of the B-ring of the steroid ring system. Irradiation of 7-dehydrocholesterol with UV-B light breaks the bond between carbons 9 and 10. The resulting 9,10 secosteroid (previtamin D3) is unstable and readily undergoes isomerization (13). Spontaneous rotation of the A-ring around the bond between carbons 5 and 6 converts unstable previtamin D3 into the more stable isomer vitamin D. This isomerization process interrupts the hydrophobic and hydrophilic interactions that hold previtamin D3 within the cell membrane so that the newly formed vitamin D3 is expelled into the interstitial fluid. Vitamin D binding protein (VDBP) in the capillaries of the dermis avidly binds vitamin D and the resulting concentration gradient of free vitamin D drags vitamin D from the interstitial fluid into the circulation (14).
Vitamin D hides little biological activity. Two sequential hydroxylation steps are necessary to convert inactive vitamin D into the biologically active metabolite 1,25(OH)2-vitamin D. The first hydroxylation reaction attaches a hydroxyl-group in position 25 and is catalyzed by the enzyme CYP27A (25-hydroxylase). The resulting 25(OH)-Vitamin D (25(OH)D) represents the most abundant circulating form of vitamin D, which is still inactive (15). Through the addition of a second hydroxyl-group in position 1 (catalyzed by CYP27B [1-hydroxylase]) the active compound 1,25(OH)2D is formed. Although the kidneys are the principal sites of 1,25(OH)2D synthesis, many other tissue types can express CYP27B1 and therefore produce 1,25(OH)2D locally (16,17). Hydroxylation in position 24 (CYP24A1) is the first step in the catabolism of 25(OH)D and 1,25(OH)2D. The resulting metabolites 24R,25(OH)2D and 1,24R,25(OH)3D are inactive (18).
Measurement of vitamin D metabolites by LC-MS/MS: pre-analytical and analytical aspects
Sample types
Sample type is a critical aspect that should always be considered when measuring biomarkers in biological fluids including plasma and serum. In the literature there are only very few studies that compared serum and plasma measurements of vitamin D and its metabolites (19). The major difference between plasma and serum is the removal of fibrinogen and other coagulation factors that are consumed by clotting of the sample. The absence of these proteins allows an increasing concentration of organic solvents and formic acid during pre-analytical sample preparation for LC-MS/MS analysis and thus enhancing protein precipitation. In general, available data show neither in 25(OH)D immunoassays nor in LC-MS/MS methods systemic differences between plasma and serum (20,21). For example, Colak et al. (22) measured 25(OH)D in serum and plasma from 15 healthy adults using an automated immunoassay from Roche Diagnostics (Roche Diagnostics, Mannheim, Germany) and found no significant difference under different storage conditions. Zhang et al. (19) compared 25(OH)D2 and 25(OH)D3 results obtained by a fully validated in-house LC-MS/MS method in matching serum and plasma samples containing either heparin or EDTA. No significant difference was observed regardless of the anticoagulant used. Similar findings were reported by Lee et al. and Mena-Bravo et al., the latter also considered 24,25 (OH)2D3. Inconsistent results have been observed for vitamin D metabolites with very low concentrations, such as 1,25(OH)2D (20,23). Mena-Bravo et al. found 60% higher 1,25(OH)2D3 results in plasma than in serum whereas Ishige et al. (21) did not observe such a difference. In contrast, Abu Kassim et al. measured nine different vitamin D metabolites by LC-MS/MS and observed higher concentrations in serum than in EDTA (24).
The use of separation gels in blood collection tubes is another important factor that may interfere with the measurement of vitamin D and its metabolites. Mena-Bravo et al. showed that neither in plasma nor in serum tubes from Becton-Dickinson the presence of a separation gel caused a difference for vitamin D3, 24,25(OH)2D3, 25(OH)D3 and 1,25(OH)2D3. However, composition of separator gels differs between vendors. Therefore, each type of gel tube should be validated individually before use in clinical practice.
Taken together, for the measurement vitamin D and 25(OH)D serum or plasma can be used safely. Also separator gels are unlikely to have an impact on the results. However, in view of the different gel compositions on the market it is prudent to test each tube type prior to clinical routine use. For other vitamin D metabolites no general recommendation can be given as data are inconsistent or lacking.
Sample preparation: protein extraction
In serum and plasma vitamin D and its metabolites are tightly bound to VDBP, albumin and lipoproteins. For example, approximately 90%, of circulating 25(OH)D is protein bound (25) and thus needs to be released and purified prior to analysis. A range of different extraction procedures have been described in the literature. All these methods use strong organic solvents to precipitate proteins and release vitamin D metabolites from carrier compounds. Subsequently, an additional extraction step is performed in order to increase purity and reduce interferences during analysis. In general, two types of extraction techniques can be distinguished, solid phase extraction (8) and liquid-liquid extraction (LLE). It should be mentioned that automated immunoassays have to use a completely different approach for the extraction of vitamin D metabolites as they can’t work with strong organic solvents.
During protein precipitation, the tertiary structure of the proteins is destroyed and vitamin D metabolites are released. Methanol, acetonitrile or zinc sulphate is typically used for protein precipitation (26). Different mixtures of these precipitants and several stabilizing additives have been described in the literature (Table 1). While methanol and acetonitrile break down predominantly proteins, zinc sulphate precipitates also lipoproteins (4,36). Beside protein precipitation, saponification is an alternative approach for the release of vitamin D from its carriers (30). Saponification is performed by adding potassium hydroxide in ethanol containing 20% (w/v) ascorbic acid to the sample, which breaks the ester linkages of triglycerides, phospholipids and esterified sterols thereby releasing vitamin D compounds. A comparison between saponification and protein precipitation revealed a more efficient extraction when protein precipitation is used. An alternative saponification method has been described by Huang et al. (45). This method uses methanol with 2% pyrogallic acid and potassium hydroxide. When compared to room temperature, heating accelerates the extraction process and allows faster sample preparation.
Table 1
| Protein precipitation |
| Methanol (MeOH) (27-29) |
| Acetonitrile (MeOH) (30-34) |
| MeCN/MeOH (9:1 v/v) (26) |
| MeOH/Isopropanol (1:1 v/v) (35) |
| Zinc sulphate/MeOH (4,36,37) |
| Liquid-liquid-extraction |
| Acetone (38) |
| Heptane (35,39,40) |
| Hexane (7,29,35) |
| Ethyl acetate (41) |
| Methyl-tert-butyl ether (36,42) |
| Solid phase extraction |
| Oasis HLB 24 µL 20 mm × 2.1 mm (4,26,31,32,43) |
| POROS R1/10 (44) |
| Zorbax Eclipse XDB-CN 5 µm, 50 mm × 2.0 mm (34) |
Sample preparation: extraction of analytes
The supernatant that remains after protein precipitation still contains multiple interfering compounds that need to be eliminated prior to analysis. Therefore, an appropriate and efficient extraction procedure is the key for an accurate quantitation of vitamin D metabolites (35). The choice of extraction procedure depends on the vitamin D metabolite of interest. Because of its relative abundance the extraction procedure has less impact on the determination of 25(OH)D than for other metabolites that circulate at low concentrations, such as 24,25(OH)2D or 1,25(OH)2D. Both, LLE and SPE are widely used in the literature and in general one is not better than the other. During LLE an organic solvent, such as heptane, hexane, ethylacetate or ethyl-tert-butyl ether is added to the supernatant obtained after protein precipitation (20). After thorough mixing and centrifugation, the supernatant, which contains the purified vitamin D metabolites, is dried under a constant flow of nitrogen or high pressure air and subsequently reconstituted in either acetonitrile or methanol. In order to amplify the detection signal some methods add a derivatization agent to these organic solvents. Sample derivatization enhances ionization and is particularly helpful when measuring analytes with a low concentration. Derivatization strategies will be discussed in more detail later. After reconstitution, a derivatization sample is ready for analysis. Although LLE is more difficult to automate and has largely been replaced by SPE, many scientists continue to use it even today due to its simplicity, flexibility and affordability. When compared to SPE, LLE often has lower extraction efficiency due to matrix effects in the sample. However, this disadvantage can be compensated by using atmospheric pressure photoionization (APPI) (38). Acetone and isopropanol appear to possess the highest extraction capacity (partition coefficient for vitamin D). However, repeatability of LLE with isopropanol unsatisfactory as imprecision has been found to be greater than 25%. Tai et al. (2) developed a method for measurement of 25(OH) vitamin D2 and D3 also with LLE using hexane-ethyl acetate (50:50). The National Institute of Standards and Technology (NIST) has used this measurement procedure to certify the concentrations of 25(OH)D3 and 25(OH)D2 in a new standard reference method (SRM) for vitamin D in human serum.
Solid-phase extraction is the most frequently used extraction technique for the measurement of vitamin D metabolites, which exploits the affinity of solutes dissolved or suspended in a liquid (mobile phase) for a solid (stationary) phase through which the sample is passed. Modified silica-based sorbents are typically used for the extraction of non-polar analytes (8). Alternatively, polymeric sorbents with higher capacity and selectivity than silica based sorbents can also be used. The analytes of interest are retained on the stationary phase. After eliminating matrix components from the stationary phase by washing with an aqueous solution, the analyte is eluted with an organic solvent and introduced into the analytical system. On-line SPE integrates the extraction procedure in the analytical system thus reducing manual intervention and increasing productivity. SPE columns differ in length (1–5 cm) and diameter (0.5–5 mm), particle size (≤5 µm) and porosity (≤100 Å). Most importantly is the amount of sorbent. Extraction efficiency improves with optimal adjustments between analyte properties, surface characteristics of the column material and mobile phase. Table 1 provides an overview about commonly used SPE columns.
SPE and LLE are both acceptable methods for the extraction of vitamin D metabolites from serum or plasma samples, because better precision, cheaper costs and the possibility of automation SPE is more frequently used than LLE.
Sample preparation: derivatization
The low ionization efficiency of vitamin D metabolites and interferences from chemically similar compounds are other analytical challenge when measuring vitamin D by LC-MS/MS. Derivatization is a frequently used strategy in analytical chemistry where the analytes of interest are chemically transformed in order to modify their physical and chemical characteristics. The goals of derivatization include improvements in thermostability, ionization and thus sensitivity. Furthermore, derivatization can increase volatility and induce mass shifts of analyte ions away from interfering signals. LC-MS/MS procedures for the measurement of vitamin D metabolites often include a derivatization step (46). Because of its relative abundance 25(OH)D does necessarily require derivatization. However, metabolites that are present at low concentrations, such as 24,25(OH)2D or 1,25(OH)2D rely on an amplification of the signal by derivatization.
Various derivatization reagents have been described for the measurement of vitamin D metabolites (26). The most frequently used derivatization reagents are Cookson-type derivatizing agents, such as 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), 4-[4-(6-methoxy-2-benzoxazolyl)phenyl]-1,2,4-triazoline-3,5-dione (MBOTAD), 4-ferrocenylmethyl-1,2,4-triazoline-3, 5-dione (FMTAD), and 4-[2-(6,7-dimethoxy-4-methyl-3-oxo-3,4-dihydroquinoxalyl)-ethyl]-1,2,4-triazoline-3,5-dione (DMEQTAD). Cookson-type reagents are 4-substituted 1,2,4-triazoline-3,5-diones with a chromophore, fluorophore or electrophore at the 4-position that form a stable Diels-Alder adduct through which ionization efficiency improves 100 to 200 times (47).
2-Nitrosopyridine (PyrNO) is an alternative derivatization reagent that contains a reactive dienophile and also forms Diels-Alder adducts (48). Derivatization with PyrNO has been found to improve multiple reaction monitoring (MRM) signals (intensity) compared to PTAD, which is used frequently. Amplifex is another derivatization agent that should be mentioned as it appears to be most efficient the measurement of 1,25-(OH)2D2 and 1,25-(OH)2D3 due to its a positively charged end group and activated dienophile moiety (49).
In summary, derivatization is frequently used for the measurement of vitamin D metabolites, especially when metabolites with a low concentration are to be determined. PTAD is the most frequently used derivatization agent but newer compounds may be more efficient for certain vitamin D metabolites. Therefore, the choice of derivatization agent should take into account the nature and concentration of the analytes of interest.
Chromatographic separation of extracted samples
LC-MS/MS combines liquid chromatography with mass spectrometry (MS) in order to increase specificity. The goal of liquid chromatography is the separation of compounds in a complex mixture thus isolating the analytes of interest from background noise. Based on its physicochemical properties each compound is retained differently on the stationary phase inside the chromatography column and is eluted at a specific time, which is called retention time. The combination of stationary phase (material, particle size and porosity), column type (length, diameter), mobile phase and flow rates determines the separation efficiency and the retention time for each compound in the sample. For the separation of hydrophobic compounds, such as vitamin D metabolites, a hydrophobic stationary phase is required, which is also referred to as reversed-phase (hydrophilic stationary phases are considered normal-phase).The stationary phase for reversed-phase liquid chromatography is an inert substance, such as silica, with non-polar side-chains attached to it (50). The most frequently used stationary phase for the measurement of vitamin D compounds is octadecyl carbon chain (C18)-bonded silica. In order to improve separation efficiency the manufacturers of chromatography columns attach pentafluorophenyl or cyano moieties to the C18 chains. These modification increase hydrophobicity and improve durability in an aggressive environment with high temperatures and an aggressive pH. The quantity of C18 chains (carbon load), the presence of spacer molecules and the surface (particle size and porosity) determine selectivity and separation performance of an LC column. Hundreds of different columns are commercially available. For the measurement of vitamin D metabolites the most frequently used LC columns are LiChrospher RP-18 (Merck, Germany), Zorbax Eclipse XDB C-18 or Zorbax SB-C18 (both from Agilent, USA), Supelcosil LC-18 (Sigma-Aldrich, Germany), X Terra C18 and ACQUITY UPLC BEH C18 (both from Waters, USA) Hypersil Gold (Thermo Scientific, USA). Apart from differences on the stationary phase material, LC columns differ in length (5–25 cm), diameter (1–5 mm), particle size (1.6–4.6 µm) and porosity (≤100 Å and until 130 Å for columns using UPLC). Separation efficiency improves with increasing column length, higher porosity, decreasing diameter and smaller particle size. However, the pressure in the column increases with increasing column length and decreasing column diameter and particle size. Therefore, the choice of LC columns depends on the chemical properties of the analytes of interest, the type of pump connected to the system and the extraction efficiency needed.
In order to apply a sample to the LC column a mobile phase is needed as a carrier. The composition of mobile phases is a key factor for the successful chromatographic separation of a sample. For the measurement of vitamin D an aqueous and an organic mobile phase are needed. The aqueous phase, which is typically water stabilized with formic acid, is used to apply the sample to the column. The organic mobile phase is needed for the elution of hydrophobic analytes from reversed phase columns. Methanol stabilized with formic acid (0.005% to 5%) or ammonium acetate (2–5 mM) is typically used as organic mobile phase. Formic acid and ammonium acetate impair contamination with bacteria or other contaminants. Alternative mobile phases are acetonitrile or ethanol diluted in water. The flow rate for chromatographic separation varies between 0.35–1.0 mL/min depending on the column used (26).
Chromatographic separation can be performed HPLC (high performance liquid chromatography), ultra-high performance liquid chromatography (UHPLC) or ultra-performance supercritical fluid chromatography systems (UPSFC). UHPLC systems use a higher pressure to pump the mobile phase through the column and the rest of the system. The columns for HPLC and UHPLC differ in particle size, length and diameter. Superior speed, resolution and sensitivity make UHPLC the preferred technique for the analysis of vitamin D metabolites. UPSFC has further improved separation and run time (51).
Mass spectrometry
MS is a highly sensitive and selective analytical technique that has the ability to overcome most of the limitations inherent to immunoassays, such as interferences from binding proteins, heterophilic antibodies and cross reactivity with other steroid compounds. After chromatographic separation, samples are introduced into the mass spectrometer where they are vaporized and ionized. Subsequently, the ions travel through three electrically charged quadropoles that filter ions based on their mass to charge ratio (m/z). The first and third of these quadrupoles allow only ions with a specific m/z to pass. All other ions are eliminated and do not reach the detector. In the second quadrupole, ions that have passed successfully the first quadrupole are fragmented by collision with an inert gas, such as nitrogen, helium or argon. In the collision cell every parent ion produces a specific spectrum of fragment ions from which two of the most abundant fragment ions are selected and filtered in the third quadrupole and reach the detector. One of these ions serves as qualifier and the other one is used for quantitation. The qualifier ensures the presence of the correct parent ion in the second quadrupole whereas the quantifier is used to calculate the concentration of the analyte in the sample (35).
Ionization and vaporization can take place in the ion source and can occur in different ways. The different forms of ionization have specific advantages and disadvantages that need to be considered when selecting a method. The most common ionization technique is electrospray ionization (ESI) where the sample is introduced into the source through an electrically charged needle with a high potential difference (2.5 to 5 kV) that forces spraying of charged droplets from the needle. As the surface of the droplets formed have the same charge as the needle they are repelled from the tip of the needle and travel towards the sampling cone on the counter electrode. While travelling the solvents of the droplets evaporate so that the droplets shrink until the surface tension can no longer sustain the charge (the Rayleigh limit) at which point a “Coulombic explosion” occurs and the droplet is ripped apart. This produces smaller droplets that can repeat the process as well as naked charged analyte molecules. ESI is considered a soft ionisation method as very little residual energy is retained by the analyte upon ionisation (52).
Further ion sources often used for mass spectrometric analysis of vitamin D metabolites are chemical ionization (1) and atmospheric-pressure chemical ionization (APCI) (53). During CI charged ions of the analyte molecules in the sample are produced through collision with high energy molecular ions of a collision gas. The collision gas that is present in large excess is ionized by bombardment with electrons that enter the source with an energy of around 200–500 eV at a pressure of 0.2–2 Torr. Common reagent gases are methane, ammonia and isobutane. Compared to other ionization techniques CI causes less fragmentation of the analytes resulting in simpler and more sensitive spectra. While CI requires high pressure inside the ion source, APCI occurs at atmospheric pressure. The mixture of analytes and solvents that exit the HPLC system enter the ion source through a capillary inside an uncharged quartz tube. At the end of the capillary the sample is nebulized and vaporized with the help of nitrogen gas and by heating to very high temperature (~350–550 °C). Subsequently the vapor passes a highly charged electrode (several kV) that ionizes nearby molecules without fragmentation. This highly charged electrode is called corona needle. Discharge of the corona needle may directly ionize analyte molecules or solvent molecules. As solvent molecules are typically present in large excess it is more likely that solvent molecules are ionized first and subsequently pass their charge onto the analyte molecules. A typical APCI ion source consists of a nebulizer probe, an ionization region with a corona discharge needle, and an ion-transfer region under intermediate pressure. Similar to CI, APCI is a soft ionization method. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1,500 Da (54).
A main difference between ESI and chemical ionization techniques is the degree of fragmentation and the production of multiple-charged ions, which effectively expands the mass range. ESI is the most frequently used ionization method for the analysis of 25(OH)D (55). However, for the detection of vitamin D metabolites with concentrations in the picomolar range ESI does not achieve a satisfactory LOD. Using ESI the LOD for 25(OH)D3 and D2 has been shown to be 2–5 times higher than with APCI. In addition, interferences from matrix effects are less in APCI than in ESI. Therefore, for the measurement of 1,25(OH)2D or 24,25(OH)2D APCI is preferred as it reduces matrix effects and improves sensitivity (56).
Interferences from isomeric and isobaric compounds represent a common problem in MS that can also affect the measurement of vitamin D metabolites in serum or plasma (27,57). This problem can be minimized by sample pre-treatment with protein precipitation, extraction and derivatization. It should also be mentioned that 3-epi-25(OH)D3 and 25(OH)D3 co-eluted from standard C18 reversed-phase chromatography columns and exhibit identical mass spectra (58). Therefore, these two vitamin D species can only be identified and quantified when high resolution chromatography is used for separation (58).
Depending on the vitamin D metabolites to be measured different mass spectrometers can be used. The main characteristics that require attention when choosing a mass spectrometer are sensitivity and resolution. While sensitivity refers to the ability to measure low concentrations, resolution describes the capability of a mass spectrometer to distinguish ions with slight differences in molecular mass and measure them separately. Today, all manufacturers offer appropriate instruments with medium and high sensitivity that can be equipped with different ionization sources.
Calibration and standardization
The use of LC-MS/MS for the measurement of vitamin D metabolites does not guarantee accurate results. Blinded method comparison studies and EQA programs have repeatedly demonstrated substantial differences between individual LC-MS/MS methods (9,10). Without proper calibration and standardization comparable results between different LC-MS/MS are hardly achievable. Therefore standard reference materials (SRM) and RMPs have been developed. In 2009 the National Institute of Standardization (NIST) has released the first SRM for the measurement of vitamin D in human serum (SRM 972). It consists of four blood serum sample pools (Level 1 – Level 4) with varying levels of 25(OH)D and has certified values for 25(OH)D2, 25(OH)D3, and 3-epi-25(OH)D3. A few years later SRM 972a was introduced, which also provides certified values for 24R,25(OH)2D3 (59,60). The first RMP for the measurement of 25(OH)D in serum has been published in 2010 by Tai et al. (2). This method is based on isotope dilution liquid chromatography tandem MS. A second RMP has been developed by Stepman et al. (61). Both RMPs measure 25(OH)D2 and D3. The method from Stepman et al. does also resolve interferences from 3-epi-25(OH)D3. A few years later, Tai and Nelson have also developed a reference method for 24R,25(OH)2D3 in human serum (62).
In order to overcome the large discrepancies between commercial 25(OH)D assays the National Institutes of Health (NIH) Office of Dietary Supplements (ODS) established the VDSP in November 2010. This program aims to standardize the measurement of vitamin D by encouraging manufacturers to produce methods traceable to these RMPs. Participating manufacturers and laboratories receive 40 serum samples from apparently healthy donors with known concentrations 25(OH)D determined by a RMP. These samples are used for method calibration. Acceptable bias and imprecision are ±5% and ±10%, respectively. While VDSP has helped to improve substantially accuracy in samples from healthy subjects without interferences, unresolved problems remain in special populations, such as pregnant women, hemodialysis patients, patients with osteoporosis or heterophilic antibodies. This fact is illustrated by EQA programs, such as the Vitamin D External Quality Assessment Scheme (DEQAS). Average differences between manufacturers can exceed 60 ng/mL (150 nmol/L) (63).
For other vitamin D metabolites SRMs and RMPs have not been developed yet, which impedes standardization between laboratories and manufacturers.
Commercial vitamin D LC-MS/MS assay
Because of the increasing demand for mass spectrometric analyses of vitamin D commercial assays become increasingly available (e.g., MassChrom 25-(OH)-Vitamin D3/D2, Chromsystems; ClinMass® LC-MS/MS Complete Kit for 25-(OH)-Vitamin D2/D3, Recipe, Vitamin D combi ImmuTube®, Immundiagnostik AG). Most of them measure 25(OH)D3 and D2. These kits include lyophilized materials for calibration and quality control, stable isotope-labeled internal standard compounds, sample preparation materials, mobile phases and analytical columns. However, not all kits contain internal standards for 25(OH)D2. Moreover, only some assays resolve and quantify 3-epi-25(OH)D3. EQAs demonstrate good accuracies for most commercial 25(OH)D LC-MS/MS assay kits. Recently, Thermo Scientific™ has released the Cascadion™ SM System, which is a fully automated LC-MS/MS random access analyser. In addition, a CE-IVD compliant ready to use reagent kit with controls and calibrators for the measurement of 25(OH)D3 and D2 is available for this instrument. A first preliminary validation study showed good linearity, a wide analytical range and acceptable precision. Accuracy data were performed using Unity Real Time controls from Bio-Rad (California, USA) (64).
A multiplex method (ImmuTube® LC-MS/MS assay) for the determination of determines 25(OH)D3, 25(OH)D2, 1,25(OH)2D3 and 24,25(OH)2D3 was recently released by Immundiagnostik AG (Bensheim, Germany). If accurate, the parallel quantitation of these metabolites (chromatograms are shown in Figure 2) would be a substantial step forward, especially for researchers studying vitamin D metabolism. An own evaluation of this product on a SCIEX QTRAP 4500 (Applied Biosystems, Framingham, MA, USA) coupled to an Agilent 1260 Infinity HPLC system (Agilent Technologies, Santa Clara, USA) confirmed acceptable dynamic ranges, LoQ and imprecision within the manufacturers claims. However, recovery was poor for 25(OH)D and 24,25(OH)2D, which raises doubts about the specificity for these metabolites (Table 2).
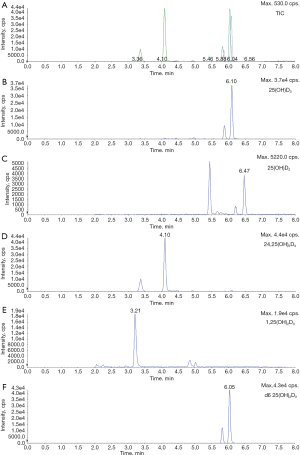
Table 2
| Vitamin D metabolites | 25(OH)D3 | 1,25(OH)2D3 | 24,25(OH)2D3 |
|---|---|---|---|
| Analytical range | 2–100 ng/mL | 5.5–215 pmol/mL | 2–50 ng/mL |
| Calibration curve | |||
| Slope | 0.0298 | 0.057 | 4.62 |
| Intercept | 0.023 | −0.002 | −0.184 |
| Correlation r2 | 0.988 | 0.998 | 0.959 |
| Intra-day precision (n=8, patients) | |||
| Mean | 19.1; 44.6 | 8.6; 33.8 | 2.3; 3.0 |
| SD | 0.35; 1.27 | 1.03; 2.49 | 0.12; 0.25 |
| Coefficient of variation [%] | 1.8; 2.9 | 12.4; 7.4 | 5.3; 8.3 |
| Inter-day precision (n=15, controls) | |||
| Mean | 24.4 (low); 125.2 (high) | 24.0 (low); 103.4 (high) | 6.2 (low); 27.1 (high) |
| SD | 2.86 (low); 8.78 (high) | 2.02 (low); 14.23 (high) | 1.05 (low); 4.69 (high) |
| Inter day variability [%] | 11.7 (low); 7.0 (high) | 8.4 (low); 13.8 (high) | 17.0 (low); 17.3 (high) |
| Recovery [%] | 1.7–3.4 | 63–73 | 1.7–3.4 |
Conclusions
LC-MS/MS is considered the golden standard for the measurement of vitamin D metabolites. In addition, LC-MS/MS allows the parallel measurement of several metabolites. However, a range of pre-analytical and analytical aspects require careful consideration. These aspects include sample type, protein precipitation, analyte extraction, derivatization, chromatographic separation ionization and capabilities of the mass spectrometer. Calibration, standardization and the use of internal standards are other important issues that impact on the accuracy of results. Only well designed methods that are continuously controlled by internal and external quality control allow an accurate and stable measurement 25(OH)D. Other metabolites, such as 1,25(OH)2D and 24,25(OH)2D, are not yet standardized and comparable results between laboratories are difficult to obtain.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.11.06). The series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases” was commissioned by the editorial office without any funding or sponsorship. Markus Herrmann served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2017 to June 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
- Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82:1942-8. [Crossref] [PubMed]
- Binkley N, Sempos CT, Vitamin DSP. Standardizing vitamin D assays: the way forward. J Bone Miner Res 2014;29:1709-14. [Crossref] [PubMed]
- Farrell CJ, Martin S, McWhinney B, et al. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem 2012;58:531-42. [Crossref] [PubMed]
- Preissner CM, Dodge LA, O'Kane DJ, et al. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clin Chem 2005;51:208-10. [Crossref] [PubMed]
- Koshida S, Asanuma K, Kuribayashi K, et al. Prevalence of human anti-mouse antibodies (HAMAs) in routine examinations. Clin Chim Acta 2010;411:391-4. [Crossref] [PubMed]
- Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 2016;103:1033-44. [Crossref] [PubMed]
- Sempos CT, Vesper HW, Phinney KW, et al. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32-40. [PubMed]
- Binkley N, Krueger DC, Morgan S, et al. Current status of clinical 25-hydroxyvitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta 2010;411:1976-82. [Crossref] [PubMed]
- Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 2011;12:19-28. [Crossref] [PubMed]
- Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev 1995;16:200-57. [PubMed]
- Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 2004;29:664-73. [Crossref] [PubMed]
- Holick MF, Chen TC, Lu Z, et al. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res 2007;22:V28-33. [Crossref] [PubMed]
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 2006;84:694-7. [Crossref] [PubMed]
- Cheng JB, Motola DL, Mangelsdorf DJ, et al. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem 2003;278:38084-93. [Crossref] [PubMed]
- Norman AW, Myrtle JF, Midgett RJ, et al. 1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 1971;173:51-4. [Crossref] [PubMed]
- Mizwicki MT, Bula CM, Bishop JE, et al. A perspective on how the Vitamin D sterol/Vitamin D receptor (VDR) conformational ensemble model can potentially be used to understand the structure-function results of A-ring modified Vitamin D sterols. J Steroid Biochem Mol Biol 2005;97:69-82. [Crossref] [PubMed]
- Norman AW, Henry HL, Malluche HH. 24R,25-Dihydroxyvitamin D3 and 1 alpha,25-dihydroxyvitamin D3 are both indispensable for calcium and phosphorus homeostasis. Life Sci 1980;27:229-37. [Crossref] [PubMed]
- Zhang SW, Jian W, Sullivan S, et al. Development and validation of an LC-MS/MS based method for quantification of 25 hydroxyvitamin D2 and 25 hydroxyvitamin D3 in human serum and plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2014;961:62-70. [Crossref] [PubMed]
- Mena-Bravo A, Priego-Capote F, Luque de Castro MD. Study of blood collection and sample preparation for analysis of vitamin D and its metabolites by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 2015;879:69-76. [Crossref] [PubMed]
- Ishige T, Satoh M, Ogawa S, et al. Improved sensitivity of serum/plasma 1alpha,25-dihydroxyvitamin D quantification by DAPTAD derivatization. Clin Chim Acta 2017;473:173-9. [Crossref] [PubMed]
- Colak A, Toprak B, Dogan N, et al. Effect of sample type, centrifugation and storage conditions on vitamin D concentration. Biochem Med (Zagreb) 2013;23:321-5. [Crossref] [PubMed]
- Lee D, Garrett TJ, Goldberger BA, et al. Quantitation of 25-hydroxyvitamin D2 and D3 in serum and plasma by LCMS/MS. Bioanalysis 2015;7:167-78. [Crossref] [PubMed]
- Abu Kassim NS, Gomes FP, Shaw PN, et al. Simultaneous quantitative analysis of nine vitamin D compounds in human blood using LC-MS/MS. Bioanalysis 2016;8:397-411. [Crossref] [PubMed]
- Kim HJ, Ji M, Song J, et al. Clinical Utility of Measurement of Vitamin D-Binding Protein and Calculation of Bioavailable Vitamin D in Assessment of Vitamin D Status. Ann Lab Med 2017;37:34-8. [Crossref] [PubMed]
- van den Ouweland JM, Vogeser M, Bacher S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord 2013;14:159-84. [Crossref] [PubMed]
- Qi Y, Geib T, Schorr P, Meier F, Volmer DA. On the isobaric space of 25-hydroxyvitamin D in human serum: potential for interferences in liquid chromatography/tandem mass spectrometry, systematic errors and accuracy issues. Rapid Commun Mass Spectrom 2015;29:1-9. [Crossref] [PubMed]
- Knox S, Harris J, Calton L, et al. A simple automated solid-phase extraction procedure for measurement of 25-hydroxyvitamin D3 and D2 by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem 2009;46:226-30. [Crossref] [PubMed]
- Newman MS, Brandon TR, Groves MN, et al. A liquid chromatography/tandem mass spectrometry method for determination of 25-hydroxy vitamin D2 and 25-hydroxy vitamin D3 in dried blood spots: a potential adjunct to diabetes and cardiometabolic risk screening. J Diabetes Sci Technol 2009;3:156-62. [Crossref] [PubMed]
- Abu Kassim NS, Shaw PN, Hewavitharana AK. Simultaneous determination of 12 vitamin D compounds in human serum using online sample preparation and liquid chromatography-tandem mass spectrometry. J Chromatogr A 2018;1533:57-65. [Crossref] [PubMed]
- Vogeser M, Kyriatsoulis A, Huber E, et al. Candidate reference method for the quantification of circulating 25-hydroxyvitamin D3 by liquid chromatography-tandem mass spectrometry. Clin Chem 2004;50:1415-7. [Crossref] [PubMed]
- Aronov PA, Hall LM, Dettmer K, et al. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 2008;391:1917-30. [Crossref] [PubMed]
- Herrmann M, Harwood T, Gaston-Parry O, et al. A new quantitative LC tandem mass spectrometry assay for serum 25-hydroxy vitamin D. Steroids 2010;75:1106-12. [Crossref] [PubMed]
- Kushnir MM, Ray JA, Rockwood AL, et al. Rapid analysis of 25-hydroxyvitamin D(2) and D(3) by liquid chromatography-tandem mass spectrometry and association of vitamin D and parathyroid hormone concentrations in healthy adults. Am J Clin Pathol 2010;134:148-56. [Crossref] [PubMed]
- Shah I, Akhtar MK, Hisaindee S, et al. Clinical diagnostic tools for vitamin D assessment. J Steroid Biochem Mol Biol 2018;180:105-17. [Crossref] [PubMed]
- Kaufmann M, Gallagher JC, Peacock M, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab 2014;99:2567-74. [Crossref] [PubMed]
- Stone J. High-Throughput Serum 25-Hydroxy Vitamin D Testing with Automated Sample Preparation. Methods Mol Biol 2016;1378:301-20. [Crossref] [PubMed]
- Musteata ML, Musteata FM. Overview of extraction methods for analysis of vitamin D and its metabolites in biological samples. Bioanalysis 2011;3:1987-2002. [Crossref] [PubMed]
- Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol 2006;125:914-20. [Crossref] [PubMed]
- Højskov CS, Heickendorff L, Moller HJ. High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25(OH) vitamin D3 and D2 in the routine clinical laboratory. Clin Chim Acta 2010;411:114-6. [Crossref] [PubMed]
- Wang Z, Senn T, Kalhorn T, et al. Simultaneous measurement of plasma vitamin D(3) metabolites, including 4beta,25-dihydroxyvitamin D(3), using liquid chromatography-tandem mass spectrometry. Anal Biochem 2011;418:126-33. [Crossref] [PubMed]
- Wagner D, Hanwell HE, Schnabl K, et al. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol 2011;126:72-7. [Crossref] [PubMed]
- Chen H, McCoy LF, Schleicher RL, et al. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay method. Clin Chim Acta 2008;391:6-12. [Crossref] [PubMed]
- Casetta B, Jans I, Billen J, et al. Development of a method for the quantification of 1alpha,25(OH)2-vitamin D3 in serum by liquid chromatography tandem mass spectrometry without derivatization. Eur J Mass Spectrom (Chichester) 2010;16:81-9. [Crossref] [PubMed]
- Huang M, Cadwallader AB, Heltsley R. Mechanism of error caused by isotope-labeled internal standard: accurate method for simultaneous measurement of vitamin D and pre-vitamin D by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2014;28:2101-10. [Crossref] [PubMed]
- Higashi T, Shimada K. Application of Cookson-type reagents for biomedical HPLC and LC/MS analyses: a brief overview. Biomed Chromatogr 2017;31: [Crossref] [PubMed]
- Gathungu RM, Flarakos CC, Reddy GS, et al. The role of mass spectrometry in the analysis of vitamin D compounds. Mass Spectrom Rev 2013;32:72-86. [Crossref] [PubMed]
- Wan D, Yang J, Barnych B, et al. A new sensitive LC/MS/MS analysis of vitamin D metabolites using a click derivatization reagent, 2-nitrosopyridine. J Lipid Res 2017;58:798-808. [Crossref] [PubMed]
- Hedman CJ, Wiebe DA, Dey S, et al. Development of a sensitive LC/MS/MS method for vitamin D metabolites: 1,25 Dihydroxyvitamin D2&3 measurement using a novel derivatization agent. J Chromatogr B Analyt Technol Biomed Life Sci 2014;953-954:62-7. [Crossref] [PubMed]
- Coskun O. Separation techniques: Chromatography. North Clin Istanb 2016;3:156-60. [PubMed]
- Jenkinson C, Taylor A, Storbeck KH, et al. Analysis of multiple vitamin D metabolites by ultra-performance supercritical fluid chromatography-tandem mass spectrometry (UPSFC-MS/MS). J Chromatogr B Analyt Technol Biomed Life Sci 2018;1087-1088:43-8. [Crossref] [PubMed]
- Banerjee S, Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int J Anal Chem 2012;2012:282574 [Crossref] [PubMed]
- Iwasaki Y, Nakano Y, Mochizuki K, et al. A new strategy for ionization enhancement by derivatization for mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1159-65. [Crossref] [PubMed]
- Gouveia MJ, Brindley PJ, Santos LL, et al. Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: a review. Metabolism 2013;62:1206-17. [Crossref] [PubMed]
- Volmer DA, Mendes LR, Stokes CS. Analysis of vitamin D metabolic markers by mass spectrometry: current techniques, limitations of the "gold standard" method, and anticipated future directions. Mass Spectrom Rev 2015;34:2-23. [Crossref] [PubMed]
- Hagenhoff S, Hayen H. LC/MS analysis of vitamin D metabolites by dielectric barrier discharge ionization and a comparison with electrospray ionization and atmospheric pressure chemical ionization. Anal Bioanal Chem 2018;410:4905-11. [Crossref] [PubMed]
- Couchman L, Benton CM, Moniz CF. Variability in the analysis of 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry: the devil is in the detail. Clin Chim Acta 2012;413:1239-43. [Crossref] [PubMed]
- Kobold U. Approaches to measurement of vitamin D concentrations - mass spectrometry. Scand J Clin Lab Invest Suppl 2012;243:54-9. [PubMed]
- Galior K, Ketha H, Grebe S, et al. 10 years of 25-hydroxyvitamin-D testing by LC-MS/MS-trends in vitamin-D deficiency and sufficiency. Bone Rep 2018;8:268-73. [Crossref] [PubMed]
- Stokes CS, Lammert F, Volmer DA. Analytical Methods for Quantification of Vitamin D and Implications for Research and Clinical Practice. Anticancer Res 2018;38:1137-44. [PubMed]
- Stepman HC, Vanderroost A, Van Uytfanghe K, et al. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 2011;57:441-8. [Crossref] [PubMed]
- Tai SS, Nelson MA. Candidate Reference Measurement Procedure for the Determination of (24R),25-Dihydroxyvitamin D3 in Human Serum Using Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem 2015;87:7964-70. [Crossref] [PubMed]
- Cavalier E, Souberbielle JC. Vitamin D and its metabolites: from now and beyond. EJIFCC 2018;29:105-10. [PubMed]
- Gutierrez B, Gao M, Sturgess J, et al. Performance Characteristics of the New Fully-Automated LC-MS/MS Total Vitamin D Assay on the CascadionTM SM Clinical Analyzer. MSACL 2018 EU. Available online: https://www.msacl.org/view_abstract/MSACL_2018_EU.php?id=610
Cite this article as: Zelzer S, Goessler W, Herrmann M. Measurement of vitamin D metabolites by mass spectrometry, an analytical challenge. J Lab Precis Med 2018;3:99.