
Recent advances in periodontitis, a prototypic osteo-immunological disease
Introduction
Bone is a dynamic tissue that balances the activities of bone formation and bone resorption. The dysbalance in favour of osteoclasts leads to pathological bone resorption as seen in osteopenic diseases including autoimmune arthritis, postmenopausal osteoporosis, or periodontitis. In addition, bone homeostasis is controlled by several regulatory systems, such as the immune system, as they share a variety of common regulatory molecules, such as cytokines. Furthermore, immune cells formed in the bone marrow interact with bone cells. An abnormal activation of the immune system often leads to bone destruction. As several tight connections have been recognized between the skeletal and immune systems, osteoimmunology has been created as an interdisciplinary research field that investigate the crosstalk between immune and skeletal systems (1-4). In this review, we will present novel advances in the understanding of bone loss due to periodontitis, which is a common and prototypic disease of osteoimmunology.
Periodontitis is a highly prevalent chronic infect-inflammatory disease that affects the tooth-supporting structures (periodontal ligament and alveolar bone), and if not treated, leads to tooth loss (5). The pathogenesis of periodontitis is multifactorial being initiated by a microbial challenge that promotes an exacerbated inflammatory response, which, in turn, is the main cause of tissue destruction (6).
In the recent years, there has been an increasing interest in the host response as a relevant factor that drives periodontal disease (PD) (7). The inflammatory immune responses, which result in the release of an array of cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α, promotes leukocyte recruitment into the gingival tissue (8). Later, T and B lymphocytes are activated by either bacteria or antigen presenting cells. Not only in the case of PD but also in other diseases, T cells have been considered important regulators of bone turnover (9). In fact, T cells can activate macrophages, or indirectly activate osteoclasts and their precursors and also they can directly express receptor activator of nuclear factor κB ligand (RANKL) (9) by the so called “pro-resorptive” cytokines (IL-1, IL-6, IL-11). These cytokines act in a network with the cells, however, their relationship with clinical manifestations of PDs is not clear yet (10,11). In addition, during the last years, the research in the field of periodontics have focused on Th-17, a subpopulation of T-lymphocytes characterized by the production of IL-17, which seems to be strongly correlated to tissue destruction (12).
Inflammation has been reported to inhibit osteoblast differentiation by modulating the Wnt signaling pathway (13) or impacting the expression of Wnt agonists and antagonists (14).
The Wnt signaling pathway is a critical bone-anabolic cascade which has recently been implicated in periodontitis (15-18). Wnt signaling stimulates osteoblast differentiation and/or function and consequently embryonic and postnatal bone formation. Increased Wnt signaling might also reduce osteoclastogenesis and bone resorption by stimulating the expression of osteoprotegerin (OPG) by osteoblasts (19-22). Inflammation has been reported to inhibit osteoblast differentiation by modulating the Wnt signaling pathway (14) or impacting the expression of Wnt agonists and antagonists (13) however, the pivotal uncoupling signal is still unknown.
In this context, this review aims to highlight the role of host response on inflammatory bone loss during periodontitis, emphasizing not only the importance of the RANKL-RANK-OPG axis but also discussing the role of Wnt signaling in periodontal bone loss.
Pathogenesis of periodontitis
PD is very prevalent and affects almost 90% of the adult population, even though it may also occur in children and adolescents. This disease is considered the second major cause of tooth loss in the world population (23).
The term “periodontal diseases” refer to the common inflammatory conditions of gingiva and/or periodontium. PD is caused by the accumulation of a biofilm that forms adjacent to the teeth and promotes localized inflammation called gingivitis, the mildest form of PD, which is readily reversible by simple oral hygiene. On the other hand, when inflammation extends deep into the tissues and causes loss of supporting connective tissue and alveolar bone, it is known as periodontitis, which is mostly an irreversible condition (5,24).
Various mechanisms contribute to the aetiopathology of PD. The oral microbial biofilm has been extensively studied and a single person can comprise several species (25). For a long time, PD was believed to be only initiated and sustained by the microorganisms of the dental biofilm, especially gram-negative anaerobic bacteria, such as Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis (26). However, nowadays, it is well established that periodontitis is not a matter of a single pathogen, but rather an imbalance of the microbial biofilm, known as dysbiosis (27). Moreover, the presence of the microbial biofilm itself might not be sufficient for the pathogenesis of PD (28). In order for PD take place, a susceptible host is also needed. It has been also suggested that gut microbiota might have a role on periodontitis due to dysregulation of immunomodulation (29,30). Similarly, also disturbance of the composition of gut microbiota induced by oral periodontopathic bacteria could be a causal mechanism connecting periodontitis and systemic disease (31).
Thus, it is now understood that the destruction of structural components of the periodontium, leading to clinical signs of periodontitis, is mainly due to an exacerbated immune-inflammatory response to the chronic presence of biofilm in the periodontal tissues (5). Therefore, PD is an interesting model to understand the role of immune system on bone loss.
In this context, both host and bacteria can elicit immune responses (32). In the beginning, chemotactic factors are released and recruit leucocytes into the tissues. Neutrophil, granulocyte, and lymphocyte infiltration into the periodontal lesion ensues: neutrophils attempt to engulf and kill bacteria, but are overwhelmed by the magnitude and chronic persistence of the microbial biofilm and various proteolytic enzymes are released promoting tissue breakdown. Meanwhile, dendritic Langerhans cells within the epithelium take up microbial antigenic material and bring it to the lymphoid tissue for presentation to lymphocytes in order to evoke both humoral antibody-mediated and cell-mediated immune responses. Although, this is usually a protective response, the sustained microbial challenge promotes the breakdown of both soft and hard tissues. Without any treatment, active periodontitis leads to tooth loss (5).
T cell-mediated responses in periodontitis
After the initial response, the infection activates the adaptive immune response where dendritic cells other than participating to the innate inflammatory response have the ability to capture and present antigens to lymphocytes of the acquired immune system. In this context, special attention has been given to the CD4+ T-helper cells (33,34).
CD4+ T-cells were initially subdivided into two subsets, designated T-helper 1 (Th1) and T-helper 2 (Th2), on the basis of their pattern of cytokine production (35). As a general rule, Th1 cytokines are associated with infectious inflammatory bone destruction (36-38), while its classic antagonists Th2 cytokines are described to minimize bone loss (10,39). Indeed, it has been demonstrated that the development of periodontitis is mainly mediated by the imbalance between the Th1 and Th2 subsets (38,40-44).
In humans, studies have supported the hypothesis that Th1 cells are associated with stable lesions and Th2 cells are associated with progressive lesions (45,46). In contrast, other studies have demonstrated that upregulation of Th1 responses or downregulation of Th2 responses are involved in periodontal tissue destruction (47-49). Moreover, others have shown a comparable presence of Th1 and Th2 cytokines in human periodontitis lesions (50-52). Therefore, it has become apparent that the pathogenesis of periodontitis cannot be fully explained through the prism of the Th1/Th2 paradigm. More detailed research into novel Th subtypes have led to the discovery of novel cytokines that could not be attributed to the classical T helper subsets, and thus resulted in the emergence of new T-cell subsets, described as Th17 and T regulatory (Treg) cells, which have overall antagonistic roles (53).
Th17 cells express the transcription factors RORγt and RORα and produce IL-17A, IL-17F, IL-21, IL-22, and IL-26. It has been reported that cytokines such as IL-1β, IL-6, IL-21, IL-23, and TGF-β are important for Th17 differentiation and development (44). Th17 cells play a protective role against extracellular bacteria and fungi. IL-17 receptor (IL-17R) deficient mice display a significant delay in neutrophil recruitment into infected sites, thus resulting in susceptibility to infection (54). When these mice were exposed to organisms such as P. gingivalis, they developed increased periodontal bone destruction (54) (Figure 1).
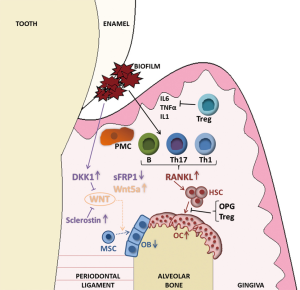
Th17 cells are also related to several autoimmune and inflammatory disorders (55). Th17 lymphocytes are defined as an osteoclastogenic T helper subset because they induce the expression of RANKL on osteoclastogenesis-supporting cells such as osteoblasts and synovial fibroblasts. Additionally, IL-17, the key cytokine of Th17 cells, enhances local inflammation and increases the production of inflammatory cytokines such as TNF, IL-1, and IL-6, which further promote RANKL expression. Finally, Th17 cells themselves express RANKL and promote osteoclastogenesis (56). Patients with periodontitis have elevated mRNA expression of Th17 inducing cytokines IL-1β, IL-6, and IL-21 when compared with the healthy control tissue (57). Therefore, Th17 cells are key mediators of periodontitis-induced bone loss.
Regulatory T cells (Tregs), on the other hand, suppress the activation, proliferation, and effector functions of a wide range of immune cells, and play a crucial role in maintaining immune homeostasis in health and under inflammatory conditions (58). To date, several types of Tregs have been identified, the two main ones being naturally occurring Foxp3+ Tregs, which develop in the thymus, and inducible Tregs (e.g., IL-10-producing Tr1 cells, TGF-β-producing cells, inducible Foxp3+ Tregs), which develop in the periphery after exposure to various signals (59). During periodontitis, the immune response needs to be controlled in order to avoid the pathogenic microorganism dissemination and, at the same time, prevent collateral tissue damage. Therefore, Tregs preferentially accumulate at infected tissues, limiting the immune responses (60). Intriguingly, Tregs also regulate bone metabolism by directly inhibiting osteoclastogenesis, which is most likely mediated by Treg-derived cytokines such as TGF-β, IL-10, and IL-4, or CTLA-4 (61). Therefore, Foxp3+ Tregs are suggested to play an essential role in the regulation of inflammation in periodontal lesions. Several reports have demonstrated increased infiltration of Tregs within the infected periodontal tissue (60,62,63) (Figure 1). Despite the increase in the number of Tregs during periodontitis, it is possible that a fraction of these cells loses their suppressive functions due to the inflammatory periodontal environment enriched in IL-6 (64). However, Ernst et al. reported that Foxp3+CD25+ cells were reduced in periodontitis lesions, but the reason for the discrepancy has not been clarified (65). In summary, the lack of function or the decreased amount of Treg cells in periodontium can contribute to the exacerbated immune response that leads to disease progression or reactivation.
Inflammation-induced bone loss in periodontitis
Osteoclasts: role of RANKL/OPG
The RANKL/OPG network plays an important role in osteoclast formation and activity, and thereby regulates bone resorption (66,67). When RANKL binds to its receptor RANK on the surface of pre-osteoclasts, a signaling cascade is initiated, which leads to activation of transcription factors such as NF-κB, nuclear factor of activated T cells (NFAT) or activator-protein 1 (AP-1) promoting the development of mature osteoclasts (68,69). RANKL-knockout mice have a severe osteopetrotic phenotype due to lack of osteoclasts (69,70). The phenotype of RANK-knockout mice is similar to that of RANKL-knockout mice (69,71). OPG, on the other hand, is a soluble decoy receptor for RANKL and inhibits its interaction with RANK, thereby blocking osteoclast differentiation. Mice overexpressing OPG develop an osteopetrotic phenotype due to inhibition of osteoclast development by inhibiting RANKL (72). Therefore, the RANKL/RANK/OPG system is critical for osteoclastogenesis, bone resorption, and bone homeostasis.
Under inflammatory conditions, the RANKL/OPG ratio is often increased leading to progressive bone loss. Also in PD, this cytokine system was subject of intense investigations. RANKL and OPG are expressed in gingival tissue and biological fluids such as saliva and serum. During gingivitis or periodontitis, RANKL expression is increased while OPG is decreased leading to an increased RANKL/OPG ratio. However, periodontal treatment does not influence the ratio as its level is constantly high even after treatment suggesting that the process of bone resorption is still ongoing and might represent a potential risk of relapsing the disease. Furthermore this implicates the potential use of the RANKL/OPG ratio just as biomarker for the untreated PD but not as a predictor of the treatment outcome (73-76). A challenging question that was answered during the last years is: where does the RANKL come from? As such, B and T lymphocytes were identified as the major sources of RANKL in the bone lesions in PD via double-color confocal microscopic analyses of healthy and periodontal gingival tissues (77,78). Interestingly, the increase of the RANKL/OPG ratio in PD appears to be related to a specific immune response. The increase of RANK- and RANKL-positive cells and the decrease of cells positive for OPG was associated with an induction of lymphocyte infiltration and high levels of MCP-1 and CCL5 suggesting that excessive infiltration of lymphocytes and a high RANKL/OPG ratio both may contribute to periodontitis-induced bone loss (76,77,79). The role of RANKL in periodontitis-induced bone loss was finally addressed in mice using OPG or specific antibodies to block RANKL. Inhibiting RANKL prevented alveolar bone loss in experimental periodontitis (76,80). Similarly, mice with an overexpression of RANK develop a periodontitis-like phenotype with profound loss of alveolar bone at the age of 5 months (81). In line with this study, also OPG-deficient (OPG−/−) mice develop distinct loss of alveolar bone and increased bone resorption. Importantly, treatment with WP9QY, a RANKL-binding peptide, suppressed osteoclastogenesis while osteoblastogenesis was induced leading to restoration of bone mass in OPG−/− animals. Compared to the conventional bisphosphonate risedronate, WP9QY additionally stimulated Wnt/β-catenin signaling and bone formation in OPG-deficient mice, whereas risedronate only decreased the number of osteoclasts in alveolar bone. Furthermore, sclerostin, an inhibitor of Wnt/β-catenin signaling, was decreased in bone tissue of OPG-deficient mice compared to wild-type mice and treatment with WP9QY further suppressed its expression. This suggests that by influencing the RANK/RANKL/OPG system for instance by inhibiting RANKL and mitigating sclerostin expression using WP9QY, periodontitis-induced bone loss can be prevented (82).
More recent studies show that not only B and T cells contribute to RANKL production in periodontal tissue, but also osteocytes. Mice with a specific deletion of RANKL in late osteoblasts and osteocytes were protected from periodontitis-induced bone loss as they failed to increase RANKL expression during periodontitis. This study further showed that in a diabetic state, which worsens periodontitis, mice with an osteocyte-specific deletion of RANKL were protected from alveolar bone loss (83).
In humans, a similar increase in RANKL and RANK immunohistochemical expression in gingival tissue of patients with periodontitis was reported, while OPG levels were decreased. Furthermore, the increased RANKL/OPG ratio was connected with the tissue destruction in periodontitis (84). Another study investigated the levels of these proteins in the gingival crevicular fluid of patients with post-menopausal osteoporosis and PD which were under bisphosphonate therapy. They focused on periodontal active sites and found no influence of bisphosphonate treatment on the levels of RANK, RANKL, and OPG, which is maybe because these cytokines are not the main targets of bisphosphonates (85). A newer study focused on the expression levels of RANKL, OPG, and TNF in chronic periodontitis as well as rheumatoid arthritis patients before and after initial periodontal treatment. They collected serum as well as gingival crevicular fluid of 17 patients with RA, 18 patients with chronic periodontitis, and 18 healthy controls. While OPG levels were reduced, RANKL was significantly increased in the gingival crevicular fluid of patients suffering from periodontitis compared to healthy control patients (77,86,87). Furthermore, local levels of OPG were higher compared to periodontitis patients and treatment of periodontitis significantly improved clinical parameters (86). In conclusion RANKL/RANK/OPG signaling is involved in the pathogenesis of PD (Figure 1) and offers potential for the treatment of bone loss as a consequence of PD.
Osteoblasts: role of Wnt signaling
One meaningful pathway that is important for the development of osteoblasts is the highly conserved Wnt pathway (88). Wnts are secreted glycoproteins that are involved in morphogenesis, embryogenesis, and cellular differentiation, and furthermore, are important regulators of bone biology (89). Wnts act via several signaling cascades that are normally divided into the canonical or the non-canonical pathway. The canonical pathway is β-catenin-dependent and is important for the maintenance of bone mass. Binding of canonical Wnt ligands such as Wnt1, Wnt3, Wnt3a, Wnt8, or Wnt10b to the receptor Frizzled (FZD)-5 and the co-receptor low-density lipoprotein receptor (LRP)-5/6 leads to stabilization of β-catenin, which in absence of Wnts would be ubiquitinylated and degraded. The stabilization leads to translocation into the nucleus and the activation of gene expression alone or in combination with T cell factor/lymphoid enhancer factor 1. Non-canonical Wnts such as Wnt4, Wnt5a, or Wnt11 activate alternative pathways such as the Wnt/Ca2+ pathway or the Wnt/planar polarity pathway, modulating cytoskeletal organization and gene expression (69,89,90). Wnt signal is regulated via secreted inhibitor proteins for instance members of the dickkopf (DKK) and secreted frizzled-related protein family, Wnt inhibitory factor 1, and sclerostin (Sost). They act via binding directly to the Wnt ligands and FZDs or by interfering with the LRP-5/6 co-receptors (89,91). Thus, members of the DKK family as well as Sost only inhibit canonical Wnt signaling while members of the sFRP family can block both, canonical and non-canonical Wnt signaling.
In periodontitis, the expression of Wnt5a and sFRP5 is regulated reciprocally compared to healthy conditions as Wnt5a is up-regulated during the disease while high concentrations of sFRP5 are found in healthy tissue (Figure 1). This was also confirmed in vitro as lipopolysaccharide (LPS) increased Wnt5a and decreased sFRP5 expression in human gingival epithelial cells, and furthermore, Wnt5a treatment induced LPS-induced inflammation which in contrast, was diminished after treatment with sFRP5. Moreover, treatment with sFRP5 led to inhibition of experimental periodontitis (92).
A study published in 2014 focused on the involvement of the two Wnt antagonists in the pathogenesis of human chronic periodontitis. Even though the number of subjects was rather low (15 healthy and 15 chronic periodontitis subjects), the mRNA expression in the periodontal tissues as well as the serum levels of sclerostin and DKK-1 were significantly higher in the periodontitis group (Figure 1). This suggests that these molecules participate in the pathogenesis of periodontitis (15). In line with this, a recent publication showed that blocking DKK-1, which is induced under inflammatory conditions, could provide a potential therapeutic opportunity to prevent bone destruction in PDs (18). MC3T3 cells treated with Escherichia coli (E. coli) LPS showed increased DKK-1 protein levels during osteogenic differentiation. After blocking DKK-1 using siRNA the E. coli LPS-inhibited osteogenic differentiation was rescued. Furthermore, DKK-1 siRNA treatment of rats with a periapical lesion decreased bone loss resulting from inflammation (18). Similar to DKK-1, also sclerostin plays a pathogenic role in periodontitis-induced bone loss. Deletion of sclerostin prevents bone loss in periostin knockout mice (93). These results are in accordance with Taut et al. showing that sclerostin antibody treatment led to a stimulation of bone regeneration in an experimental model of periodontitis in rats (94). Moreover, there are more studies focussing on the involvement on the Wnt/β-catenin signaling inhibitor sclerostin and its blockade. For instance, in an ovariectomized rat model of induced experimental periodontitis, treatment with sclerostin antibody led to increased alveolar crest height and bone mass accrual (95). Finally, sclerostin inhibition using an antibody alone or in combination with DKK-1 co-inhibition led to an increase of alveolar bone volume and architecture in rats suffering from alveolar bone loss suggesting that DKK-1 as well as sclerostin play a role in alveolar bone accrual during estrogen-deficient and edentulous states (96). As sclerostin inhibition has been associated with a compensatory increase in DKK-1 and vice versa (97-99), the dual inhibition of sclerostin and DKK-1 using bispecific antibodies are particularly promising to treat diseases with excessive suppression of bone formation.
Taken together, these data indicate that Wnt signaling and especially its inhibition by sclerostin or DKK-1 plays an important role in the pathogenesis of periodontitis and that reactivation of Wnt signal may be an effective therapy to ameliorate alveolar bone loss in periodontitis.
Conclusions
Periodontitis is a disease that affects the tooth-supporting structures and the host response has been considered one of the main etiological factors of this disease. Besides a dysbalance in the classical Th1 and Th2 subsets, Th17 and Tregs have emerged as new players in periodontitis. These cells do not only contribute to the exacerbation of the inflammatory process, but also directly affect bone cell activity to promote bone loss. As such, the RANKL-RANK-OPG axis and more recently Wnt signaling have been identified as key pathways that mediate the pathogenesis of periodontitis-induced bone loss. In this sense, due to the close relationship between bone tissue and immune system, periodontitis is a valuable disease model to expand the knowledge on osteoimmunology.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to ST and MR, and the Brazilian National Council for Scientific and Technological Development (CNPq) to PG.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Markus Herrmann and Barbara Obermayer-Pietsch) for the series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2018.12.01). The series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Periodontal Res 2005;40:287-93. [Crossref] [PubMed]
- Tompkins KA. The osteoimmunology of alveolar bone loss. Connect Tissue Res 2016;57:69-90. [Crossref] [PubMed]
- Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol 2007;143:31-48. [Crossref] [PubMed]
- Rauner M, Sipos W, Thiele S, et al. Advances in Osteoimmunology: Pathophysiologic Concepts and Treatment Opportunities. Int Arch Allergy Immunol 2013;160:114-25. [Crossref] [PubMed]
- Pihlstrom BL, Michalowicz B, Johnson N. Periodontal diseases. Lancet 2005;366:1809-20. [Crossref] [PubMed]
- Giannobile WV. Host-Response Therapeutics for Periodontal Diseases. J Periodontol 2008;79:1592-600. [Crossref] [PubMed]
- Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci 2015;23:329-55. [Crossref] [PubMed]
- Kayal RA. The Role of Osteoimmunology in Periodontal Disease. Biomed Res Int 2013;2013:639368 [Crossref] [PubMed]
- Brunetti G, Colucci S, Pignataro P, et al. T Cells Support Osteoclastogenesis in an In Vitro Model Derived From Human Periodontitis Patients. J Periodontol 2005;76:1675-80. [Crossref] [PubMed]
- Hienz SA, Paliwal S, Ivanovski S. Mechanisms of Bone Resorption in Periodontitis. J Immunol Res 2015;2015:615486 [Crossref] [PubMed]
- Souza PP, Lerner UH. The role of cytokines in inflammatory bone loss. Immunol Invest 2013;42:555-622. [Crossref] [PubMed]
- Dar HY, Azam Z, Anupam R, et al. Osteoimmunology: The Nexus between bone and immune system. Front Biosci (Landmark Ed) 2018;23:464-92. [Crossref] [PubMed]
- Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteoporos Rep 2014;12:9-16. [Crossref] [PubMed]
- Tang Y, Zhou X, Gao B, et al. Modulation of Wnt/β-catenin signaling attenuates periapical bone lesions. J Dent Res 2014;93:175-82. [Crossref] [PubMed]
- Napimoga MH, Nametala C, Da Silva FL, et al. Involvement of the Wnt-β-catenin signaling antagonists, sclerostin and dickkopf-related protein 1, in chronic periodontitis. J Clin Periodontol 2014;41:550-7. [Crossref] [PubMed]
- Sousa LH, Linhares EVM, Alexandre JT, et al. Effects of Atorvastatin on Periodontitis of Rats Subjected to Glucocorticoid-Induced Osteoporosis. J Periodontol 2016;87:1206-16. [Crossref] [PubMed]
- Liu X, Zhang Z, Pan S, et al. Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res 2018;53:842-52. [Crossref] [PubMed]
- Tan X, Huang D, Zhou W, et al. Dickkopf-1 may regulate bone coupling by attenuating wnt/β-catenin signaling in chronic apical periodontitis. Arch Oral Biol 2018;86:94-100. [Crossref] [PubMed]
- Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Dev Cell 2005;8:751-64. [Crossref] [PubMed]
- Glass DA, Karsenty G. Canonical Wnt Signaling in Osteoblasts Is Required for Osteoclast Differentiation. Ann N Y Acad Sci 2006;1068:117-30. [Crossref] [PubMed]
- Spencer GJ. Wnt signaling in osteoblasts regulates expression of the receptor activator of NFκB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 2006;119:1283-96. [Crossref] [PubMed]
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007;13:156-63. [Crossref] [PubMed]
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000 2012;60:15-39. [Crossref] [PubMed]
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Prim 2017;3:17038. [Crossref] [PubMed]
- Lourenço TG, Heller D, Silva-Boghossian CM, et al. Microbial signature profiles of periodontally healthy and diseased patients. J Clin Periodontol 2014;41:1027-36. [Crossref] [PubMed]
- Lovegrove JM. Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodontol 2004;87:7-21. [PubMed]
- Feres M, Teles F, Teles R, et al. The subgingival periodontal microbiota of the aging mouth. Periodontol 2000 2016;72:30-53. [Crossref] [PubMed]
- Kinane DF, Mark Bartold P. Clinical relevance of the host responses of periodontitis. Periodontol 2000 2007;43:278-93. [Crossref] [PubMed]
- Medina-Gomez C. Bone and the gut microbiome: a new dimension. J Lab Precis Med 2018;3:96. [Crossref]
- Xiao E, Mattos M, Vieira GH, et al. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017;22:120-8.e4. [Crossref] [PubMed]
- Nakajima M, Arimatsu K, Kato T, et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 2015;10:e0134234 [Crossref] [PubMed]
- Benakanakere M, Kinane DF. Innate Cellular Responses to the Periodontal Biofilm. Front Oral Biol 2012;15:41-55. [Crossref] [PubMed]
- Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med 2001;12:125-35. [Crossref] [PubMed]
- Campbell L, Millhouse E, Malcolm J, et al. T cells, teeth and tissue destruction - what do T cells do in periodontal disease? Mol Oral Microbiol 2016;31:445-56. [Crossref] [PubMed]
- Seymour GJ, Gemmell E, Kjeldsen M, et al. Cellular immunity and hypersensitivity as components of periodontal destruction. Oral Dis 1996;2:96-101. [Crossref] [PubMed]
- Kotake S, Nanke Y, Mogi M, et al. IFN-γ-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol 2005;35:3353-63. [Crossref] [PubMed]
- Stashenko P, Gonçalves RB, Lipkin B, et al. Th1 Immune Response Promotes Severe Bone Resorption Caused by Porphyromonas gingivalis. Am J Pathol 2007;170:203-13. [Crossref] [PubMed]
- Fukada SY, Silva TA, Garlet GP, et al. Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol Immunol 2009;24:25-31. [Crossref] [PubMed]
- Chen Z, Andreev D, Oeser K, et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat Commun 2016;7:11596. [Crossref] [PubMed]
- Alayan J, Ivanovski S, Farah CS. Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodontal Res 2007;42:97-103. [Crossref] [PubMed]
- Zhao L, Zhou Y, Xu Y, et al. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J Clin Periodontol 2011;38:509-16. [Crossref] [PubMed]
- Gonzales JR, Gröger S, Boedeker RH, et al. Expression and secretion levels of Th1 and Th2 cytokines in patients with aggressive periodontitis. Clin Oral Investig 2012;16:1463-73. [Crossref] [PubMed]
- Souto GR, Queiroz-Junior CM, de Abreu MH, et al. Pro-inflammatory, Th1, Th2, Th17 Cytokines and Dendritic Cells: A Cross-sectional Study in Chronic Periodontitis. PLoS One 2014;9:e91636 [Crossref] [PubMed]
- Okui T, Aoki Y, Ito H, et al. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res 2012;91:574-9. [Crossref] [PubMed]
- Tokoro Y, Matsuki Y, Yamamoto T, et al. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol 1997;107:166-74. [Crossref] [PubMed]
- Sigusch B, Klinger G, Glockmann E, et al. Early-Onset and Adult Periodontitis Associated With Abnormal Cytokine Production by Activated T Lymphocytes. J Periodontol 1998;69:1098-104. [Crossref] [PubMed]
- Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000 1994;5:112-41. [Crossref] [PubMed]
- Takeichi O, Haber J, Kawai T, et al. Cytokine Profiles of T-lymphocytes from Gingival Tissues with Pathological Pocketing. J Dent Res 2000;79:1548-55. [Crossref] [PubMed]
- Ukai T, Mori Y, Onoyama M, et al. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol 2001;46:901-8. [Crossref] [PubMed]
- Wassenaar A, Reinhardus C, Thepen T, et al. Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect Immun 1995;63:2147-53. [PubMed]
- Nakajima T, Yamazaki K, Cullinan MP, et al. T-cell antigen specificity in humans following stimulation with Porphyromonas gingivalis. Arch Oral Biol 1999;44:1045-53. [Crossref] [PubMed]
- Berglundh T, Liljenberg B, Lindhe J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol 2002;29:705-9. [Crossref] [PubMed]
- Gaffen SL, Hajishengallis G. A New Inflammatory Cytokine on the Block: Re-thinking Periodontal Disease and the Th1/Th2 Paradigm in the Context of Th17 Cells and IL-17. J Dent Res 2008;87:817-28. [Crossref] [PubMed]
- Yu JJ, Ruddy MJ, Wong GC, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 2007;109:3794-802. [Crossref] [PubMed]
- Stadhouders R, Lubberts E, Hendriks RW. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J Autoimmun 2018;87:1-15. [Crossref] [PubMed]
- Ganesan R, Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol 2017;91:134-44. [Crossref] [PubMed]
- Dutzan N, Vernal R, Vaque JP, et al. Interleukin-21 Expression and Its Association With Proinflammatory Cytokines in Untreated Chronic Periodontitis Patients. J Periodontol 2012;83:948-54. [Crossref] [PubMed]
- Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest 2003;112:1310-2. [Crossref] [PubMed]
- Alvarez C, Rojas C, Rojas L, et al. Regulatory T Lymphocytes in Periodontitis: A Translational View. Mediators Inflamm 2018;2018:7806912 [Crossref] [PubMed]
- Cardoso CR, Garlet GP, Moreira AP, et al. Characterization of CD4 + CD25 + natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol 2008;84:311-8. [Crossref] [PubMed]
- Bozec A, Zaiss MM. T Regulatory Cells in Bone Remodeling. Curr Osteoporos Rep 2017;15:121-5. [Crossref] [PubMed]
- Nakajima T, Ueki-Maruyama K, Oda T, et al. Regulatory T-cells Infiltrate Periodontal Disease Tissues. J Dent Res 2005;84:639-43. [Crossref] [PubMed]
- Araujo-Pires AC, Vieira AE, Francisconi CF, et al. IL-4/CCL22/CCR4 Axis Controls Regulatory T-Cell Migration That Suppresses Inflammatory Bone Loss in Murine Experimental Periodontitis. J Bone Miner Res 2015;30:412-22. [Crossref] [PubMed]
- Takahashi K, Takashiba S, Nagai A, et al. Assessment of Interleukin-6 in the Pathogenesis of Periodontal Disease. J Periodontol 1994;65:147-53. [Crossref] [PubMed]
- Ernst CWO, Lee JE, Nakanishi T, et al. Diminished forkhead box P3/CD25 double-positive T regulatory cells are associated with the increased nuclear factor-kB ligand (RANKL+) T cells in bone resorption lesion of periodontal disease. Clin Exp Immunol 2007;148:271-80. [Crossref] [PubMed]
- Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745-55. [Crossref] [PubMed]
- Kondo T, Kitazawa R, Yamaguchi A, et al. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J Cell Biochem 2008;103:335-45. [Crossref] [PubMed]
- Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004;428:758-63. [Crossref] [PubMed]
- Pietschmann P. Principles of Osteoimmunology. Springer Vienna, 2012.
- Martin TJ, Gillespie MT. Receptor activator of nuclear factor kappa B ligand (RANKL): another link between breast and bone. Trends Endocrinol Metab 2001;12:2-4. [Crossref] [PubMed]
- Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev 1999;13:2412-24. [Crossref] [PubMed]
- Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309-19. [Crossref] [PubMed]
- Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol 2012;39:239-48. [Crossref] [PubMed]
- Taubman MA, Kawai T, Han X. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J Clin Periodontol 2007;34:367-9. [Crossref] [PubMed]
- Bostanci N, Ilgenli T, Emingil G, et al. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: Implications of their relative ratio. J Clin Periodontol 2007;34:370-6. [Crossref] [PubMed]
- Xiao W, Li S, Pacios S, et al. Bone Remodeling under Pathological Conditions. Front Oral Biol 2016;18:17-27. [Crossref] [PubMed]
- Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 2006;169:987-98. [Crossref] [PubMed]
- Chen B, Wu W, Sun W, et al. RANKL expression in periodontal disease: Where does RANKL Come from? Biomed Res Int 2014;2014:731039 [PubMed]
- Gibertoni F, Sommer MEL, Esquisatto MAM, et al. Evolution of Periodontal Disease: Immune Response and RANK/RANKL/OPG System. Braz Dent J 2017;28:679-87. [Crossref] [PubMed]
- Jin Q, Cirelli JA, Park CH, et al. RANKL Inhibition Through Osteoprotegerin Blocks Bone Loss in Experimental Periodontitis. J Periodontol 2007;78:1300-8. [Crossref] [PubMed]
- Sojod B, Chateau D, Mueller CG, et al. RANK/RANKL/OPG signalization implication in periodontitis: New evidence from a RANK transgenic mouse model. Front Physiol 2017;8:338. [Crossref] [PubMed]
- Ozaki Y, Koide M, Furuya Y, et al. Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis. PLoS One 2017;12:e0184904 [Crossref] [PubMed]
- Graves DT, Alshabab A, Albiero ML, et al. Osteocytes play an important role in experimental periodontitis in healthy and diabetic mice through expression of RANKL. J Clin Periodontol 2018;45:285-92. [Crossref] [PubMed]
- Giannopoulou C, Martinelli-Klay CP, Lombardi T. Immunohistochemical expression of RANKL, RANK and OPG in gingival tissue of patients with periodontitis. Acta Odontol Scand 2012;70:629-34. [Crossref] [PubMed]
- Verde ME, Bermejo D, Gruppi A, et al. Effect of Bisphosphonates on the Levels of Rankl and Opg in Gingival Crevicular Fluid of Patients With Periodontal Disease and Post-menopausal Osteoporosis. Acta Odontol Latinoam 2015;28:215-21. [PubMed]
- Balci Yuce H, Gokturk O, Aydemir Turkal H, et al. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J Oral Sci 2017;59:397-404. [Crossref] [PubMed]
- Bostanci N, Ilgenli T, Emingil G, et al. Differential expression of receptor activator of nuclear factor-κB ligand and osteoprotegerin mRNA in periodontal diseases. J Periodontal Res 2007;42:287-93. [Crossref] [PubMed]
- Bodine PVN, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord 2006;7:33-9. [Crossref] [PubMed]
- Westendorf JJ, Kahler R, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene 2004;341:19-39. [Crossref] [PubMed]
- Behrens J. Cross-regulation of the Wnt signaling pathway: a role of MAP kinases. J Cell Sci 2000;113:911-9. [PubMed]
- Kawano Y, Kypta R. Secreted antagonists of the Wnt signaling pathway. J Cell Sci 2003;116:2627-34. [Crossref] [PubMed]
- Maekawa T, Kulwattanaporn P, Hosur K, et al. Differential Expression and Roles of Secreted Frizzled-Related Protein 5 and the Wingless Homolog Wnt5a in Periodontitis. J Dent Res 2017;96:571-7. [Crossref] [PubMed]
- Ren Y, Han X, Ho SP, et al. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J 2015;29:2702-11. [Crossref] [PubMed]
- Taut AD, Jin Q, Chung JH, et al. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 2013;28:2347-56. [Crossref] [PubMed]
- Chen H, Xu X, Liu M, et al. Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone 2015;76:141-8. [Crossref] [PubMed]
- Liu M, Kurimoto P, Zhang J, et al. Sclerostin and DKK1 Inhibition Preserves and Augments Alveolar Bone Volume and Architecture in Rats with Alveolar Bone Loss. J Dent Res 2018;97:1031-8. [Crossref] [PubMed]
- Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 2016;7:11505. [Crossref] [PubMed]
- Colditz J, Thiele S, Baschant U, et al. Postnatal skeletal deletion of Dickkopf-1 increases bone formation and bone volume in male and female mice, despite increased sclerostin expression. J Bone Miner Res 2018;33:1698-707. [Crossref] [PubMed]
- Witcher PC, Miner SE, Horan DJ, et al. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight 2018; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Thiele S, Rauner M, Goes P. Recent advances in periodontitis, a prototypic osteo-immunological disease. J Lab Precis Med 2018;3:101.


