
Diagnostic performance of protein induced by vitamin K absence II for chronic hepatitis B-related hepatocellular carcinoma
Introduction
Viral hepatitis infection is a leading cause of liver fibrosis worldwide, a progressive disease that can lead to liver cirrhosis and eventually devastating hepatocellular carcinoma (HCC). HCC is the third most common cause of cancer-related death on the globe, with 782,500 new cases and 745,500 deaths during the year of 2012 across the world, with a majority of these cases occur in Asian countries where hepatitis B virus (HBV) infection is highly prevalent (1-6). It is estimated that China alone accounts for as high as 50% of the total number of HCC cases with 80% of cases attributable to HBV infection (7-9). Therefore, there is little doubt that the health and economic burden of HBV-related HCC is particularly challenging and serious in China (1,3). Moreover, HCC is usually first diagnosed at its advanced stage when treatment options are very limited and 5-year survival rate is considerably low in China (10). Thus, screening for, early detecting, and diagnosing of HCC among patients with chronic hepatitis B (CHB) is urgently needed. Currently, α-fetoprotein (AFP) and liver ultrasound are wildly used in screening for HCC among patients at high risk (11). However, several limitations have been identified, such as highly operator independence in liver ultrasound with the accuracy of its results influenced by the experience and skills of the operating physicians. In fact, application of liver ultrasound is restricted to conditions and some patients who cannot be imaged (12). AFP is the most commonly used non-invasive biomarker for HCC surveillance and diagnosis, and serum AFP levels greater than 200 ng/mL was considered to have diagnostic significance for HCC (2). However, at its cutoff value of 200 ng/mL the sensitivity and specificity for screening for HCC are far from satisfactory. In fact, an estimated 40% of HCC patients have normal serum level of AFP, which frequently results in false negative for HCC (13). Therefore, there is an urgent need to unveil better serological biomarkers either alone or in combination with AFP for the early diagnosis of HCC, especially non-invasive molecular markers with better ability to discrimination HCC from hepatitis viral infection.
Prothrombin induced by vitamin K absence-II (PIVKA-II), also referred to as des-gamma carboxyprothrombin, has been identified as a promising serum biomarker for HCC, which has been backed up by a wealth of scientific evidence since the first study by Liebman and colleagues, showing that serum levels of PIVKA-II were significantly increased in HCC patients and PIVKA-II was first proposed for the laboratory diagnosis of primary HCC (14). Since then, extensive studies have been conducted, indicating that PIVKA-II were abnormally expressed in the liver tissues, elevated in the serum of the HCC patients, and its sensitivity and specificity for the diagnosis of HCC may be superior to AFP with the combined test of PIVKA-II and AFP more sensitive and special, compared to AFP or PIVKA-II alone (15-23). In Japan, PIVKA-II in couple with AFP has been routinely used for HCC screening with the cost covered by Japan’s national health insurance (10,24). However, AFP was also found to be more sensitive than PIVKA-II for the diagnosis of early and very early stage HCC (25-28). Thus, the diagnostic performance of PIVKA-II is still controversial. In China, Cui et al. found that PIVKA-II can be a potential serum biomarker for HCC screening and diagnosis and the combination of AFP and PIVKA-II may improve the diagnostic sensitivity in Chinese HCC patients (19,20). However, to date, PIVKA-II has not yet used in clinical diagnosis of HCC in China (10). Until now, the role of serum PIVKA-II in the early detection of HCC in patients with chronic HBV infection with negative or mildly elevated AFP has not yet been clearly assessed.
In this study, we aimed to assess the diagnostic performance of serum PIVKA-II for HBV-related HCC in a Southern Chinese population. The results obtained from this study may provide more scientific evidence for the diagnostic value of PIVKA-II as an alternative non-invasive biomarker for HBV-related HCC in China and other Asian countries where HBV infection is highly prevalent.
Methods
Human subjects and study design
A cohort of 134 HCC patients was retrospectively enrolled in this study between September 2016 to January 2017 at the First Affiliated Hospital of Sun Yat-sen University. A total of 119 non-HCC patients with HBV infection and 100 healthy individuals were included in this study as controls. The HCC subjects were initially diagnosed by ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), or selective celiac angiography, and confirmed by pathological examination of the liver biopsies. Chronic HBV infection was defined as the persistent existence of hepatitis B surface antigen (HBsAg) for at least 1 year or HBV DNA concentrations more than 105. Healthy controls were those individuals who took the regular physical examination in the First Affiliated Hospital of Sun Yat-sen University and had normal liver and kidney function without a history of liver disease and malignant tumors. The exclusion criteria for serum samples were as follows: (I) patients who have alcoholic liver disease, obstructive jaundice or bruising cirrhosis; (II) patients with warfarin or vitamin K or cephalosporin antibiotics recent before testing; (III) HCC patients who had undergone treatments (such as hepatectomy, liver transplantation, radiofrequency ablation, radiotherapy, or chemotherapy) before blood collection; (IV) serum samples contaminated, substandard produce agglutination, or insufficient for testing.
The stages of the HBV-related HCC subjects were determined in accordance with the Barcelona Clinic Liver Cancer (BCLC) system (29). BCLC stage 0 was defined as a single lesion ≤2 cm; BCLC stage A was defined as a single lesion between 2 and 5 cm, or ≤3 lesions and each ≤3 cm; BCLC stage B was defined as a single lesion >5 cm, or more than 3 lesions, without the presence of macrovascular invasion or metastasis; and BCLC stage C was those with the presence of macrovascular invasion or metastasis. We combined the stage 0 and stage A into early stage HCC, and stage B and stage C into advanced stage HCC.
The study protocol was reviewed and approved by the Human Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. All research procedures involving human subjects were in compliance with the 1964 Declaration of Helsinki guidelines. All patients had given written informed consent after the nature of the research had been explained to them, including a review of their medical records and the use of their samples.
Examination of serum PIVKA II and AFP
Peripheral blood samples of the patients were obtained with BD Vacutainer® Blood Collection Tubes, and immediately centrifuged. All the sera were separated out and stored at −80 °C until experimental examination. Serum levels of PIVKA-II and AFP were tested by chemiluminescent microparticle immunoassay (CMIA) (Abbott Diagnostics, Abbott Park, IL, USA) according to the manufacturer’s instructions. The limit of assay imprecision (coefficient of variance (CV)) was set at ≤10%. The cut-off values of serum PIVKA-II was 40 mAU/mL, according to the manufacturer’s instructions. The values of serum AFP less than 20 ng/mL (<20 ng/mL) were designated as normal or negative for HBV-related HCC, while levels of AFP of 20–200 ng/mL (<200 ng/mL) were considered to be mildly elevated AFP.
Biochemical analyses
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, conjugated bilirubin, and unconjugated bilirubin were examined with an Automatic Biochemical Analyzer in the central clinical laboratory at the First Affiliated Hospital of Sun Yat-sen University. Levels of HBsAg and hepatitis B e antigen (HBeAg) were quantified with a platform of I2000 assay (Abbott Diagnostics, USA). Serum HBV DNA loads were measured using real-time polymerase chain reaction (PCR) on an ABI 7500 sequence detector (Applied Biosystems, USA) following the manufacturer’s instructions.
Statistical analysis
The statistical analysis was performed using the IBM SPSS Statistics version 20.0 from SPSS Inc. (Chicago, IL, USA), MedCalc version 15.0, and GraphPad Prism version 5.02. The Kolmogorov-Smirnov test was conducted for an initial examination of the normality of all continuous variables. The continuous variables with a normal distribution were presented as the mean ± standard deviation (SD), whereas those that were not normally distributed were expressed as median (range). Categorical variables were presented in absolute values and percentages. A one-way analysis of variance (ANOVA) test was used for continuous variables and a Chi square test was used for categorical variables. To assess differences between the groups, we used the Mann-Whitney U test for pairwise comparisons between two groups and Kruskal-Wallis test comparison of multiple groups. The diagnostic performance of serum PIVKA-II for HBV-related HCC was determined using receiver operating characteristic (ROC) curve analysis, and the area under ROC curve (AUC), sensitivity, specificity, and the 95% confidence intervals (CI) were calculated. The optimal cut-offs for serum PIVKA-II were derived from the ROC curves with both maximum sensitivity and specificity. In this study, a two-sided P value less than 0.05 (P<0.05) was considered statistically significant.
Results
Demographic and clinical characteristics of the study subjects
This study enrolled a cohort of 134 HBV-related HCC patients, 119 CHB patients, and 100 healthy individuals in the period from September 2016 to January 2017. The baseline demographic and clinical characteristics were summarized in Tables 1,2. Of the HBV-related HCC patients, the mean age was 54.5±11.8 years, which was significantly higher than 36.0±10.8 years for the 119 CHB patients (P<0.05), and 49.2±9.7 years for healthy individuals (P<0.05). In the HBV-related HCC group, the percentage of male was 85%, which was more dominant than 67% of male in the CHB group, and 51% in the normal group. Biochemical examinations at enrollment showed that levels of total protein, albumin, platelets and hemoglobin were significantly lower than those in the CHB and normal groups (P<0.05), while serum levels of aminotransferases (ALT and AST) and bilirubin (total bilirubin, conjugated bilirubin, and unconjugated bilirubin) were significantly greater compared with the other two groups (P<0.05). Based on the serum levels of AFP, the HBV-related HCC patients were subdivided into three subgroups: AFP <20 ng/mL (n=49, 36.6%), AFP 20–200 ng/mL (n=25, 18.6%), and AFP >200 ng/mL (n=60, 44.8%). In addition, the stages of the HBV-related HCC patients were evaluated according to the Barcelona Clinic Liver Cancer (BCLC) classification system, that were distributed among the following subgroups: stage 0 (n=15, 11.2%), stage A (n=27, 20.1%), stage B (n=42, 31.3%), and stage C (n=50, 37.4%). The male patients in the advanced HBV-related HCC (n=83, 90.2%) were predominant compared with those in the early stage HBV-related HCC (n=31, 73.8%) (P<0.05). Tumor size in the early stage HBV-related HCC patients was smaller than that in the advanced stage HBV-related HCC patients (P<0.001) with 19 (45.2%) patients in the early stage HBV-related HCC had a tumor size in diameter less than 2 cm, whereas 90 (97.8%) patients in the advanced stage HBV-related HCC had a tumor diameter greater than 2 cm. A majority of patients (n=31, 73.8%) in the early stage HBV-related HCC group had a single tumor in contrast to more than half of patients (n=50, 54.4%) in the advanced stage HBV-related HCC had multiple tumors. In addition, portal vein thrombosis, intrahepatic or distant metastases were more frequently occurred in the advanced stage HBV-related HCC versus the early stage HBV-related HCC.
Table 1
| Variables | HCC (n=134) | Hepatitis B (n=119) | Healthy individuals (n=100) |
|---|---|---|---|
| Age (years) | 54.5±11.8 | 36.0±10.8 | 49.2±9.7 |
| Gender, n (%) | |||
| Male | 114 (85) | 80 (67) | 51 (51) |
| Female | 20 (15) | 39 (33) | 49 (49) |
| Etiology, n (%) | |||
| HBsAg (+) | 106 (79.1) | 119 (100.0) | |
| HBeAg (+) | 34 (25.4) | 59 (49.6) | |
| Anti-HCV (+) | 4 (3.0) | ||
| HBsAg (+) and anti-HCV (+) | 3 (2.2) | ||
| Others | 21 (15.7) | ||
| Liver cirrhosis, n (%) | 97 (72.4) | ||
| ALT (U/L) | 79.2±275.9 | 34.9±36.9 | 23.9±14.8 |
| AST (U/L) | 96.8±159.8 | 34.1±34.4 | 23.9±6.54 |
| Total protein (g/L) | 66.62±6.33 | 75.63±4.65 | 73.72±4.58 |
| Albumin (g/L) | 36.18±5.59 | 45.83±2.78 | 45.07±3.85 |
| Total bilirubin (ìmol/L) | 31.67±63.31 | 15.39±6.51 | 12.34±3.96 |
| Free bilirubin (ìmol/L) | 13.06±38.02 | 2.85±1.41 | 3.67±15.0 |
| Conjugated bilirubin (ìmol/L) | 17.56±16.63 | 12.54±5.37 | 11.77±16.43 |
| Prothrombin time (s) | 12.76±1.40 | 12.86±2.01 | 13.01±1.10 |
| Platelets (×1,000/mm3) | 134.3±20.0 | 145.3±16.9 | 145.4±15.6 |
| Hemoglobin (g/L) | 177.3±82.8 | 215.0±48.2 | 243.2±54.5 |
| AFP (ng/mL) | |||
| AFP <20 ng/mL | 49 (36.6) | 117 (98.3) | 100 (100.0) |
| AFP 20–200 ng/mL | 25 (18.6) | 2 (1.7) | 0 (0.0) |
| AFP >200 ng/mL | 60 (44.8) | 0 (0.0) | 0 (0.0) |
| BCLC stage n (%) | |||
| Stage 0 | 15 (11.2) | ||
| Stage A | 27 (20.1) | ||
| Stage B | 42 (31.3) | ||
| Stage C | 50 (37.4) | ||
Data are presented as n (%) or means ± standard deviation for normally distributed continuous variables, median (range) for continuous variables that are not normally distributed. HCC, hepatocellular carcinoma; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; HBsAg, hepatitis B virus surface antigen; HBeAg, hepatitis B virus e antigen; Anti-HCV, anti-hepatitis C virus. Kruskal-Wallis test and Chi square test were used.
Table 2
| Variables | Early stage HCC (n=42) | Advanced stage HCC (n=92) | P value |
|---|---|---|---|
| Age (years) | 57.2±10.4 | 53.3±12.2 | 0.0727 |
| Gender, n (%) | |||
| Male | 31 (73.8) | 83 (90.2) | 0.013 |
| Female | 11 (26.2) | 9 (9.8) | |
| Etiology | |||
| HBsAg (+) | 30 (71.4) | 76 (82.6) | 0.14 |
| HBeAg (+) | 10 (23.8) | 24 (26.1) | 0.779 |
| Anti-HCV (+) | 2 (4.8) | 2 (0.02) | 0.589 |
| HBsAg (+) and anti-HCV (+) | 2 (4.8) | 1 (0.01) | 0.231 |
| Others | 9 (21.4) | 12 (13.0) | 0.215 |
| Tumor size, n (%) | |||
| <2 cm | 19 (45.2) | 2 (2.2) | <0.001 |
| >2 cm | 23 (54.8) | 90 (97.8) | |
| Number of nodules, n (%) | |||
| Single | 31 (73.8) | 42 (45.6) | 0.002 |
| Multiple | 11 (26.2) | 50 (54.3) | |
| Liver cirrhosis, n (%) | 28 (66.7) | 69 (75.0) | 0.317 |
| Portal vein thrombosis, n (%) | 0 (0.0) | 48 (52.2) | <0.001 |
| Metastasis, n (%) | 0 (0.0) | 18 (19.6) | 0.02 |
Data are presented as n (%) or means (± standard deviation). Mann-Whitney test and Chi square test were used. HBsAg, hepatitis B virus surface antigen; HBeAg, hepatitis B virus e antigen; HCC, hepatocellular carcinomas; HCV, hepatitis C virus.
Serum levels of PIVKA-II in HBV-related HCC and CHB patients
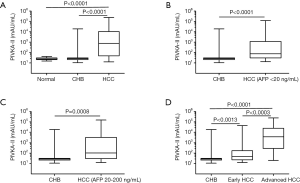
We initially examined serum levels of PIVKA-II in the study subjects and the data were presented in Figure 1. The precision of PIVKA II assay in this study was 4%, which satisfy the requirement of test. The HBV-related HCC patients had the median PIVKA-II concentrations of 774.90 mAU/mL, which were significantly increased in contrast to 26.98 mAU/mL in the CHB patients (P<0.05) or 24.26 mAU/mL in the healthy individuals (P<0.05), whereas there was no significant difference in levels of PIVKA-II between the CHB patients and healthy individuals (26.98 vs. 24.26 mAU/mL, P>0.05) (Figure 1A). When the HBV-related HCC patients were classified into the early stage and advanced stage groups, both groups displayed significant higher serum levels of PIVKA-II compared to those in the CHB patient (47.20 vs. 26.98 mAU/mL, P<0.05; 3,649 vs. 26.98 mAU/mL, P<0.05). Furthermore, we found that the advanced stage HBV-related HCC patients had greater PIVKA-II levels than the early stage HBV-related HCC patients (3,649 vs. 47.20 mAU/mL, P<0.05).
Diagnostic performance of PIVKA-II, AFP and the combined test of PIVKA-II and AFP
We used the ROC curves to evaluate the diagnostic performance of PIVKA-II, AFP and the combined test of the two biomarkers. For the combination procedure, the two biomarkers were used as predictors and got every predicting possibility as the combining biomarker value using logistic regression in SPSS binary logistic session. The recommended clinical cutoff value for AFP (20 ng/mL) and the manufacturer’s recommended cutoff value for PIVKA-II (40 mAU/mL) were used in our study. AUC, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PIVKA-II, AFP and the combined test were presented in Table 3.
Table 3
| Variables | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Total stage HCC vs. controls | |||||
| AFP | 0.902 (0.866–0.931) | 63.4 | 99.1 | 97.7 | 81.6 |
| PIVKA-II | 0.885 (0.848–0.917) | 79.9 | 96.4 | 93.0 | 88.7 |
| AFP + PIVKA-II | 0.925 (0.892–0.950) | 78.4 | 96.8 | 93.7 | 88.0 |
| Early stage HCC vs. controls | |||||
| AFP | 0.875 (0.828–0.912) | 50.0 | 99.1 | 91.3 | 91.2 |
| PIVKA-II | 0.720 (0.662–0.774) | 57.2 | 96.4 | 75.0 | 92.1 |
| AFP + PIVKA-II | 0.879 (0.833–0.916) | 81.0 | 84.0 | 49.3 | 95.8 |
| Advanced stage HCC vs. controls | |||||
| AFP | 0.914 (0.878–0.943) | 69.6 | 99.1 | 97.0 | 88.6 |
| PIVKA-II | 0.962 (0.934–0.980) | 90.2 | 96.4 | 91.2 | 95.9 |
| AFP + PIVKA-II | 0.946 (0.914–0.968) | 83.7 | 97.7 | 92.9 | 94.2 |
| HBsAg (+) HCC vs. CHB | |||||
| AFP | 0.923 (0.879–0.954) | 66.4 | 98.3 | 97.2 | 77.0 |
| PIVKA-II | 0.897 (0.849–0.934) | 83.6 | 95.0 | 93.5 | 86.3 |
| AFP + PIVKA-II | 0.943 (0.904–0.970) | 83.6 | 94.1 | 92.6 | 86.8 |
| HBsAg (+) early HCC vs. CHB | |||||
| AFP | 0.888 (0.826–0.934) | 53.3 | 98.3 | 88.9 | 89.3 |
| PIVKA-II | 0.713 (0.633–0.784) | 56.7 | 95.0 | 73.9 | 89.7 |
| AFP + PIVKA-II | 0.886 (0.824–0.933) | 80.0 | 89.1 | 64.9 | 94.6 |
| HBsAg (+) advanced HCC vs. CHB | |||||
| AFP | 0.935 (0.891–0.966) | 71.4 | 98.3 | 96.5 | 84.2 |
| PIVKA-II | 0.966 (0.930–0.987) | 93.5 | 95.0 | 92.3 | 95.8 |
| AFP + PIVKA-II | 0.966 (0.930–0.987) | 89.6 | 95.0 | 92.0 | 93.4 |
| HCC (AFP <20 ng/mL) vs. CHB | |||||
| PIVKA-II | 0.790 (0.697–0.883) | 65.3 | 94.9 | 84.2 | 86.7 |
| HCC (AFP 20–200 ng/mL) vs. CHB | |||||
| PIVKA-II | 0.775 (0.638–0.912) | 60.0 | 98.3 | 71.4 | 91.7 |
HCC, hepatocellular carcinoma; AFP, α-fetoprotein; CHB, chronic hepatitis B; AUC, area under the ROC curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; HBsAg, hepatitis B virus surface antigen; HBeAg, hepatitis B virus e antigen.
In our study, PIVKA-II and AFP concentrations were above the cutoff value in 107 and 85 patients in HCC group and in 9, and 2 patients in control group (hepatitis B group, and healthy controls, respectively). As data shown in Table 3, for differentiating total stage HCC from controls, sensitivity and NPV of PIVKA-II were higher than AFP (79.9% vs. 63.4%, 88.7% vs. 81.6%, respectively), but specificity and PPV were lower (96.4% vs. 99.1%, 93.0% vs. 97.7%, respectively). Similarly, the combined test could improve the sensitivity and NPV, compared to AFP (78.4% vs. 63.4%, 88.0% vs. 81.6%, respectively). The same trend could be observed in early stage HCC and advanced stage HCC. For advanced stage HCC, PIVKA-II shown the superior sensitivity and NPV to AFP (90.2% vs. 69.6%, 95.9% vs. 88.6%, respectively), but specificity and PPV were inferior (96.4% vs. 99.1%, 91.2% vs. 97.0%, respectively), and the combined test also had higher sensitivity and NPV (83.7% vs. 69.6%, 94.2% vs. 88.6%, respectively) and lower specificity and PPV than AFP alone (97.7% vs. 99.1%, 92.9% vs. 97.0%, respectively). As for early stage HCC, PIVKA-II had lower specificity and PPV (96.4% vs. 99.1%, 75.0% vs. 91.3%, respectively), and higher sensitivity and NPV (57.2% vs. 50.0%, 92.1% vs. 91.2%, respectively), compared to AFP, and the combined test was more sensitive and less specific than AFP.
Analysis of correlation of serum PIVKA-II with CHB-related HCC with negative or mildly elevated AFP
Mann-Whitney tests were performed. There was a trend toward higher levels of serum PIVKA-II with increasing stages of the CHB-related HCC subjects. Serum levels of PIVKA-II were significantly associated with levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin (all P<0.05). Subsequently, we analyzed the relationships between PIVKA-II levels and HCC pathological characteristics, and data were summarized in Table 4. Serum PIVKA-II were significantly associated with tumor size (P<0.001), portal vein thrombosis (P<0.001), and tumor metastasis (P=0.048), tumor stage (P<0.001).
Table 4
| Variables | AFP* | R | P value | PIVKA-II* | R | P value |
|---|---|---|---|---|---|---|
| Tumor size | 0.5748 | <0.0001 | ||||
| <2 cm | 12.1 (3.8–87.1) | 0.3315 | 0.0009 | 31.0 (18.4–51.8) | ||
| >2 cm | 328.3 (8.9–9,661) | 1,626 (108–20,840) | ||||
| Number of nodules | 0.149 | NS | ||||
| Single | 30.6 (4.6–2,829) | 0.1992 | 0.0217 | 307.4 (40.6–7,591) | ||
| Multiple | 328.3 (13.2–10,420) | 1,604 (66.5–22,870) | ||||
| Portal vein thrombosis | 0.3766 | <0.0001 | ||||
| Yes | 1101 (36.3–35,480) | 0.344 | <0.0001 | 7,005 (768.2–42,340) | ||
| No | 22.6 (4.7–837.5) | 123.7 (33.23–2,489) | ||||
| Metastasis | 0.1735 | 0.0488 | ||||
| Yes | 301.7 (18.9–10,120) | 0.05685 | NS | 7,242 (635.1–21,060) | ||
| No | 79.3 (5.8–4,590) | 545.8 (45.42–8,927) | ||||
| Tumor stage | 0.5249 | <0.0001 | ||||
| Early stage | 18.5 (4.8–164.9) | 0.2888 | 0.0009 | 47.2 (22.6–172.9) | ||
| Advanced stage | 465.1 (9.2–11,510) | 3,649 (279.5–24,730) | ||||
| Liver cirrhosis | 0.1029 | 0.2367 | ||||
| Yes | 292.6 (9.1–8,681) | 0.1922 | 0.0261 | 1,252 (58.5–9,641) | ||
| No | 13.0 (4.4–482.4) | 296.6 (33.1–12,110) | ||||
| HBsAg | 0.2083 | 0.0164 | ||||
| Positive | 159.2 (8.9–6,576) | 0.1245 | NS | 1,168 (70.9–19,240) | ||
| Negative | 29.7 (3.5–4,216) | 107.5 (32.4–2,398) |
*, values are expressed as median (range). HBsAg, hepatitis B virus surface antigen; NS, not significant; AFP, α-fetoprotein; HCC, hepatocellular carcinoma.
Discriminatory ability of serum PIVKA II to detect CHB-related HCC with negative or mildly elevated AFP relative to CHB patients
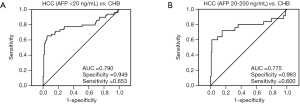
ROC curve analysis was carried out to detect HBV-related HCC in CHB patients. Furthermore, the area under the ROC curve (AUC) was calculated to assess the performance of serum PIVKA-II levels in detection of HBV-related HCC with negative or mildly elevated AFP relative to CHB patients. The HBV-related HCC patients with negative or mildly elevated AFP were defined as those with AFP concentrations <20 ng/mL and AFP of 20–200 ng/mL, and the data were compared between the HBV-related HCC cases vs. CHB patients. As shown in Figure 1B,C, serum PIVKA-II levels were significantly higher in the HBV-related HCC patients with both AFP <20 ng/mL and AFP of 20–200 ng/mL compared to CHB patients (P<0.0001, P=0.0008, respectively). Of 166 study subjects with AFP <20 ng/mL (49 HBV-related HCC, 117 CHB), the AUC value was 0.790 for PIVKA-II in distinguishing HCC from CHB, with a sensitivity of 65.3% and a specificity of 94.9% (Figure 2A). Similar AUC value (0.814), sensitivity (65.3%) and specificity (98.0%) were observed for PIVKA-II in detecting HBV-related HCC from normal controls. And AUC value (0.801), sensitivity (65.3%) and specificity (96.3%) were observed for PIVKA-II in detecting HBV-related HCC from controls (CHB + normal controls). Moreover, in the 144 study subjects with 25 HBV-related HCC with mildly elevated AFP (20–200 ng/mL) and 119 CHB, we calculated the value of AUC for PIVKA-II in discriminating HBV-related HCC from CHB, which was 0.775, with a sensitivity of 60.0% and a specificity of 98.3% (Figure 2B). Similar results were observed for the AUC value (0.795), sensitivity (72%), and specificity (90%) in discriminating HBV-related HCC from normal subjects.
Discussion
Chronic HBV infection is the undoubtedly major causative factors of HCC in China, where both mobility and mortality of HBV-related HCC remain considerably high. Early detection of HCC is key to combating the cancer more effectively (1,10). Thus, non-invasive serum markers for HBV-associated HCC has particularly clinical significance in China and other Asian countries with high prevalence of HBV infection. However, it has turned out that the current non-invasive maker AFP has high false-negative rate, missing as high as 40% cases in the diagnosis of HCC (13,30). The present study of a Chinese population of HBV-related HCC and CHB patients had the main novel findings, which were summarized as follows: (I) serum levels of PIVKA-II were significantly greater in the HBV-related HCC patients compared to the CHB patients (P<0.05), with a trend toward higher levels of serum PIVKA-II with increasing stages, and with a significant positive correlation between PIVKA-II levels and tumor size, portal vein thrombosis, and tumor metastasis (P<0.05); (II) the sensitivity and specificity for PIVKA-II in discriminating HBV-related HCC from CHB study subjects (AFP <20 ng/mL) were 65.3% and 94.9%, respectively; (III) for the study subjects with AFP of 20–200 ng/mL, the serum PIVKA-II displayed sensitivity of 60.0% and a high specificity of 98.3% in distinguishing HBV-related HCC from CHB. Our results suggest that the serum PIVKA-II is able to detect HBV-related HCC among CHB patients with negative or mildly elevated AFP.
Recent years have witnessed an emerging body of research on serum PIVKA-II as a promising non-invasive serum biomarker for HCC screening, early detection, and diagnosis, which was supported by extensive comparative studies on the performance of PIVKA-II vs. AFP for HCC. However, controversial results were noticed on whether PIVKA-II is better than AFP for HCC diagnosis. In a large case-control study conducted by Marrero et al. (18), PIVKA-II was found to be better performed than AFP. Pote et al. (21) showed that PIVKA-II had higher sensitivity and specificity than AFP and PIVKA-II was more efficient than AFP for the diagnosis of early HCC. Volk et al. (17), Li et al. (22), Tateishi et al. (23) draw the same conclusion. For another, Lok et al. (31) found that neither AFP nor PIVKA-II was the optimal biomarker for HCC diagnosis. In the study conducted by Pote et al., they indicated that the result which might lead to the poor performance of AFP was that early stage HCC accounted for the majority in their study (21). Conversely, in our present study, majority of (68.7%) HCC patients were advanced stage HCC, which might cause the elevation of AFP and resulted in a better performance of AFP. There were still many differences between our results and the previous studies conclusions, which might have been because of different numbers, characteristics of patients, diagnostic criteria and detection methods. In addition, our study limited to the southern China population, but the previous study mainly focus on Japan, Korea, Europe and the United States population. In addition to the above conflicting results, the discriminatory ability of serum PIVKA-II for HBV-related HCC in a Chinese population, in particular for those cases missed by AFP method with negative AFP (<20 ng/mL) or mildly elevated AFP (20–200 ng/mL) need to be determined.
It was of note in this study that 49 (36.6%) of HBV-related HCC patients had serum levels of AFP <20 ng/mL, while 25 (18.6%) had serum levels of AFP 20–200 ng/mL (Table 1), which is consistent with previous findings that approximately 40% of HCC cases were diagnosed, in support of the inadequacy of sensitivity and specificity of AFP as a tumor marker shown by previous reports. To further investigated the clinical utility of PIVKA-II for HCC with low AFP levels, we used the ROC curve to analyze the diagnostic value of PIVKA-II for HCC with AFP <20 and 20–200 ng/mL. Results shown that AUC for PIVKA-II was 0.801, with sensitivity and specificity of 65.3% and 94.9% in discriminating patients with AFP levels <20 ng/mL from controls. This result was slightly more sensitive and specific than the previous study conducted by Choi et al. showing sensitivity and specificity of 57.9% and 95.9% with a cutoff value of 40 mAU/mL (26). For HCC patients with AFP of 20–200 ng/mL, PIVKA-II shown an AUC of 0.775, with sensitivity and specificity of 60.0% and 98.3%, which was similar to Choi et al. (26). Thus, PIVKA-II could improve the detection rate for HCC without increased AFP levels, indicating that PIVKA-II could be used as a complementary biomarker for low AFP HCC detection. In the correction analysis (Table 4), we found that serum PIVKA-II was in correction with tumor size, portal vein thrombosis, tumor metastasis, tumor stage and HBV infection, indicating that serum levels of PIVKA-II may be related to the prognosis of HCC. This result was in line with Pote et al. (21). We also found that PIVKA-II was not related to liver cirrhosis, indicating that liver cirrhosis has little effect on PIVKA, whereas AFP was associated with liver cirrhosis and levels of AFP in HCC cases with liver cirrhosis was higher than in those without liver cirrhosis. In our study, 72.4% of HCC patients were with liver cirrhosis, which may lead to the false increase AFP in HCC patients and improve the diagnostic performance of AFP.
Our study has a few limitations. First, of the 134 HBV-related HCC subjects, a low proportion (42/134) was categorized as early stage HCC with a majority of cases at advanced stage of HCC, which may result in higher serum levels of PIVKA-II compared with the previous studies. A large sample size with early stage of HBV-related HCC may require in the future study. Second, a wide variety of liver-related diseases, such as acute hepatitis, chronic hepatitis, liver hemangioma, liver cirrhosis, liver cyst, fatty liver and intrahepatic cholestasis, etc. have been reported to be linked to HCC, only hepatitis B patients were studied as the controls, and thus more control-diseases should be included in the follow-up study. The third limitation was in relation to the retrospective nature of this study, a prospective study is underway in our department to further assess and validate the value of serum PIVKA-II for screening and diagnosing of HCC among patients chronically infected with HBV.
In conclusion, our study demonstrates the great potential for serum PIVKA-II as a useful tool to prioritize or detect HBV-related HCC patients among patients chronically infected with HBV. Thus, our findings may add an important non-invasive marker for diagnosis of HCC induced by chronic HBV infection. This study has determined, for the first time, the diagnostic value of serum PIVKA-II in a southern Chinese population. PIVKA-II turns out to have high diagnostic performance with high sensitivity and specificity. PIVKA-II could improve the detection rate for HBV-related HCC without negative and mildly increased AFP. Thus, PIVKA-II is highly recommended to be a serum biomarker for HBV-related HCC.
Acknowledgments
Funding: This work was supported by a grant from the Natural Science Foundation of Guangdong Province, China (Grant No. 2015A030313035 to LSL). We were grateful to all patients for their willingness to participate in this study. We also would like to extend our appreciation to the editors in Medjaden Bioscience Limited for their assistance in editing and proofreading this manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.02.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the Human Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet 1981;2:1129-33. [Crossref] [PubMed]
- Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212-9. [Crossref] [PubMed]
- Lu FM, Zhuang H. Management of hepatitis B in China. Chin Med J (Engl) 2009;122:3-4. [PubMed]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Goldstein ST, Zhou F, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329-39. [Crossref] [PubMed]
- Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: The major impact of China. Hepatology 2014;60:2099-108. [Crossref] [PubMed]
- Song P, Gao J, Inagaki Y, et al. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer 2013;2:31. [Crossref] [PubMed]
- Rd BA, D'Angelica MI, Abrams TA, et al. Hepatobiliary cancers, version 2.2014. Journal of the National Comprehensive Cancer Network Jnccn 2014;12:1152-82. [Crossref] [PubMed]
- Zhang K, Song P, Gao J, et al. Perspectives on a combined test of multi serum biomarkers in China: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Drug Discov Ther 2014;8:102-9. [Crossref] [PubMed]
- Malek NP, Schmidt S, Huber P, et al. The diagnosis and treatment of hepatocellular carcinoma. Dtsch Arztebl Int 2014;111:101-6. [PubMed]
- Liebman HA, Furie BC, Tong MJ, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984;310:1427-31. [Crossref] [PubMed]
- Nomura F, Ishijima M, Kuwa K, et al. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. Am J Gastroenterol 1999;94:650-4. [Crossref] [PubMed]
- Lamerz R, Runge M, Stieber P, et al. Use of serum PIVKA-II (DCP) determination for differentiation between benign and malignant liver diseases. Anticancer Res 1999;19:2489-93. [PubMed]
- Volk ML, Hernandez JC, Su GL, et al. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark 2007;3:79-87. [Crossref] [PubMed]
- Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110-8. [Crossref] [PubMed]
- Cui R, He J, Zhang F, et al. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer 2003;88:1878-82. [Crossref] [PubMed]
- Cui R, Wang B, Ding HG, et al. Usefulness of determining a protein induced by vitamin K absence in detection of hepatocellular carcinoma. Chin Med J (Engl) 2002;115:42-5. [PubMed]
- Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848-54. [Crossref] [PubMed]
- Li C, Zhang Z, Zhang P, et al. Diagnostic accuracy of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res 2014;44:E11-25. [Crossref] [PubMed]
- Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17-30. [Crossref] [PubMed]
- Kudo M. Japan’s Successful Model of Nationwide Hepatocellular Carcinoma Surveillance Highlighting the Urgent Need for Global Surveillance. Liver Cancer 2012;1:141-3. [Crossref] [PubMed]
- Kim J, Ki SS, Lee SD, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol 2006;101:2051-9. [Crossref] [PubMed]
- Choi JY, Jung SW, Kim HY, et al. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol 2013;19:339-46. [Crossref] [PubMed]
- Tameda M, Shiraki K, Sugimoto K, et al. Des-γ-carboxy prothrombin ratio measured by P-11 and P-16 antibodies is a novel biomarker for hepatocellular carcinoma. Cancer Sci 2013;104:725-31. [Crossref] [PubMed]
- Ertle JM, Heider D, Wichert M, et al. A Combination of alpha-Fetoprotein and Des-gamma-Carboxy Prothrombin Is Superior in Detection of Hepatocellular Carcinoma. Digestion 2013;87:121-31. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Marrero JA. Screening tests for hepatocellular carcinoma. Clin Liver Dis 2005;9:235-51. [Crossref] [PubMed]
- Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 2010;138:493-502. [Crossref] [PubMed]
Cite this article as: Wan J, Su J, Ye Z, Huang C, Liang J, Liu M, Luo J, Li L. Diagnostic performance of protein induced by vitamin K absence II for chronic hepatitis B-related hepatocellular carcinoma. J Lab Precis Med 2019;4:10.


