
Sclerostin, bone morphogenetic protein, Wnt and the lung: a potential role beyond bone metabolism?
Introduction
Sclerostin
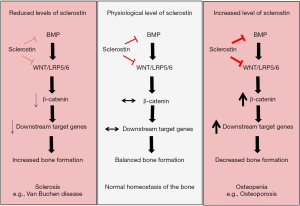
Sclerostin is a secreted glycoprotein, encoded by the SOST gene (1,2). SOST was identified by linkage analysis in patients with Van Buchen disease (MIM 269500) in 2001, a condition where sclerosis of the skeleton occurs with progressive bone mass increase (3-5). It has been shown, that a homozygous mutation in the SOST or in the enhancer element (Chr 17p21)—which drives SOST expression—is responsible for the skeletal sclerosis (6). Sclerostin has been hence described as a negative regulator of bone growth and mineralization, and the human genetic phenotype was confirmed in animal models. Over-expression of SOST in mice causes strong osteopenia (7,8), while SOST knock-out mice develop sclerosis of the skeleton mirroring the human disease (9). As indicated by the strong phenotype induced by the lack or over-expression of SOST, sclerostin is extremely abundant in osteocytes, where it activates bone resorption (10).
Sclerostin: mechanism of action in the bone
Bone morphogenetic protein (BMP) pathway
Sclerostin belongs to the differential screening-selected gene aberrant in the neuroblastoma (DAN) protein family (2,3). As the other DAN proteins such as gremlin or noggin, sclerostin presents a cysteine knot structure which gives the possibility to antagonize BMP activity by competing with the BMP receptor (2). BMP signalling is constituted of BMP ligands (BMP2, 4 and 9) binding to BMP receptor type 1 (BMPR1) and BMP receptor type 2 (BMPR2) which in turn activate the transcription factor SMAD1 and 5 and hence downstream target genes (11). It was first reported that sclerostin exerts its negative effect on the bone by inhibiting BMP effect on osteoblasts (12). Later, it was discovered that sclerostin is actually not expressed by osteoblasts, but by osteocytes and once secreted it induces osteoblast apoptosis by inhibiting BMP6 induced signalling (8). It was also observed that the inhibitory effect of sclerostin on BMP was not direct but rather an indirect mechanism, suggesting that sclerostin may inhibit BMP signalling by interacting with an intermediate factor or pathway which in turn affects BMP signalling (10).
Wnt pathway
Wnt signalling consists of β-catenin-dependent (canonical) and -independent (non-canonical) pathways. In the β-catenin-dependent pathway, when there is no Wnt ligand, the cytoplasmic β-catenin is phosphorylated and therefore targeted for ubiquitination via proteasome-mediated proteolysis. When a Wnt ligand is present, it binds to LRP5/6 receptor and frizzled (FZD) co-receptor which leads to a stabilization of the cytoplasmic β-catenin. β-catenin then translocates into the nucleus and activates downstream target genes involved in cell proliferation and survival. The β-catenin-independent pathway is LRP5/6-independent and causes increased intracellular Ca2+, activation of calcium-dependent proteins and the rearrangement of cytoskeleton, leading to cell proliferation and migration (13). More recently, sclerostin has been reported to bind to the receptor LRP5/6, sequestering it from the frizzled (FZD) co-receptor and thereby leading to inhibition of the wingless (Wnt) canonical pathway (14). In light of this study, van Bezooijen et al. have proposed and expanded the concept that sclerostin is an indirect antagonist of BMP signalling by showing that the BMP-inhibitory property of sclerostin lies in the modulation of Wnt pathway (15). The authors showed that sclerostin—by binding to LRP5/6—antagonizes Wnt ligand and inhibit BMP-induced activation of Wnt signalling, which is necessary for alkaline phosphatase activation during bone formation (15) (Figure 1).
Beyond the bone: focus on the lung
Pulmonary hypertension (PH): BMP and Wnt signalling
BMP and Wnt pathways are extremely important for bone remodelling, however, their expression and signalling are present in other organs and tissues as well, therefore their actions are not exclusively relevant for the bone. Interestingly, it has been shown that alterations on BMP and Wnt signalling can lead to pathological manifestations, such as PH (16). PH is a rare disease characterized by increased pulmonary vascular resistance which leads to remodelling of the smooth muscle and endothelial layer of the pulmonary arteries leading to narrowing or occlusion of the vessel lumen. As a consequence, the right ventricle of the heart is subjected to an excessive strain which ultimately causes right heart failure (17). The cause of PH is unknown, however the pathogenesis of PH have been often linked to abnormalities of the BMP and Wnt signalling (16). The BMP pathway is extremely important for maintaining the pulmonary vasculature homeostasis, and an attenuation of BMP signalling is a frequent observation in the PH pathogenesis (18,19). Loss of function mutations in BMPR2 are associated with both hereditary and non-hereditary PH as well as in the animal models of the disease (19-21). It has been shown that BMP signalling is important on one hand for the survival of endothelial cell (EC) and on the other hand for counteracting the pro-proliferative effect of TGF-β on smooth muscle cell (SMC) of the lung vasculature (22,23). Attenuation of the BMP signalling would then leads to decreased survival of EC and hyper-proliferation of smooth muscle cells; both being hallmarks of PH pathogenesis (24).
The importance of BMP signalling in this aspect has also been proven by the findings that the DAN protein gremlin, acting as a BMP antagonist, is elevated in pulmonary arteries of PH patients and in animal models of PH (25,26). The inhibition of gremlin, by a blocking antibody, reversed the remodelling and the increased pulmonary pressure in the animal models of PH (27). These findings indicate that the action of BMP antagonists could explain the development of PH in the absence of BMPR2 mutation.
The Wnt pathway has also been recently associated to PH development (28-30). Several studies reported activation of both Wnt canonical and non-canonical pathways in PH patients and in the corresponding monocrotaline and hypoxic animal models (31,32). The β-catenin protein levels are elevated in the smooth muscle cells of PH patients, suggesting a pro-proliferative status of these cells (28). Additionally, similarly to the bone metabolism, recent studies have suggested that BMP and Wnt signalling are tightly interconnected during the pathomechanism of PH as well. It has been shown that the protective effect of BMP on EC survival is mediated via β-catenin activation (30). Additionally, a BMP-dependent activation of Wnt signalling has been shown to affect the proliferation and migration of pulmonary arterial SMC (33). These studies suggest that a molecule having potential to modulate both BMP and Wnt pathways, might influence the molecular mechanism governing altered homeostasis of the vascular cells in PH.
Expression of sclerostin: regulation and implications for the lung
Sclerostin has been discovered as an essential bone-related molecule, involved in bone remodelling and homeostasis (34).
However, recently it has been shown that sclerostin is not only confined to the skeletal compartment, but it is present in other organs as well. Expressional analysis revealed that sclerostin is expressed in the cartilage, liver, kidney, heart and in the lung (3,35,36). Additionally, in the cardiovascular system, sclerostin has been detected in the aorta (36), specifically in the vascular SMC where it is often associated with vascular calcification (37). As sclerostin is a negative regulator of mineralization, sclerostin expression could be up-regulated to counteract the ongoing calcifying mechanisms. Importantly, expression studies have revealed that several factors important in the pathogenesis of PH affect sclerostin levels. In the bone, it has been shown that sclerostin expression is down-regulated by nitric oxide (NO) production. This evidence is particularly important in relation to the lung, where NO is one of the major messenger molecules for pulmonary vasodilation (38). However, in PH patients the endothelial production of NO is often impaired in the pulmonary vasculature, due to endothelial dysfunction (39). Therefore, the decreased vascular NO could be the cause of increased sclerostin levels in the PH pulmonary arteries. Additionally, expression of sclerostin is modulated by hypoxia and cytokines, such as IL-6 (40,41). Contrary to the systemic circulation, hypoxia induces vasoconstriction in the pulmonary vasculature (42). Hypoxic vasoconstriction is a necessary response in order to limit the circulation in the hypoxic lung regions and divert the blood flow towards better oxygenated regions, thereby increasing the efficiency of gas exchange (42). Due to the vasoconstrictive status and substantial remodelling of their pulmonary arteries, PH patients are often hypoxic, and this decrease in oxygen concentration could lead to sclerostin upregulation. Similarly, IL-6 is a cytokine shown to be elevated in PH (43,44) and over-expression of IL-6 in mice results in spontaneous PH development (45). The increased level of IL-6 could contribute to increased sclerostin production. These abovementioned factors are only few known stimuli inducing sclerostin levels, however, we do not know whether other molecules with an established role in PH development (such as PDGF-BB) could also affect sclerostin expression. Nitric oxide, hypoxia and IL-6 could enhance sclerostin production triggering then sclerostin-action on BMP and Wnt pathways leading to perpetuation of the disease.
Sclerostin: potential involvement in PH
Several studies have delineated disturbances of BMP and Wnt pathways in PH, however, the underlying molecular mechanisms responsible for these alterations are not yet known. One could speculate that excess sclerostin levels due to upregulation by NO, hypoxia, IL-6 or other factors, could disturb the BMP and Wnt pathways helping the perpetuation of the disease in a vicious circle. To date, the role of sclerostin on vascular cells has not yet been investigated. Sclerostin could affect the physiological homeostasis of EC and SMC driving endothelial dysfunction and vascular remodelling and thereby contributing to the pathophysiology of PH.
Here, we suggest a possible mechanism of action of sclerostin on SMC and EC which might take part to the pathomechanism underlying PH.
In SMC sclerostin elevation could lead to inhibition of the canonical Wnt pathway by binding to LRP5/6. This would activate the non-canonical pathway leading to migration and intracellular calcium increase. Increase of intracellular calcium is a very important signalling event which induces contraction of SMC (46). It has been shown that SMC isolated from the pulmonary artery of PH patients present higher intracellular calcium than SMC isolated from healthy lungs. This leads to increased susceptibility for SMC contraction which consequently translates for higher pulmonary vascular tone (46). Additionally, elevated intracellular calcium in SMC, is often associated to increased proliferative potential (Figure 2).
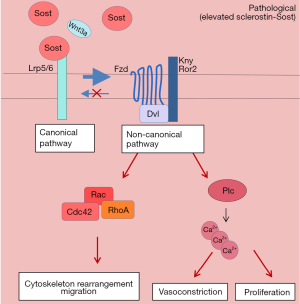
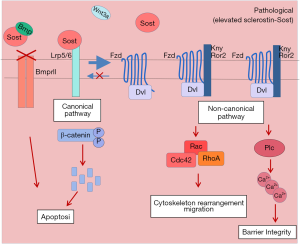
Sclerostin could also act on the EC of the pulmonary vessels. In this vascular cell type it could induce apoptosis by binding to LRP5/6 and inhibiting the canonical Wnt pathway. At the same time, as BMP has been shown to induce β-catenin activation, sclerostin would also block BMP-induced survival of EC. Additionally, similarly to SMCs, the blockage of the canonical pathway would lead to activation of the non-canonical pathway, affecting the cytoskeleton and intracellular calcium of EC leading to alterations of cell to cell contact and impairment of the barrier integrity of the endothelial layer (Figure 3).
Concluding remarks
The current research is mostly focused on the role of sclerostin in bone remodelling and hence it is commonly known as an osteocyte-specific protein. However, a growing body of evidence shows that sclerostin is expressed and plays an important role in other tissues and organs as well. Although sclerostin has been reported to be present in the lung and in the cardiovascular system, its role in their physiological and pathological conditions has not been studied yet. In light of the simultaneous effect of sclerostin on both BMP and Wnt pathways and the involvement of these two pathways in the pathogenesis of PH, one can speculate that sclerostin could be a very interesting candidate for the pathogenesis of PH. In vitro functional studies should elucidate the influence of sclerostin on vasoreactivity and barrier integrity—the main physiological function of smooth muscle and ECs. Additionally, ex vivo vascular force measurements by wire myography and lung dynamic assessments by isolated-perfused lung system would facilitate to understand the role of sclerostin in vascular tone, resistance, as well as vascular permeability and oedema formation in a more physiological context. Eventually, in vivo studies (e.g., Sugen/Hypoxia rat model) would be essential to prove the relevance of sclerostin in PH. These studies would open up new avenues, where sclerostin could be investigated on a much broader horizon beyond bone remodelling.
Acknowledgments
The authors acknowledge Dr. Grayzna Kwapiszewska for the helpful discussion.
Funding: The study was supported by Austrian Science Fund (FWF) (T1032-B34).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases”. The article has undergone external peer review.
Conflicts of Interest: The series “Clinical and Analytical Aspects of Bone and Intersystemic Diseases” was commissioned by the editorial office without any funding or sponsorship. Barbara Obermayer-Pietsch served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Bezooijen RL, ten Dijke P, Papapoulos SE, et al. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 2005;16:319-27. [Crossref] [PubMed]
- Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 2001;10:537-43. [Crossref] [PubMed]
- Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 2001;68:577-89. [Crossref] [PubMed]
- Van Hul W, Balemans W, Van Hul E, et al. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am J Hum Genet 1998;62:391-9. [Crossref] [PubMed]
- Balemans W, Van Den Ende J, Freire Paes-Alves A, et al. Localization of the gene for sclerosteosis to the van Buchem disease-gene region on chromosome 17q12-q21. Am J Hum Genet 1999;64:1661-9. [Crossref] [PubMed]
- Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 2002;39:91-7. [Crossref] [PubMed]
- Loots GG, Kneissel M, Keller H, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 2005;15:928-35. [Crossref] [PubMed]
- Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003;22:6267-76. [Crossref] [PubMed]
- Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 2008;23:860-9. [Crossref] [PubMed]
- van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004;199:805-14. [Crossref] [PubMed]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 2001;187:265-76. [Crossref] [PubMed]
- Kusu N, Laurikkala J, Imanishi M, et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem 2003;278:24113-7. [Crossref] [PubMed]
- Skronska-Wasek W, Gosens R, Konigshoff M, et al. WNT receptor signalling in lung physiology and pathology. Pharmacol Ther 2018;187:150-66. [Crossref] [PubMed]
- Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 2005;280:19883-7. [Crossref] [PubMed]
- van Bezooijen RL, Svensson JP, Eefting D, et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 2007;22:19-28. [Crossref] [PubMed]
- Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002;105:1672-8. [Crossref] [PubMed]
- Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed) 2016;69:177. [Crossref] [PubMed]
- Goumans MJ, Zwijsen A, Ten Dijke P, et al. Bone Morphogenetic Proteins in Vascular Homeostasis and Disease. Cold Spring Harb Perspect Biol 2018;10. [PubMed]
- Morty RE, Nejman B, Kwapiszewska G, et al. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 2007;27:1072-8. [Crossref] [PubMed]
- Best DH, Austin ED, Chung WK, et al. Genetics of pulmonary hypertension. Curr Opin Cardiol 2014;29:520-7. [Crossref] [PubMed]
- Takahashi H, Goto N, Kojima Y, et al. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;290:L450-8. [Crossref] [PubMed]
- Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 2006;98:209-17. [Crossref] [PubMed]
- Upton PD, Morrell NW. The transforming growth factor-beta-bone morphogenetic protein type signalling pathway in pulmonary vascular homeostasis and disease. Exp Physiol 2013;98:1262-6. [Crossref] [PubMed]
- Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S20-31. [Crossref] [PubMed]
- Cahill E, Costello CM, Rowan SC, et al. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 2012;125:920-30. [Crossref] [PubMed]
- Wellbrock J, Harbaum L, Stamm H, et al. Intrinsic BMP Antagonist Gremlin-1 as a Novel Circulating Marker in Pulmonary Arterial Hypertension. Lung 2015;193:567-70. [Crossref] [PubMed]
- Ciuclan L, Sheppard K, Dong L, et al. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol 2013;183:1461-73. [Crossref] [PubMed]
- Takahashi J, Orcholski M, Yuan K, et al. PDGF-dependent beta-catenin activation is associated with abnormal pulmonary artery smooth muscle cell proliferation in pulmonary arterial hypertension. FEBS Lett 2016;590:101-9. [Crossref] [PubMed]
- de Jesus Perez V, Yuan K, Alastalo TP, et al. Targeting the Wnt signaling pathways in pulmonary arterial hypertension. Drug Discov Today 2014;19:1270-6. [Crossref] [PubMed]
- de Jesus Perez VA, Alastalo TP, Wu JC, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol 2009;184:83-99. [Crossref] [PubMed]
- Sklepkiewicz P, Schermuly RT, Tian X, et al. Glycogen synthase kinase 3beta contributes to proliferation of arterial smooth muscle cells in pulmonary hypertension. PLoS One 2011;6:e18883 [Crossref] [PubMed]
- Laumanns IP, Fink L, Wilhelm J, et al. The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2009;40:683-91. [Crossref] [PubMed]
- Perez VA, Ali Z, Alastalo TP, et al. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J Cell Biol 2011;192:171-88. [Crossref] [PubMed]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276-87. [Crossref] [PubMed]
- Hernandez P, Whitty C, John Wardale R, et al. New insights into the location and form of sclerostin. Biochem Biophys Res Commun 2014;446:1108-13. [Crossref] [PubMed]
- Didangelos A, Yin X, Mandal K, et al. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics 2010;9:2048-62. [Crossref] [PubMed]
- Lv W, Guan L, Zhang Y, et al. Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4. Int Urol Nephrol 2016;48:2043-50. [Crossref] [PubMed]
- Delgado-Calle J, Riancho JA, Klein-Nulend J. Nitric oxide is involved in the down-regulation of SOST expression induced by mechanical loading. Calcif Tissue Int 2014;94:414-22. [Crossref] [PubMed]
- Tonelli AR, Haserodt S, Aytekin M, et al. Nitric oxide deficiency in pulmonary hypertension: Pathobiology and implications for therapy. Pulm Circ 2013;3:20-30. [Crossref] [PubMed]
- Chen D, Li Y, Zhou Z, et al. HIF-1alpha inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS One 2013;8:e65940 [Crossref] [PubMed]
- Pathak JL, Bakker AD, Luyten FP, et al. Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif Tissue Int 2016;98:596-608. [Crossref] [PubMed]
- Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017;151:181-92. [Crossref] [PubMed]
- Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151:1628-31. [Crossref] [PubMed]
- Jasiewicz M, Knapp M, Waszkiewicz E, et al. Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage. Cytokine 2015;76:187-92. [Crossref] [PubMed]
- Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009;104:236-44, 28p following 44.
- Kuhr FK, Smith KA, Song MY, et al. New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol 2012;302:H1546-62. [Crossref] [PubMed]
Cite this article as: Biasin V, Obermayer-Pietsch B. Sclerostin, bone morphogenetic protein, Wnt and the lung: a potential role beyond bone metabolism? J Lab Precis Med 2018;3:102.


