
A case of malaria diagnosed accidentally with Sysmex XN-9000 automated analyzer
Introduction
Malaria is an acute infectious disease caused by one of the four species of protozoa belonging to the genus Plasmodium (Plasmodium vivax, Plasmodium ovale, Plasmodium falciparum and Plasmodium malariae) transmitted to humans by female mosquitoes of the genus Anopheles. It represents the third leading cause of mortality in the world, with over 200 million infections a year and more than 400 thousand deaths a year as reported in the World Malaria Report 2018 (1). Although malaria infection is a problem specially in tropical regions, in 2015 in 26 countries of the European Union 6,199 cases of malaria were confirmed as reported by The European Surveillance System (TESSy) on 30 June 2017 (2). A proportion of 99.8% of the cases were acquired during travels, while other seven cases were reported as locally acquired in three countries: five reported by Greece, one by Belgium and one by The Netherlands (2).
The diagnosis Malaria should be made through the combination of case history, clinical observation and diagnostic tests. Since 1990s new methods to detect malaria have been introduced, such as: molecular diagnosis, antigen detection using rapid diagnostic tests and indirect immuno-fluorescence (IFA) or enzyme-linked immuno-sorbent assay (ELISA) to measure past malaria experience. However, the gold standard for confirming the presence and the identification of malaria parasites is still microscopic examination of peripheral blood smears (3).
In several studies it has been reported that the use of automated hematology analyzers, especially in non-endemic areas, supports the diagnosis of malaria, in particular in those cases where there is no clinical suspicion (4,5). A study by Mohapatra et al. showed a sensitivity and specificity of 74.2% and 91.1% respectively using XE-2100 in the detection of parasitosis (6). In this regard, we report a case of Plasmodium falciparum detected thanks to the automated blood cell count without a specific request for the research of the malarial parasite.
Case presentation
A 33-year-old male patient came to the Emergency Room for sudden loss of consciousness followed by stupor and confusional state. He has been complained for 5 days a clinical picture of high fever, headache and repeated diarrheal discharges. Spinal tap was performed, with extraction of about 10 mL of clear liquor for the chemical-physical, microscopic, cultural and virological examinations. Liquor examinations was negative. He has submitted to encephalic CT scan showing no lesions and blood tests, which revealed an increase C-reactive protein 26.1 mg/dL, total bilirubin 4.61 mg/dL, conjugated bilirubin 3.17 mg/dL and alanine aminotransferase 48 U/L. His complete blood counts were: white blood cell (WBC) 12.81×109/L, red blood cell (RBC) 3.21×1012/L, hemoglobin 94 g/L, hematocrit 0.28 and platelets 47×109/L. The WBC differential count provided by the instrument showed normal values for lymphocyte 1.66×109/L, monocyte 0.50×109/L, eosinophil 0.08×109/L and basophil 0.0× cells, except for the number of neutrophil cells that were increased 10.56×109/L.
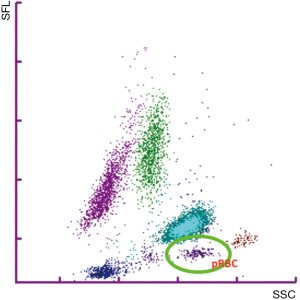
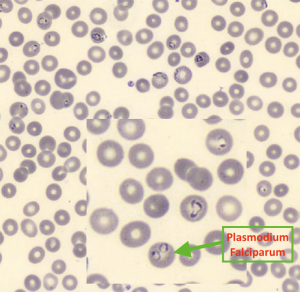
The instrument signaled the presence of an abnormal scattergram and the presence of parasitic RBCs (pRBC) (Figure 1). In this case the analyzer flagged no pseudoeosinophlia. Microscopic examination of the peripheral blood smear confirmed thrombocytopenia and the intraerythrocyte presence of Plasmodium falciparum (Figure 2). From these data, subsequent specific tests may be required to speed up the appropriate therapeutic approach quicklier and avoid more serious complications of the disease.
Discussion
Because the malaria is non-endemic in Europe, the search for the parasite is usually done only under specific request if there is a clinical suspicion and sometimes it can be omitted if the patient presents a non-specific febrile symptomatology. However, migratory flows and the increased frequency of travels to tropical regions have brought a growing number of cases of malaria accessing to the Emergency Room.
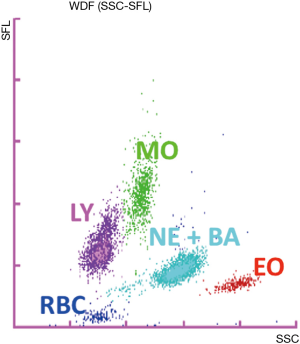
The Sysmex XN-9000 automated analyzer (Sysmex, Kobe, Japan) is a flow cytometer that uses the impedance principle associated with an optical system based on a beam of light emitted by a laser. The count and the volumetric measurements of RBCs and platelets are made using the resistive or impedance detection method. The determination of the WBC count and differentiation are obtained using 3 different signals: forward scatter light (FSC), side scatter light (SSC) and side fluorescence light (SFL). The intensity of the FSC indicates the volume of the cell, while the SSC indicates the cell contents such as nucleus and granules. The SFL indicates the amount of DNA and RNA presented. These measurements are performed in different analytical channels: “WNR” in which the count of the erythroblasts, basophils and WBC is performed; “WDF” to obtained information about the leukocyte formula; and “WPC” for white progenitor cell. In the WDF channel most information is obtained for morphological and functional characterization of the leukocytes (Figure 3).
In our case, a fundamental contribution to the malarial parasite detection is given by blood cells count through automated hematology analyzers XN-9000, which represents an important support to a rapid diagnosis of malaria also in absence of a specific clinical request. A careful observation of the WDF-scattergram provided by the analyzer pointed out a cells cluster in an abnormal position between eosinophil and neutrophil cells normally absent (Figure 2). In WDF channel RBC are not completely lysed, so even a small number of parasite RBC (inclusions trophozoite and schizont) increase fluorescence, leading to an overlap with neutrophil or eosinophil. This can lead to incorrect results, such as false high neutrophil or eosinophil count. The activation of the pRBC flag allows the correct neutrophil or eosinophil count obtained from WNR channel avoiding false results when trophozoites and/or gametocytes and schizont are present.
Therefore, the presence of a low platelets number in association with a leukocyte population with abnormal characteristics of fluorescence and cellular complexity present in the WDF-scattergram can be the first indication of Plasmodium infection in patients with febrile episodes in non-endemic areas.
In conclusion, detection of malaria by automated hematology analyzers represents an advantage that aids in the diagnosis of malaria in particular when clinical suspect is low.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Malaria Report 2018. Geneva: World Health Organization; 2018
- European Centre for Disease Prevention and Control. Malaria - Annual epidemiological report for 2015. Stockholm: ECDC; 2018.
- Wongsrichanalai C, Barcus MJ, Muth S, et al. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 2007;77:119-27. [Crossref] [PubMed]
- Campuzano-Zuluaga G, Alvarez-Sánchez G, Escobar-Gallo GE, et al. Design of malaria diagnostic criteria for the Sysmex XE-2100 hematology analyzer. Am J Trop Med Hyg 2010;82:402-11. [Crossref] [PubMed]
- Huh HJ, Oh GY, Huh JW, et al. Malaria detection with the Sysmex XE-2100 hematology analyzer using pseudoeosinophilia and abnormal WBC scattergram. Ann Hematol 2008;87:755-9. [Crossref] [PubMed]
- Mohapatra S, Samantaray JC, Arulselvi S, et al. Automated detection of malaria with haematology analyzer Sysmex XE-2100. Indian J Med Sci 2011;65:26-31. [Crossref] [PubMed]
Cite this article as: Roccaforte V, Liuzzi G, Porreca WP, Russo RM, Zavaroni E, De Angelis ML, Farioli L, Bussetti M, Buonocore R, Burati S, Tosca MC, Perno CF, Pastori S. A case of malaria diagnosed accidentally with Sysmex XN-9000 automated analyzer. J Lab Precis Med 2019;4:13.


