
Diagnosis of hypertensive disorders in pregnancy: an update
Classification and definition of hypertensive disorder in pregnancy
Hypertensive disorders complicate 5–10% of pregnancy and, according to “The FIGO Textbook of Pregnancy Hypertension” (1) and the International Society for the Study of Hypertension in Pregnancy (ISSHP) (2), are classifiable in 4 categories:
- Pre-existing hypertension;
- Gestational hypertension (GH);
- Pre-eclampsia (PE) and eclampsia;
- Other conditions.
Pre-existing hypertension can be found in approximately 1% of woman and is defined either when systolic blood pressure (SBP) is higher or equal to 140 mmHg and/or diastolic blood pressure (DBP) is higher or equal to 90 mmHg before the 20th of gestation or persisting after 12th week after delivery. GH, that occurs in above 3% of pregnancy, is defined as the de novo presence of hypertension arising after the 20th week of gestation without the characteristics that define PE (Figure 1).
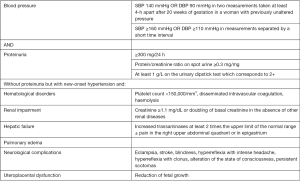
PE can complicate GH, chronic hypertension or can develop in women with normal blood pressure (BP) until diagnosed.
Other conditions that can be diagnosed during pregnancy are:
- White coat hypertension: findings of SBP ≥140 mmHg or DBP ≥90 mmHg in office but repeatedly less than 135/85 mmHg at home, measured by ambulatory BP measurement (ABPM) or Home BP monitoring (HBPM). It is a widespread condition, up to 30% of women diagnosed with GH and 30% of women in the first trimester of pregnancy (3);
- Masked hypertension: the presence of normal BP values in the clinic but high values at home or when ABPM is performed.
Therefore, it is necessary to measure BP before pregnancy for differential diagnosis, otherwise, diagnosis cannot be sure.
Other scientific societies use other slightly different classifications. American College of Obstetrician and Gynaecologist (ACOG), Society of Obstetricians and Gynaecologists of Canada (SOGC), Royal College of Obstetricians and Gynaecologists (RCOG), Society of Obstetric Medicine of Australia and New Zealand (SOMANZ), in line with ISSHP, describe chronic hypertension as a disturbance detected before 20 weeks of gestation. GH for ACOG and RCOG is the new-onset elevation of BP after 20 weeks of gestation without proteinuria. SOMANZ and SOGC take into consideration the absence of proteinuria and or fetal features of PE (abnormal fetal heart rate, oligohydramnios, stillbirth, abruption with evidence of maternal or fetal compromise, presence of reverse ductus venous A wave) to define GH ACOG definition of PE takes into consideration the presence of proteinuria (more than 300 mg/day) or target organ damage but the criteria of platelet (PLT) count is less than 100,000/µL (4) (ISSHP use a cut-off of 150,000/µL) (5). RCOG considers PE the presence of hypertension with proteinuria and eclampsia a convulsive condition associated with PE or haemolysis, elevated liver enzymes, and low PLT count syndrome (6).
How to measure BP in pregnancy
BP measurement in pregnancy does not deviate from the measurement outside this period: women should be positioned seated, without crossed legs, with feet touching the floor and the back completely resting on the chair. The lying or supine or side position can underestimate the pressure value (7). Women must refrain from talking, reading, consulting the telephone or watching television. The arm should not be stretched and should be at heart level. If not, a pillow can be used. The first measurement must be taken after at least 5 minutes of sitting position at rest. The cap should not be placed over clothes and should be of the right size. The length should cover two-thirds of the distance between the shoulder and the elbow; the lower edge of the cuff should be 1–2 cm above the fold of the elbow. The width must be such that the inflatable part of the cuff should be around 80% of the woman’s arm. This avoids overestimating the pressure if it is too tight (the error can be as much as 7–13 mmHg for SBP and 5–10 mmHg for DBP). BP should be measured several times. By sphygmomanometer, the first measurement must be discarded, and the average value of the two successive measurements should be considered. By automatic devices, the average of two successive measurements one minute apart must be considered (1,8).
Finally, the role of the device by which the pressure is measured appears pivotal. The gold standard device remains the mercury sphygmomanometer but the measurement with automatic instruments eliminates some errors due to the observer. However, only a few devices have been validated in pregnancy (9-12), particularly if PE is diagnosed (11,13,14).
Recently the FIGO has validated a risk score for early screening of PE that can be extensively read in “FIGO initiative on pre-eclampsia” (15). For this purpose BP should be measured during the first trimester (11th–13th week of gestation) by validated automated and semi-automated devices (http://www.dableducational.org/sphygmomanometers/devices_1_clinical.html#ClinTable) in sitting position, with arms supported at the level of the heart with an appropriate size adult cuff depending on the mid-arm circumference, after rest for 5 minutes, in both arms simultaneously. Two sets of recordings should be performed at 1-minute intervals (16). The four sets of measurements are required to insert into the risk calculator and the final mean arterial pressure measurement (average of four sets of measurements) will be automatically calculated for the calculation of patient-specific risk.
Laboratory testing for early screening
Since 2019 the pharmacological prevention of PE with low dose aspirin was based on analysis of risk factors from maternal demographic characteristics and medical history (2,5,17-21). Factors to be considered for assuming low dose aspirin are hypertensive disease in previous pregnancy, chronic hypertension, chronic renal disease, diabetes mellitus, autoimmune disease or any two of the moderate-risk factors (nulliparity, age ≥40 years, body mass index (BMI) ≥35 kg/m2, family history of PE, or inter-pregnancy interval >10 years). The detection rate with the risk factors mentioned above was proven to be very low (39% for preterm PE and 34% for term PE) with a quite high risk of false-positive rate (10.3%) (4). An alternative approach to screening for PE was recently validated and estimate patient-specific risk using Bayes theorem. The tools take into consideration maternal characteristics and medical history (the a priori risk) and the results of biophysical and biochemical measurements (22). The biomarkers considered in this validated tool are placental growth factor (PlGF) and pregnancy-associated plasma protein-A (PAPP-A). Unbalanced expression of angiogenic factors is pivotal in the pathophysiology of PE because are involved in endothelial injury and capillary permeability (23). Numerous biomarkers have been tested for early screening with different results in specificity and sensibility (24). PlGF is a proangiogenic factor, member of the vascular endothelial growth factor (VEGF) sub-family whose main source is the placental trophoblast (25). It binds to VEGF receptor 1 (VEGFR-1) and exerts vasculogenic and angiogenic functions. PlGF levels are reduced in PE by several weeks to months before the onset of PE, correlate with disease severity, and normalize after delivery (26). According to a recent meta-analysis, comparing PlGF, human chorionic gonadotropin (hCG), placental protein-13 (PP-13), PAPP-A measured singularly, the concentrations of PlGF measured in the first trimester have the highest detection rate and perform better in predicting early-onset PE than late-onset PE (sensitivity 40%, specificity 90%) (27). PAPP-A is a metalloproteinase that joins the insulin-like growth factor. Reduced levels of this molecule have been linked with high risk to develop PE (28-31). Impaired placentation and/or placental dysfunction, caused by an inadequate trophoblastic invasion of the maternal spiral arteries, are the pathogenic mechanisms of PE. Measuring biomarkers before 20 weeks of gestation seems useful in early prediction of PE, but how reliable they are in predicting PE development so far from the event is still uncertain and undoubtedly not advisable in routine clinical practice (24). The recent published “FIGO initiative on pre-eclampsia” suggests the use of a combination of biochemical (PlGF and PAPP-A), anamnestic (age, BMI, mean arterial pressure, ethnicity, obstetric history, inter-pregnancy interval, family history of PE, method of conception, history of chronic hypertension or diabetes or systemic lupus erythematosus or antiphospholipid syndrome, smoking habit), clinical parameter (mean arterial pressure and uterine pulsatility index) to obtain a reliable estimation of risk (15). Evidence is based on 3 studies on more than 120,000 pregnancies at 11–13 weeks of gestation (22,32,33). The detection rate at the screen-positive rate of 10% of early-PE, preterm-PE, and all-PE was about 90%, 75%, and 50% (34).
Diagnosis of PE
To be diagnosed PE requires BP criteria and significant proteinuria or organ dysfunction. Proteinuria is considered significant when is higher than 300 mg/day. During pregnancy, an increase in glomerular filtration secondary to the augmented circulating volume and to the modification of protein handling in the nephron results in a slight increment in proteinuria (normal range between 150 and 300 mg/day) (35). These changes resolve with the end of the pregnancy. The value of 300 mg/24 h represents the 95th percentile of the proteinuria confidence interval in pregnancy (36) but does not necessarily constitute a pathological level; more likely the risk of maternal and fetal adverse events increases with proteinuria higher than 500 mg/24 h (37). Proteinuria can be both glomerular and tubular type and the single most represented protein is the Tamm-Horsfall protein but albumin, thyroxine-binding pre-albumin, immunoglobulins, α1-antitrypsin, transferrin, β-lipoprotein and low-molecular-weight proteins can be detected (38). All pregnant women should be screened for proteinuria in early pregnancy, to search for pre-existing renal disease and to acquire a baseline value to follow during the evolution of pregnancy, especially in women at increased risk of PE (1). There are various methods for measuring proteinuria (on spot samples: urinary dipstick testing, heat coagulation test, urinary protein/creatinine ratio, or urinary albumin/creatinine ratio and 24-h collection). Two are the most reliable samples: the 24-h sample and the spot sample in which to measure the urinary protein/creatinine ratio. The 24-h collection is the gold standard method (threshold 300 mg/24 h) but it is time-consuming and often not accurate. A study conducted by Côté et al. in 2008 demonstrates that 24-h urine collection is frequently inaccurate based on 24-h urinary creatinine excretion, especially in lean bodyweight women (39). The ratio has the advantage of being simpler to obtain than 24-h urine collection. The threshold value that best corresponds to 24-h proteinuria greater than 300 mg/24 h, according to the most recent meta-analysis is 34 mg/mmol (0.30 mg/mg), with sensitivity and specificity above 80% (24). The international federation of obstetrician gynaecologists advises considering 30 mg/mmol without forgetting that the evaluation of proteinuria is only one of the aspects to consider when it is necessary to manage a gestation complicated by hypertension (1). In the case of twin pregnancies, the level to consider not pathological is higher but the threshold value is still discussed. PE can occur even in the absence of proteinuria but with a sign of target organ damage. In the suspicion of PE, women should undergo laboratory tests to search for renal, liver, metabolic or haematological features of PE. The laboratory tests also allow to explore the severity of the organ damage and to check for differential diagnoses (Table 1). A complete blood count is useful for at least three parameters. Haemoglobin concentration can be higher than the normal value in case of intravascular volume depletion (and consequent uteroplacental circulation deficiency) or lower in the case of haemolysis elevated liver enzyme low platelet (HELLP) syndrome (a form of severe PE that includes in the diagnostic criteria haemolysis). Look for haemoglobin is mandatory for differential diagnoses to search for signs of dehydration (for various causes such as vomiting or diarrhoea), or haemolysis for causes other than HELLP syndrome [enzyme deficiencies, hemoglobinopathies, membrane defects of red blood cells, liver disease, infections like malaria or sepsis, autoimmune, micro-angiopathic like thrombotic thrombocytopenic purpura (TTP) or haemolytic uremic syndrome (HUS)] or for search indices of chronic anaemia or acute bleeding. White blood count can help in support or exclude infections when suspected causes of reduced or augmented levels of haemoglobin. PLT must be checked as an index of PE. Moreover, their reduction is generally associated with poor outcome (40,41). PLT count can be reduced also because of gestational thrombocytopenia (a mild, asymptomatic condition in which PLT count is usually not lower than 70,000/mm3, not associated with risk of bleeding and that usually occurs in late gestation and resolves spontaneously after delivery; it is not combined with other alteration of biochemical indices) or for chronic liver disease with hypersplenism or in case of immune thrombocytopenia and in myelodysplastic condition. Even viral infections (like rubella, mumps, varicella, parvovirus, hepatitis C, and Epstein-Barr virus), drugs, congenital thrombocyte defects, and TTP can reduce PLT count. Coagulation tests can be useful for differential diagnosis and to evaluate the severity of PE. Disseminated intravascular coagulation (DIC) is a threatening condition typical features of abruption placenta and severe sepsis; activated partial thromboplastin time can be prolonged in antiphospholipid syndrome and acute fatty liver of pregnancy. Finally, fibrinogen level can help because it is not altered in PE but is reduced in other causes of DIC. Elevated serum creatinine is another possible feature of PE as a marker of acute kidney failure and severity of PE. It is important to test it also for rule out other acute or chronic kidney disease, renal failure in malignant hypertension and TTP-HUS (in case of coincidence of thrombocytopenia) or acute fatty liver of pregnancy (mixed with liver dysfunction). Regarding serum uric acid in PE it is associated with poor perinatal outcomes but not with adverse maternal outcomes (42). It is generally attributed to a reduction in glomerular filtration rate and increased reabsorption in the proximal renal tubules (43). It can also rise in case of dehydration, use of diuretics and genetic causes (44). Another important organ to evaluate is the liver through liver enzyme [alanine aminotransferase/alanine aminotransferase (ALT/AST)] and bilirubin. AST or ALT elevation is associated with adverse maternal outcome. In PE the cause is probably due to a reduction in the hepatic blood flow that causes ischemia and haemorrhage in the peri-portal spaces. Hepatocyte integrity may also be threatened by peri-portal and sinusoidal fibrin deposition and fat accumulation (45,46). Bilirubin increase is an index of liver failure, whereas indirect bilirubin level suggests the presence of haemolysis (in the context of HELLP or not). Liver involvement in PE must be differentiated from other hepatic chronic or acute complication typical of pregnancy status or not: in particular acute fatty liver of the pregnancy is a serious condition that usually determines a more severe liver involvement with hypoglycaemia, hyper-ammoniemia, and DIC that are very rare in PE. Liver transaminases can also arise in a benign condition, the intrahepatic cholestasis of pregnancy or conditions not associated with pregnancy (like viral hepatitis or cholecystitis). Finally, lactate dehydrogenase is also useful, especially because is associated with adverse maternal outcome and with the severity of PE/HELLP. It can also be elevated in TTP-HUS, and acute fatty liver of pregnancy.
Table 1
| LAB test | Levels in PE | Differential diagnosis |
|---|---|---|
| Hemoglobin | Can be higher than the normal values in case of intravascular volume depletion (consequent uteroplacental circulation deficiency) |
High in any causes of volume depletion: vomiting; diarrhoea; dehydration |
| Platelet count | Low | Low in: Gestational thrombocytopenia (>70,000/mm3); chronic liver disease; immune thrombocytopenia; myelodysplasia; viral infections (rubivirus, paramyxovirus, HHV-3, parvovirus, HCV, EBV); adverse reaction to drugs; TTP (usually <20,000/mm3); SLE reactivation |
| PT-INR | Prolonged in case of superimposed DIC | Prolonged in APS, DIC from other causes (sepsis) |
| aPTT | Prolonged in case of superimposed DIC | Prolonged in AFLP |
| Fibrinogen | Usually normal | Low in: DIC for all causes; AFLP |
| Creatinine blood test | Can be high | High in: acute or chronic kidney disease (e.g. Malignant hypertension); TTP-HUS; AFLP |
| AST/ALT | Can be high | High in: AFLP; intrahepatic cholestasis of pregnancy; viral hepatitis; cholecystitis |
| Uric acid | Can be high | High in: dehydration; medication (e.g., diuretics); genetic causes |
| Bilirubin | Can be high | High in: AFLP; haemolytic anaemia; liver disease (acute or chronic); genetic diseases (Gilbert disease, Dubin-Johnson syndrome); biliary tract obstructions |
| LDH | Can be high | High in: AFLP; haemolysis |
PE, pre-eclampsia; HELLP syndrome, haemolysis elevated liver enzyme low platelet syndrome; HHV-3, human herpes virus-3; HCV, hepatitis C virus; EBV, Epstein-Barr virus; TTP, thrombotic thrombocytopenic purpura; SLE, systemic lupus erythematosus; PT-INR, prothrombin time-international normalized ratio; aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; APS, antiphospholipid syndrome; AFLP, acute fatty liver of pregnancy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HUS, haemolytic uremic syndrome; LDH, lactate dehydrogenase.
Conclusions
Hypertensive disorders of pregnancy are associated with short and long-term complications both for the mother and for the foetus. Clinical and biochemical reliable screening for early treatment with the only, but effective, drugs that can prevent PE (i.e., acetylsalicylic acid) is crucial for reducing maternal and fetal sequelae. Correct classification and differential diagnosis of PE is central to manage in the most proper way this potentially life-threatening condition.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Lippi, Martina Montagnana and Zhi-De Hu) for the series “Laboratory Medicine in Pregnancy” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.11.04). The series “Laboratory Medicine in Pregnancy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Magee L, von Dadelszen P, Stones W. The FIGO Textbook of Pregnancy Hypertension. London: The Global Library of Women’s Medicine, 2016.
- Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104. [Crossref] [PubMed]
- Brown MA, Mangos G, Davis G, et al. The natural history of white coat hypertension during pregnancy. BJOG 2005;112:601-6. [Crossref] [PubMed]
- O'Gorman N, Wright D, Poon LC, et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol 2017;49:756-60. [Crossref] [PubMed]
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 2014;4:105-45. [Crossref] [PubMed]
- NICE. Hypertension in pregnancy: diagnosis and management. NICE Guidelines, 2019.
- Wichman K, Ryden G, Wichman M. The influence of different positions and Korotkoff sounds on the blood pressure measurements in pregnancy. Acta Obstet Gynecol Scand Suppl 1984;118:25-8. [Crossref] [PubMed]
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697-716. [Crossref] [PubMed]
- Nouwen E, Snijder M, van Montfrans G, et al. Validation of the Omron M7 and Microlife 3BTO-A blood pressure measuring devices in preeclampsia. Hypertens Pregnancy 2012;31:131-9. [Crossref] [PubMed]
- Chung Y, de Greeff A, Shennan A. Validation and compliance of a home monitoring device in pregnancy: microlife WatchBP home. Hypertens Pregnancy 2009;28:348-59. [Crossref] [PubMed]
- Chung Y, Brochut MC, de Greeff A, et al. Clinical accuracy of inflationary oscillometry in pregnancy and pre-eclampsia: Omron-MIT Elite. Pregnancy Hypertens 2012;2:411-5. [Crossref] [PubMed]
- James L, Nzelu D, Hay A, et al. Validation of the Omron MIT Elite blood pressure device in a pregnant population with large arm circumference. Blood Press Monit 2017;22:109-11. [Crossref] [PubMed]
- Reinders A, Cuckson AC, Lee JT, et al. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: the Microlife 3BTO-A. BJOG 2005;112:915-20. [Crossref] [PubMed]
- Nathan HL, de Greeff A, Hezelgrave NL, et al. An accurate semiautomated oscillometric blood pressure device for use in pregnancy (including pre-eclampsia) in a low-income and middle-income country population: the Microlife 3AS1-2. Blood Press Monit 2015;20:52-5. [Crossref] [PubMed]
- Poon LC, Shennan A, Hyett JA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet 2019;145:1-33. [Crossref] [PubMed]
- Poon LC, Zymeri NA, Zamprakou A, et al. Protocol for measurement of mean arterial pressure at 11-13 weeks' gestation. Fetal Diagn Ther 2012;31:42-8. [Crossref] [PubMed]
- Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol 2015;55:e1-29. [Crossref] [PubMed]
- WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Geneva: WHO, 2011.
- National Collaborating Centre for Women's Children's Health. Hypertension in Pregnancy. RCOG Press, 2010.
- US Preventive Services Task Force. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:1661-7. [Crossref] [PubMed]
- Committee Opinion No. 638: First-Trimester Risk Assessment for Early-Onset Preeclampsia. Obstet Gynecol 2015;126:e25-7. [Crossref] [PubMed]
- O'Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol 2016;214:103.e1-103.e12. [Crossref] [PubMed]
- Llurba E, Crispi F, Verlohren S. Update on the pathophysiological implications and clinical role of angiogenic factors in pregnancy. Fetal Diagn Ther 2015;37:81-92. [Crossref] [PubMed]
- Montagnana M, Danese E, Lippi G, et al. Blood laboratory testing for early prediction of preeclampsia: chasing the finish line or at the starting blocks? Ann Med 2017;49:240-53. [Crossref] [PubMed]
- Gobble RM, Groesch KA, Chang M, et al. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta 2009;30:869-75. [Crossref] [PubMed]
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672-83. [Crossref] [PubMed]
- Zhong Y, Zhu F, Ding Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: systematic review and meta-analysis. BMC Pregnancy Childbirth 2015;15:191. [Crossref] [PubMed]
- Poon LC, Maiz N, Valencia C, et al. First-trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound Obstet Gynecol 2009;33:23-33. [Crossref] [PubMed]
- Smith GC, Stenhouse EJ, Crossley JA, et al. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 2002;87:1762-7. [Crossref] [PubMed]
- Yaron Y, Heifetz S, Ochshorn Y, et al. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn 2002;22:778-82. [Crossref] [PubMed]
- Ong CY, Liao AW, Spencer K, et al. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 2000;107:1265-70. [Crossref] [PubMed]
- O'Gorman N, Wright D, Poon LC, et al. Accuracy of competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks' gestation. Ultrasound Obstet Gynecol 2017;49:751-5. [Crossref] [PubMed]
- Tan MY, Wright D, Syngelaki A, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol 2018;51:743-50. [Crossref] [PubMed]
- Wright D, Tan MY, O'Gorman N, et al. Predictive performance of the competing risk model in screening for preeclampsia. Am J Obstet Gynecol 2019;220:199.e1-199.e13. [Crossref] [PubMed]
- Taylor R, Roberts J, Cunningham F, et al. editors. Chesley’s hypertensive disorders in pregnancy. 4th edition. Academic Press, 2015.
- Higby K, Suiter CR, Phelps JY, et al. Normal values of urinary albumin and total protein excretion during pregnancy. Am J Obstet Gynecol 1994;171:984-9. [Crossref] [PubMed]
- Bramham K, Poli-de-Figueiredo CE, Seed PT, et al. Association of proteinuria threshold in pre-eclampsia with maternal and perinatal outcomes: a nested case control cohort of high risk women. PLoS One 2013;8:e76083 [Crossref] [PubMed]
- Holt JL, Mangos GJ, Brown MA. Measuring protein excretion in pregnancy. Nephrology (Carlton) 2007;12:425-30. [Crossref] [PubMed]
- Côté AM, Firoz T, Mattman A, et al. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol 2008;199:625.e1-6. [Crossref] [PubMed]
- von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet 2011;377:219-27. [Crossref] [PubMed]
- Ngwenya S. Severe preeclampsia and eclampsia: incidence, complications, and perinatal outcomes at a low-resource setting, Mpilo Central Hospital, Bulawayo, Zimbabwe. Int J Womens Health 2017;9:353-7. [Crossref] [PubMed]
- Livingston JR, Payne B, Brown M, et al. Uric Acid as a Predictor of Adverse Maternal and Perinatal Outcomes in Women Hospitalized With Preeclampsia. J Obstet Gynaecol Can 2014;36:870-7. [Crossref] [PubMed]
- Lam C, Lim KH, Kang DH, et al. Uric acid and preeclampsia. Semin Nephrol 2005;25:56-60. [Crossref] [PubMed]
- Merriman TR. An update on the genetic architecture of hyperuricemia and gout. Arthritis Res Ther 2015;17:98. [Crossref] [PubMed]
- Minakami H, Oka N, Sato T, et al. Preeclampsia: a microvesicular fat disease of the liver? Am J Obstet Gynecol 1988;159:1043-7. [Crossref] [PubMed]
- Dani R, Mendes GS, Medeiros Jde L, et al. Study of the liver changes occurring in preeclampsia and their possible pathogenetic connection with acute fatty liver of pregnancy. Am J Gastroenterol 1996;91:292-4. [PubMed]
Cite this article as: Tagetti A, Fava C. Diagnosis of hypertensive disorders in pregnancy: an update. J Lab Precis Med 2020;5:8.


