
Atherogenic postprandial remnant lipoproteins—a causal lipoproteins for the initiation of obesity and atherosclerosis
Introduction
The Framingham Heart Study first proposed the concept of “risk factors” for cardiovascular disease (CVD) (1). The term “risk factors” have been used for the diagnosis and the term “causal factors” used for the therapy. “Risk” can be defined for diagnostic purpose by the results based on the prospective studies, while “causal” factor for therapeutic purpose of CVD may be particularly difficult to define. With the many emerging methodological improvements, it has been shown that the postprandial remnant lipoproteins (RLP) are the risk factor as the diagnostic purpose and also a therapeutic target related to the causal factors of CVD. Therefore, we defined “risk” as the target of diagnosis for the prevention and “cause” as the target for the therapy in this review.
Oxidized low density lipoprotein (Ox-LDL) has been proposed as the major atherosclerosis-causal lipoproteins by Steinberg et al. (2). However, Ox-LDL in plasma was found to be less than 0.01% of LDL even in patients with CVD, indicating that it is at too low a concentration to be a risk factor of CVD when compared with RLP (3). We presented an evidence that RLP containing both apoB100 and apoB48 carrying particles has striking similarity with Ox-LDL and are likely the causal lipoproteins for the initiation and progression of atherosclerosis (Table 1). All these bioactive properties of Ox-LDL and RLP were observed under very similar concentrations by in vitro studies (3). Therefore, very low concentration of Ox-LDL in plasma can’t present the bioactive properties comparable to RLP concentration in plasma. Also, the plasma concentration of RLP is similar with the effective concentration of bioactive properties shown by in vitro studies.
Table 1
| Ox-LDL and RLP supported macrophage foam cell formation |
|---|
| Ox-LDL and RLP were chemotactic for monocytes, T cells and tissue macrophages |
| Ox-LDL and RLP enhance the expression of adhesion molecules on monocyte and endothelium |
| Ox-LDL and RLP were mitogenic for smooth muscle cells and macrophages |
| Ox-LDL and RLP altered inflammatory gene expression in vascular cells through a redox-sensitive mechanism |
| Ox-LDL and RLP induced tissue factor, prothrombogenic molecules expression and platelet aggregation |
| Ox-LDL and RLP impaired EDR by altering NO activity |
| Ox-LDL and RLP were cytotoxic and could induced apoptosis in endothelial and smooth muscle cells |
This review article intends to review RLP as causal lipoproteins for the initiation of atherosclerosis and compare RLP to Ox-LDL and chylomicron (CM) remnants which was proposed as postprandial atherogenic lipoproteins by Zilversmit et al. (5).
RLP increases and decreases significantly in plasma after fat intake at a dynamic phase of lipoprotein metabolism and both in vitro and in vivo studies showed RLP has similar bioactive properties with Ox-LDL (3). On the other hand, LDL doesn’t significantly change after fat intake, and LDL itself has no significant atherogenic properties from in vitro studies. All the atherogenic properties of LDL were shown by Ox-LDL which was derived from the oxidation modification of LDL (2). Therefore, actively fluctuating markers such as blood sugar and RLP with bioactive properties, instead of LDL, could be the major cause of initiation of diseases in diabetes and atherosclerotic diseases.
Zilversmit et al. (5) first proposed the hypothesis that postprandial lipoproteins are the major risk of atherosclerosis in patients besides familial hypercholesterolemia. He proposed that CM remnants were the major “risk factor” of atherosclerosis in postprandial plasma while did not emphasize the role of VLDL remnants. We sought to elucidate the characteristics of postprandial remnant lipoproteins by using the isolation method of RLP, especially after fat load (6). Namely, RLP is not simply a risk factor of atherosclerosis, but was shown to be likely a causal factor for both initiation and progression of atherosclerosis, based on its striking similarity in bioactive properties with Ox-LDL (Table 1) (3). The epidemiological and clinical investigations have shown the atherogenic characteristics of lipoproteins. In contrast, we investigated the characteristics of RLP in postprandial plasma using the immuno-separation method and analyzed the composition and biological and biochemical properties of lipoproteins (6). Although Zilversmit et al. proposed CM remnants as the major atherogenic remnants after fat intake, Havel and we identified that VLDL remnants are the major postprandial remnant lipoproteins which was shown by the ratio of apoB100 and apoB48 in RLP and their particle sizes (4,6-8). Therefore, we reconsidered the difference of therapeutic and diagnostic target in the context of LDL and VLDL remnants. LDL has been the target of CVD therapy, while VLDL remnants may be the target of CVD prevention rather than the therapy of CVD. VLDL remnants are the initial metabolites of postprandial lipoproteins induced by the excessive fat intake, specifically composed of long chain fatty acids in CM and VLDL, and is key to the initiation of lipoprotein metabolism (Figure 1). The initial step of lipoprotein metabolism can be controlled by taking less fat and more exercise which can reduce VLDL remnants in plasma (9). This step succeeds to the obesity and its associated insulin resistance. LDL is the final metabolite of postprandial lipoproteins and can be controlled if VLDL remnants are not excessively formed, secreted into circulation and accumulated in adipose tissue. However, if excessive VLDL remnants keep remaining in circulation, plasma LDL will increase as a result. Therefore, LDL fits better as the secondary target for therapy, but not for prevention of atherosclerosis.
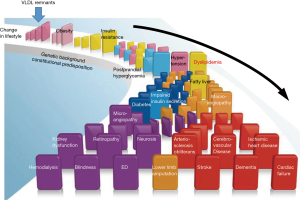
Fluctuation of plasma lipids and lipoproteins after food intake and the role of lipoprotein lipase (LPL)
As non-fasting plasma TG concentration is now recognized as a risk for cardiovascular diseases (10-12), TG concentration in postprandial plasma rather than in fasting plasma, gained more attention to investigate the role of TG on atherosclerosis and CVD. We reported that non-fasting TG was more significantly correlated with RLP (RLP-C and RLP-TG) compared to fasting TG (8,13). Approximately 80% or more of the increased TG (postprandial TG – fasting TG) after fat intake consists of RLP-TG (Table 2, Figure 2). The postprandial RLP showed their main peak at particle size of VLDL, but not of large CM (Figure 3). Interestingly, the particle size of apoB48 carrying RLP was shown to be very similar with apoB100 carrying RLP in 4 hours after fat load in a typical hyperlipidemic patient (Figure 4). These results may indicate the possibility that CM apoB48 remnants are incorporated into liver after fat load and reconstructed into VLDL apoB48 in liver and secreted with VLDL apoB100 from liver and form VLDL remnants in circulation with the same pathway. Plasma RLP as well as TG increase significantly during most of the day except in early morning. These increases depend significantly on the kinds of food taken. The typical foods in Japan did not significantly increase TG and RLP concentration in plasma during a given day compared to fat-rich foods in Western countries (16-18).
Table 2
| Parameter | 0 h | 2 h | 4 h | 6 h |
|---|---|---|---|---|
| TC (mg/dL) | 225 (184–250) | 230 (180–260) | 230 (180–250) | 230 (180–260) |
| TG (mg/dL) | 113 (66–160) | 140 (110–220)* | 180 (140–380)* | 160 (80–300)* |
| HDL-C (mg/dL) | 67 (45–80) | 70 (40–80) | 70 (40–80) | 70 (40–80) |
| LDL-C (mg/dL) | 128 (105–150) | 130 (100–150) | 130 (100–140) | 130 (100–150) |
| RLP-C (mg/dL) | 5.6 (3.9–6.9) | 6.3 (4.9–8.4)* | 8.1 (5.4–13.8)* | 7.5 (4.7–20)* |
| RLP-TG (mg/dL) | 13.8 (5.5–29.3) | 37.7 (35.4–68.4)* | 86.2 (38.4–224.4)* | 77.7 (16.3–142.4)* |
| RLP-TG/RLP-C | 2.1 (1.3–4.9) | 7.2 (5.7–8.5)* | 11.6 (6.4–15.9)* | 5.6 (3.4–11.4)* |
| RLP-TG/TG | 0.11 (0.08–0.19) | 0.32 (0.27–0.34)* | 0.47 (0.31–0.59)* | 0.36 (0.19–0.55)* |
| apo B100 (mg/dL) | 111.9 (88.8–162.4) | 126.4 (87.7–173) | 126.2 (95–160.2) | 126.6 (101–168.3) |
| Apo B-48 (μg/mL) | 6 (3.1–10.3) | 10.8 (6.3–14)* | 13 (6.4–18.3)* | 9.3 (3.8–22.8)* |
| LPL (ng/mL) | 23.7 (19.4–25.7) | 23 (18.8–28.0) | 20.6 (18.0–23.0) | 22.2 (16.9–25.9) |
| LPL/RLP-TG | 1.92 (0.9–4.4) | 0.5 (0.38–0.75)* | 0.48 (0.22–0.8)* | 0.65 (0.13–1.32) |
Data are shown as median (25%tile – 75%tile). 0 h vs. 2 h, 4 h, and 6 h by Dunn test, *P<0.05.

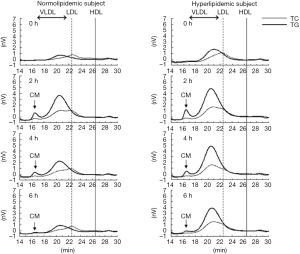
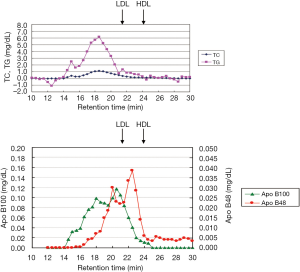
Schneeman et al. (19) reported the postprandial increase of apoB-48 and apoB-100 carrying lipoprotein particles in TG-rich lipoproteins (TRL). The increase of apoB-48 in TRL showed a 3.5-fold in concentration compared to a 1.6-fold increase in apoB-100 in TRL. However, apoB-100 increased in TRL occupied more than 80% in lipoprotein particles, indicating the rich of VLDL remnants. We also reported that apoB-100 particles in RLP after fat intake significantly more increased than apoB-48 particles in RLP in the postprandial plasma (6,8,20) (Figure 5).
It has been generally believed when CM and VLDL particles are hydrolyzed by LPL and converted to smaller particles such as intermediate density lipoproteins (IDL) identified as typical remnant lipoproteins. However, several studies, including our own (21-23) reported that RLP is predominantly composed of large size VLDL mostly derived from VLDL1, which remained comparatively large in particle size when given the excessive supply of CM and VLDL after fat load. Also we recently noticed that the presence of postprandial RLP in plasma at blood sampling may exhibit the large particle size RLP, which is different from the remnant definition of smaller particle size. Interestingly, LPL didn’t increase in circulation after fat intake reported by Ishiyama et al. (15,24). The significant increase of RLP particle size manifested as increased RLP-TG which resulted in less LPL per postprandial RLP. Although Karpe et al. (25) reported that LPL activity increased after fat load associated with the increase of TG, the increase of LPL concentration and activity after fat-rich meal was not evident in our study (15). It is possible that the LPL activity assay used by Karpe et al. lacked the sensitivity and/or accuracy compared with our method (22,24). Although insulin is reported to increase LPL in plasma (26), plasma LPL concentration didn’t change after glucose, carbohydrate load or combined food intake of fat and glucose (cookie test) (24,27).
Increased formation of remnants by cholesteryl ester transfer protein (CETP) in postprandial plasma
Fielding et al. (28) and Guerin et al. (29) reported that plasma CETP activity significantly increased almost in parallel with the increase of TG concentration after fat load. The increase in RLP concentrations (both RLP-C and RLP-TG) in plasma was positively correlated with CETP activity in patients with proteinuria (30). All these findings indicate that CETP activity is closely correlated with remnant lipoprotein formation in plasma.
Patients with CETP deficiency show a phenotype of low LDL-C with high HDL-C and particularly with apoE-rich HDL particles. ApoE-rich large HDL provides cholesteryl ester (CE) and apoE to CM or VLDL during lipolysis, accelerating formation of RLP in the postprandial phase. Inazu et al. (31) investigated CETP deficiency in one homozygote and three heterozygotes of apoE2 and controls with an apoE3/3 phenotype and reported the role of CETP in postprandial lipoprotein metabolism. After fat load, TG, RLP-TG and apoB48 concentration expressed as area under the curve (AUC) increased significantly lower in 2, 4, 6 h in heterozygous CETP deficient subjects compared to the controls. Moreover, the homozygous deficient subjects showed a significantly lower AUC for the increase of TG, RLP-TG and apoB-48 concentration after fat load. However, HPLC profiles in homozygous deficient subjects showed that RLP-C increased after fat load was not composed of VLDL size, but of large HDL size, namely, apoE-rich HDL. In heterozygous deficient subjects, a bimodal distribution of the RLP particle size increased after fat load was found to be from VLDL and large HDL. The subjects with CETP deficiency clearly showed the low responder to TRL and reduced remnant lipoprotein formation after a fat-load than controls.
Okamoto et al. (32) reported the role of CETP for RLP formation using CETP inhibitor (JTT-705) by in vitro assays. The results clearly showed that CETP enhanced the formation of RLP by transferring CE from HDL to TRL. CETP inhibitor was shown to inhibit RLP formation in the in vitro assay system. Therefore, the inhibition of RLP formation by CETP inhibitor is through a different mechanism from statins and fibrates. We believe that the most applicable use of CETP inhibitor is to suppress the postprandial increase of VLDL remnants, rather than to increase of the cholesterol efflux capacity by HDL.
TG in RLP (RLP-TG) is the marker for the postprandial remnants, while cholesterol in RLP (RLP-C) is for the marker for fasting remnants in plasma
RLP-C has been shown as an independent risk factor for cardiovascular diseases reported during last two decades as summarized in (3,6,33). Most of the reports described the RLP-C concentration as an independent CVD risk in the fasting plasma. We further established a sensitive RLP-TG assay to determine the normal range of RLP-TG in the fasting and postprandial plasma of Japanese population (34). The measurement of TG and cholesterol in RLP provided a RLP-TG/RLP-C ratio which was highly correlated with the RLP particle size exhibited by the HPLC profiles reported by Okazaki et al. (35). The RLP-TG/ RLP-C ratio exhibited various RLP particle sizes in lipid disorders; Type III exhibited a significantly low RLP-TG/RLP-C ratio which indicated the higher cholesterol and comparatively lower TG in RLP (reflecting IDL fraction) (36). However, the RLP-TG/RLP-C ratio was significantly high in the postprandial plasma, indicating the increased TG content in RLP (reflecting large VLDL fraction) (Table 2). By calculating the RLP-TG/RLP-C ratio, it is feasible to predict the size of RLP particle which is comparable with the HPLC analysis and can distinguish the kind of abnormal lipoproteins such as LpX (37).
Although RLP-C increased after fat load, the changes in the RLP-C/total TG or RLP-C/total TC ratio in the postprandial plasma were small and not significantly different from the fasting RLP-C/TC ratio. This is because RLP-C in the total TG (RLP-C/TG ratio) was approximately 5% in the fasting and rather decrease to 4% in the postprandial plasma in normal controls. Only RLP-C/TG ratio above 10% in the fasting plasma is now used as a diagnostic marker for type III hyperlipidemia (36,38). In contrast to RLP-C/TG, the RLP-TG/TG ratio was around 10% in the fasting plasma and more than 40% in the postprandial plasma in healthy subjects (Table 2). We preferred to determine the postprandial increase of RLP as the RLP-TG/TG ratio after fat load (15,24,34). The postprandial RLP-TG/TG ratio increased 3 fold in 2 h and RLP-TG increased approximately 50% of the total TG. The most of the increased TG was represented by the increased RLP-TG after fat load (Table 2).
The particle size shown by the RLP-TG/RLP-C ratio reveals the time dependently increased TG in RLP in the postprandial plasma. RLP-TG/RLP-C ratio increased more than 5-fold in 4 h, while RLP-C increased 1.5-fold (Table 2). Therefore, we think that RLP-TG reflects more dynamic physiological feature of the postprandial lipoprotein metabolism than RLP-C for the purpose of direct comparison with total TG metabolism. A higher RLP-TG/TG ratio is associated with higher RLP-C in plasma with an increased risk for CHD (39,40).
RLP-LPL complex is the new characteristics of remnant lipoproteins
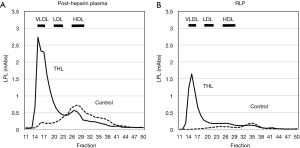
The characteristics of RLP was investigated since 1993 by using an immunoaffinity gel separation method we developed and investigated the bioactive properties and clinical significance of plasma remnant lipoproteins (3,8,41,42). We found that the most of lipoprotein lipase (LPL) presented in RLP fraction in non-heparin plasma which we have isolated and indicated as remnant lipoproteins (43). The characteristics of RLP-LPL are summarized as follows: (I) Approximately 80% of LPL in non-heparin plasma was found in RLP fraction as RLP-LPL complex (Table 3). (II) The LPL found in RLP in the pre- and post-heparin plasma was shown to be inactive. (III) When LPL activity was inhibited by tetrahydrolipstatin (THL) in the post-heparin plasma, most LPL was found to elute at VLDL particle size, identical with RLP (Figure 6). After the hydrolysis of CM and VLDL by LPL, most of the LPL is dissociated from endothelium into circulation as the RLP-LPL complex with inactive forms. A small amount of LPL bound to non-RLP fraction were also found in non-heparin plasma. Majority of circulating plasma LPL containing activity are believed to be in dimeric form, but Beigneux et al. recently reported that active LPL is a monomeric form rather than dimeric form (44). They suspect that ANGPTL4 likely inactivates LPL by promoting the unfolding of active LPL monomers as opposed to by converting catalytically active homodimers into inactive monomers. Those results indicate the possibility that most LPL is present more than one molecule on one RLP in pre-heparin plasma.
Table 3
| Parameter | Pre-heparin | Post-heparin | Pre-heparin – Post-heparin | P value |
|---|---|---|---|---|
| TC (mg/dL) | 174±33 | 169±32 | −5±8 | NS |
| TG (mg/dL) | 139±78 | 103±58 | −36±24 | P<0.01 |
| LDL-C (mg/dL) | 102±28 | 100±27 | −2±5 | NS |
| HDL-C (mg/dL) | 49±13 | 47±12 | −2±3 | NS |
| RLP-C (mg/dL) | 7.2±4.6 | 6.7±4.1 | −0.5±1.2 | NS |
| RLP-TG (mg/dL) | 45±367 | 26±24 | −19.1±18.3 | P<0.01 |
| RLP-TG/RLP-C | 6±1.7 | 3.5±1.4 | −58%±13% | P<0.01 |
| Plasma LPL (ng/mL) | 81±27 | 438±95 | 357±89 | P<0.01 |
| RLP−LPL (ng/mL) | 62±26 | 117±30 | 64±23 | P<0.01 |
| RLP-LPL/plasma LPL (%) | 77±11 | 27±5 | NC | P<0.01 |
Chylomicronemia is a typical condition lacking LPL mass or lipolytic activity (45,46). These patients can’t form remnant lipoproteins, therefore, large nascent CM and VLDL remain in plasma. For example, typical RLP is not formed when LPL activity is completely blocked by autoantibodies against GPIHBP1 (47) (Figure 7, Table 4). As these very large particles don’t carry LPL, these lipoproteins can’t be defined as remnant lipoproteins although patients have very high CM and VLDL concentration in plasma. The scenario with TG above 10 mmol/L (880 mg/dL) have been controversial on whether it is atherogenic or non-atherogenic. It could be differentiated by the presence or absence of LPL activity in abnormally high TG patients. Many of the LPL deficient patients are exposed to high risk of acute pancreatitis (45,46), but rarely the risk of atherosclerosis due to the lack of RLP formation.
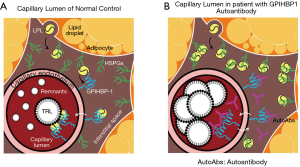
Table 4
| ID | TG (mg/dL) | GPIHBP1 autoAbs (AU/mL) | GPIHBP1 (pg/mL) | LPL (ng/mL) | ANA | Mutation |
|---|---|---|---|---|---|---|
| 38 | 2660 | 327 | 85 | 9.8 | – | Normal range |
| 101 | 1,213 | 70 | 29 | 4.6 | +++ | TG <150 mg/dL |
| 102 | 6,500 | 2,366 | 4 | 5.8 | +++ | LPL 40–85 ng/mL |
| 103 | 9,090 | 81 | NT | NT | ++ | GPIHBP1 315–1,200 pg/mL |
| 111 | 1,389 | 98 | 9 | 32.1 | – | |
| 157 | 549.0 | 134 | 156 | 16.9 | +++ | |
| 164 | 4,784 | 196 | ? | NT | +++ | |
| 3 | >25,000 | ND | 36 | 7.3 | GPIHBP1 nulla (homozygous) | |
| 6 | 6,480 | ND | 31 | 13.1 | GPIHBP1-C68Ya (homozygous) | |
| 39 | 468.1 | ND | 11 | 18.5 | GPIHBP1-C89F/nullb (compound het) | |
| 11 | 1,524 | ND | 3 | 70.6 | GPIHBP1-C89X (homozygous) | |
| 15 | 4,665 | ND | 6 | 11.9 | GPIHBP1-C89X (homozygous) | |
| 21 | 3,164 | ND | 7 | 7.9 | GPIHBP1-S107Cc (homozygous) | |
| 27 | 842 | ND | 9 | 10 | GPIHBP1-S107Cc (homozygous) |
All cases showed very low serum LPL concentration. Those cases had no severe atherosclerosis, but some had the history of acute pancreatitis. a: Rios JJ, Shastry S, Jasso J, et al. J Inherit Metab Dis 2012;35:531-40. b: Charrière S, Peretti N, Bernard S, et al. J Clin Endocrinol Metab 2011;96:E1675-9. c: Plengpanich W, Young SG, Khovidhunkit W, et al. J Biol Chem 2014;289:19491-9. ND, not detected; NT, not tested.
RLP-Lp(a) complex may cause the oxidized characteristics of remnant lipoproteins
Diffenderfer et al. (48) suggested the hypothesis that Lp(a) is bound to RLP which may cause the oxidized form of RLP, because Lp(a), specifically phospholipids bound to apo(a), is known to be oxidized significantly (49,50). Nagasawa et al recently reported that most of the non-covalent bound apo(a) in Lp(a) binds to RLP. RLP-apo (a) increased significantly in postprandial plasma although total Lp(a) concentration barely changes (51). The RLP-apo(a) complex may explain why RLP is oxidized in plasma without any oxidation in vitro, unlike oxidized LDL. Tsimikas et al. (14) reported close correlation between Ox-LDL and Lp(a) concentration in plasma, implying the presence of common oxidized molecules. In particular, this may explain why the increased postprandial RLP concentration is associated with the alterations of endothelial function. Shige et al. (52), Maggi et al. (53) and Funada et al. (54) performed the studies of alterations in endothelial function after fat load followed by time-dependent blood sample collection. Flow mediated dilation (FMD) of the brachial artery for the determination of endothelial function was assessed concurrently. The postprandial increase of RLP-C showed the significantly high correlation with decrease in FMD. They concluded that RLP is strongly associated with the endothelial dysfunction in postprandial hyperlipemia that occurs after fat load. These results support the hypothesis that the RLP-apo(a) complex increased after a fat load may cause endothelial dysfunction as reported by Doi et al. (55), possibly through the interaction with VLDL receptor on endothelial cells (56). Karpe et al. (57) reported previously that most of the postprandial remnant particles were cleared in adipose tissue and muscle in humans. Therefore, we recognize that the postprandial remnant lipoproteins are mostly cleared by the VLDL receptor in adipose tissue and muscle. Since Lp(a) is known to be one of the ligands for the VLDL receptor (58), the interaction of RLP-apo(a) and RLP-LPL with VLDL receptor (43) may be potential initiation of obesity and insulin resistance and associated more advanced atherosclerotic diseases.
Japan Eicosapentaenoic Acid Lipid Intervention Study (JELIS) presented the positive results of EPA in hypercholesterolemia from randomized clinical trial of cardiovascular events
Epidemiological and clinical studies clarified that the intake of long-chain n-3 fatty acids protects against coronary artery diseases and reduce the mortality (59-62). JELIS is the study of long-term use of eicosapentaenoic acid (EPA) that investigated its effect for prevention of major coronary events in hypercholesterolaemic patients who took statins in Japan (63).
The 18,645 patients with a total cholesterol of 6.5 mmol/L or greater were recruited by physicians throughout Japan between 1996 and 1999. Patients were randomly assigned to receive either 1,800 mg of EPA daily with statin (EPA group) or statin only (controls) during a 5-year follow-up. At the mean follow-up of 4.6 years, the primary endpoint was detected in 262 (2.8%) patients in the EPA group and 324 (3.5%) in controls-a 19% relative reduction in major coronary events (P=0.011) by taking EPA. Unstable angina and non-fatal coronary events were also significantly reduced in the EPA group. In patients with a history of coronary artery disease, major coronary events were reduced by 19% by taking EPA. Therefore, EPA was shown to be a promising treatment for prevention of major coronary events, and especially non-fatal coronary events, in Japanese hypercholesterolaemic patients.
Those results revealed that EPA plus statin was significantly more effective against cardiovascular events than statin alone, probably because EPA prevents the formation of RLP raised by the excessive fat intake and lack of exercise at the initial step of lipoprotein metabolic pathway. As RLP is known to be a precursor of sdLDL-C, it was speculated that EPA reduced plasma RLP significantly (64) and statins reduced sdLDL-C (65), and this combination of EPA and statins prevented the initiation and progression of atherosclerotic diseases. RLP is a key factor which can control the generation of sdLDL and other downstream lipoproteins known as atherogenic lipoproteins.
The flow of metabolic domino; increased postprandial VLDL remnants in plasma appear prior to the obesity and induce insulin resistance, but not the consequence of insulin resistance
The prevalent opinion on postprandial dyslipidemia places it after insulin resistance in the flow of metabolic domino, a similar position to postprandial hyperglycemia. It is believed that dyslipidemia including elevated RLP is commonly observed in patients with insulin resistance due to the decreased LPL activity (33). In line with this theory, elevated postprandial RLP is deemed as the consequence of insulin resistance. However, more recent findings point to the proposed cascade that VLDL remnants appear prior to obesity and insulin resistance in the flow of metabolic domino (Figure 1). Because the elevation of plasma VLDL remnants after fat-rich meal intake occurs at the initial steps in the metabolic pathway. Subsequently, the excessive VLDL remnants are delivered to peripheral tissues, in particular to visceral adipocytes and enlarged adipocytes to induce insulin resistance (66). These events occur in the “eat more and exercise less” life style. By improving life style, we are able to prevent the increase of VLDL remnants in plasma and the induction of obesity and following metabolic domino. VLDL remnants are the true residual risk factor need to be controlled. Increased sdLDL in plasma is the result of increased VLDL remnants formation (22). Therefore, sdLDL-C enhances the progression of atherosclerotic diseases, but not the initiation of atherosclerosis. VLDL remnants anchor at the very initial step of metabolic domino and are the most appropriate target to control the initiation and the progression of atherosclerotic diseases.
Conclusions
Ox-LDL and RLP share the striking similarity of pro-atherogenic and pro-inflammatory characteristics. However, Ox-LDL was not found at sufficient concentration in plasma as RLP to initiate and advance of atherosclerosis. RLP is the first product of lipoprotein metabolism circulating in plasma after fat-rich meal for energy delivery to the peripheral tissues. However, when RLP presents continuously and excessively in plasma, visceral adipocytes incorporate and accumulate it as TG and enlarge the adipocytes which cause insulin resistance. Once insulin resistance is induced, the metabolic domino fires and enhance severer dyslipidemia leading to various atherosclerotic diseases down the road. Therefore, the persistent elevation of RLP following intake of fat-rich meal is the initiator of atherosclerosis and the target to be tightly controlled in daily life.
Acknowledgments
We would like to thank Drs. Richard Havel, John Brunzell and Ernst Schaefer for their long term collaboration with the remnant lipoprotein research.
Funding: This work was supported in part by a Grant-in-Aid 26460640 for General Scientific Research from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2020.03.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mahmood SS, Levy D, Vasan RS, et al. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014;383:999-1008. [Crossref] [PubMed]
- Steinberg D, Parthasarathy S, Crew TE, et al. Beyond cholesterol: modification of low-density lipoprotein that increase its atherogenecity. N Engl J Med 1989;320:915-24. [Crossref] [PubMed]
- Nakajima K, Nakano T, Tanaka A. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidized LDL and remnant lipoproteins in plasma. Clin Chim Acta 2006;367:36-47. [Crossref] [PubMed]
- Nakano T, Tanaka A, Okazaki M, et al. Particle size of apoB-48 carrying lipoproteins in remnant lipoproteins isolated from postprandial plasma. Ann Clin Biochem 2011;48:57-64. [Crossref] [PubMed]
- Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation 1979;60:473-85. [Crossref] [PubMed]
- Nakajima K, Nakano T, Tokita Y, et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta 2011;412:1306-18. [Crossref] [PubMed]
- Havel RJ. Postprandial hyperlipidemia and remnant lipoproteins Curr. Opin. Lipidol 1994;5:102-9. [Crossref] [PubMed]
- Nakajima K, Nakano T, Tokita Y, et al. The characteristics of remnant lipoproteins in the fasting and postprandial plasma. Clin Chim Acta 2012;413:1077-86. [Crossref] [PubMed]
- Havel RJ. Remnat lipoproteis as therapeutic targets. Curr Opin Lipidol 2000;11:615-20. [Crossref] [PubMed]
- Iso H, Naito Y, Sato S, et al. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol 2001;153:490-99. [Crossref] [PubMed]
- Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. [Crossref] [PubMed]
- Bansal S, Buring J, Rifai N, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309-16. [Crossref] [PubMed]
- Nakajima K, Tokita Y, Sakamaki K, et al. Triglyceride content in remnant lipoproteins is significantly increased after food intake and is associated with plasma lipoprotein lipase. Clin Chim Acta 2017;465:45-52. [Crossref] [PubMed]
- Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in a cute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation 2004;109:3164-70. [Crossref] [PubMed]
- Nakajima K, Tokita Y, Sakamaki K, et al. Triglyceride content in remnant lipoproteins is significantly increased after food intake and is associated with plasma lipoprotein lipase. Clin Chim Acta 2017;465:45-52. [Crossref] [PubMed]
- Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241-7. [Crossref] [PubMed]
- Ooi TC, Cousins M, Ooi DS, et al. Postprandial remnant-like lipoproteins in hypertriglyceridemia. J Clin Endocrinol Metab 2001;86:3134-42. [PubMed]
- Tanaka A, Tomie N, Nakano T, et al. Measurement of postprandial remnant-like particles (RLP) following a fat-loading test. Clin Chim Acta 1998;275:43-52. [Crossref] [PubMed]
- Schneeman BO, Kotite L, Todd KM, et al. Relationships between the responses of triglyceride rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc Natl Acad Sci USA 1993;90:2069-73. [Crossref] [PubMed]
- Nakajima K, Nakajima Y, Takeichi S, et al. ApoB-100 carrying lipoprotein, but not apoB-48, is the major subset of proatherogenic remnant-like lipoprotein particles detected in plasma of sudden cardiac death cases. Atherosclerosis 2007;194:473-82. [Crossref] [PubMed]
- Campos E, Nakajima K, Tanaka A, et al. Properties of an apolipoprotein E-enriched fraction of triglyceride-rich lipoprotein isolated from human blood plasma with a monoclonal antibody to apolipoprotein B-100. J Lipid Res 1992;33:369-80. [PubMed]
- Shirakawa T, Nakajima K, Shimomura Y, et al. Comparison of the effect of post-heparin and pre-heparin lipoprotein lipase and hepatic triglyceride lipase on remnant lipoprotein metabolism. Clin Chim Acta 2015;440:193-200. [Crossref] [PubMed]
- Zheng C, Murdoch SJ, Brunzell JD, et al. Lipoprotein Lipase Bound to Apolipoprotein B Lipoproteins Accelerates Clearance of Postprandial. Lipoproteins in Humans. Arterioscler Thromb Vasc Biol 2006;26:891-96. [Crossref] [PubMed]
- Ishiyama N, Sakamaki K, Shimomura Y, et al. Lipoprotein lipase does not increase significantly in the postprandial plasma. Clin Chim Acta 2017;464:204-10. [Crossref] [PubMed]
- Karpe F, Olivecrona T, Walldius G, et al. Lipoprotein lipase in plasma after an oral fat load: relation to free fatty acids. J Lipid Res 1992;33:975-84. [PubMed]
- Chan BL, Lisanti M, Rodriguez-Boulan E, et al. Insulin-stimulated release of lipoprotein lipase by metabolism of its phosphatidylinositol anchor protein. Science 1988;241:1670-72. [Crossref] [PubMed]
- Harano Y, Miyawaki T, Nabiki J, et al. Development of cookie test for the simulta-neous determination of glucose intolerance, hyperinsulinemia, insulin resistance and postprandial dyslipidemia. Endocr J 2006;53:173-80. [Crossref] [PubMed]
- Fielding CJ, Havel RJ, Todd KM, et al. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. J Clin Invest 1995;95:611-8. [Crossref] [PubMed]
- Guerin M, Egger P, Soudant C, et al. Cholesteryl ester flux from HDL to VLDL-1 is preferentially enhanced in type IIB hyperlipidemia in the postprandial state J. Lipid Res 2002;43:1652-60. [Crossref] [PubMed]
- Deighan CJ, Caslake MJ, McConnell M, et al. The atherogenic lipoprotein phenotype: small dense LDL and lipoprotein remnants in nephrotic range proteinuria. Atherosclerosis 2001;157:211-20. [Crossref] [PubMed]
- Inazu A, Nakajima K, Nakano T, et al. Decreased post-prandial triglyceride response and diminished remnant lipoprotein formation in cholesteryl ester transfer protein (CETP) deficiency. Atherosclerosis 2008;196:953-7. [Crossref] [PubMed]
- Okamoto H, Miyai A, Sasase T, et al. Cholesteryl ester transfer protein promotes the formation of cholesterol-rich remnant like lipoprotein particles in human plasma. Clin Chim Acta 2007;375:92-8. [Crossref] [PubMed]
- Twickler TB, Dallinga-Thie GM, Cohn JS, et al. Elevated remnant-like particle cholesterol concentration: a characteristic feature of the atherogenic lipoprotein phenotype. Circulation 2004;109:1918-25. [Crossref] [PubMed]
- Nakano T, Tokita Y, Nagamine T, et al. Measurement of serum remnant-like lipoprotein particle-triglyceride (RLP-TG) and RLP-TG/total TG ratio using highly sensitive triglyceride assay reagent. Clin Chim Acta 2011;412:71-8. [Crossref] [PubMed]
- Okazaki M, Usui S, Tada N, et al. Relation between RLP- triglyceride to RLP-cholesterol ratio and particle size distribution in RLP-cholesterol profiles by HPLC. Clin Chim Acta 2000;296:135-49. [Crossref] [PubMed]
- Wang T, Nakajima K, Leary ET, et al. Ratio of remnant-like particle-cholesterol to serum total triglycerides is an effective alternative to ultracentrifugal and electrophoretic methods in the diagnosis of familial type III hyperlipoproteinemia. Clin Chem 1999;45:1981-7. [PubMed]
- Nakajima K, Nakano T, Nakazato K, et al. Isolation and Detection of Serum Lp-X by Immunoaffinity Separation Method. J Clin Lab Invest Update 2015;3:1-6. [Crossref]
- Nakajima K, Saito T, Tamura A, et al. A new approach for the detection of type III hyperlipoproteinemia by RLP-cholesterol assay. J Atheroscler Thromb 1994;1:30-6. [Crossref] [PubMed]
- McNamara JR, Shah PK, Nakajima K, et al. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis 2001;154:229-36. [Crossref] [PubMed]
- Karpe F, Boquist S, Tang R, et al. Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res 2001;42:17-21. [PubMed]
- Nakajima K, Saito T, Tamura A, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gel. Clin Chim Acta 1993;223:53-71. [Crossref] [PubMed]
- Nakajima K, Okazaki M, Tanaka A, et al. Separation and determination of remnant-like particles in serum from diabetes patients using monoclonal antibodies to apo B-100 and apo A-I. J Clin Ligand Assay 1996;19:177-83.
- Sato K, Okajima F, Miyashita K, et al. The majority of lipoprotein lipase in plasma is bound to remnant lipoproteins: A new definition of remnant lipoproteins. Clin Chim Acta 2016;461:114-25. [Crossref] [PubMed]
- Beigneux AP, Allan CM, Sandoval NP, et al. Lipoprotein lipase is active as a monomer. Proc Natl Acad Sci U S A 2019;116:6319-28. [Crossref] [PubMed]
- Sugandhan S, Khandpur S, Sharma VK. Familial chylomicronemia syndrome. Pediatr Dermatol 2007;24:323-5. [Crossref] [PubMed]
- Tada H, Kawashiri MA, Nakahashi T, et al. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J Clin Lipidol 2015;9:519-24. [Crossref] [PubMed]
- Beigneux AP, Miyashita K, Ploug M, et al. Autoantibodies against GPIHBP1 as a cause of hypertriglyceridemia. N Engl J Med 2017;376:1647-58. [Crossref] [PubMed]
- Diffenderfer MR, Lamon-Fava S, Marcovina SM, et al. Distinct metabolism of apolipoproteins (a) and B-100 within plasma lipoprotein (a). Metabolism 2016;65:381-90. [Crossref] [PubMed]
- Leibundgut G, Scipione C, Yin H, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J Lipid Res 2013;54:2815-30. [Crossref] [PubMed]
- Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein (a) as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 2008;49:2230-9. [Crossref] [PubMed]
- Nagasawa T, Nakajima K, Miyashita K, et al. Increase of Lp(a) in remnant lipoproteins after fat load. The 29th World Congress of World Association of Societies of Pathology and Laboratory Medicine WASPaLM 2017, Abstract p18-8 (220) in Kyoto (*, This article described the presence of apo(a) on RLP).
- Shige H, Ishikawa T, Suzukawa M, et al. Endothelium-dependent flow-mediated vasodilation in the postprandial state in type 2 diabetes mellitus. Am J Cardiol 1999;84:1272-74. [Crossref] [PubMed]
- Maggi FM, Raselli S, Grigore L, et al. Lipoprotein remnants and endothelial dysfunction in the postprandial phase J Clin Endocrinol Metab 2004;89:2946-50. [Crossref] [PubMed]
- Funada J, Sekiya M, Otani T, et al. The close relationship between postprandial remnant metabolism and insulin resistance. Atherosclerosis 2004;172:151-54. [Crossref] [PubMed]
- Doi H, Kugiyama K, Ohgushi M, et al. Membrane active lipids in remnant lipoproteins cause impairment of endothelium-dependent vasorelaxation. Arterioscler Thromb Vasc Biol 1999;19:1918-24. [Crossref] [PubMed]
- Wyne KL, Pathak K, Seabra MC, et al. Expression of the VLDL receptor in endothelial cells. Arterioscler Thromb Vasc Biol 1996;16:407-15. [Crossref] [PubMed]
- Karpe F, Humphreys SM, Samra JS, et al. Clearance of lipoprotein remnant particles in adipose tissue and muscle in humans. J Lipid Res 1997;38:2335-43. [PubMed]
- Argraves KM, Kozarsky KF, Fallon JT, et al. The atherogenic lipoprotein Lp(a) is internalized and degraded in a process mediated by the VLDL receptor. J Clin Invest 1997;100:2170-81. [Crossref] [PubMed]
- Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med 1985;312:1205-9. [Crossref] [PubMed]
- Siscovik DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995;274:1363-7. [Crossref] [PubMed]
- Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 1997;336:1046-53. [Crossref] [PubMed]
- Albert CM, Hennekens CH, O'Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA 1998;279:23-8. [Crossref] [PubMed]
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090-8. [Crossref] [PubMed]
- Nakamura N, Hamazaki T, Kobayashi M, et al. Effects of eicosapentaenoic acids on remnant-like particles, cholesterol concentrations and plasma fatty acid composition in patients with diabetes mellitus. In Vivo 1998;12:311-4. [PubMed]
- Florentin M, Liberopoulos EN, Moutzouri E, et al. The effect of simvastatin alone versus simvastatin plus ezetimibe on the concentration of small dense low-density lipoprotein cholesterol in subjects with primary hypercholesterolemia. Curr Med Res Opin 2011;27:685-92. [Crossref] [PubMed]
- Nakajima K, Tokita Y, Tanaka A. Hypothesis: Postprandial remnant lipoproteins are the causal factors that induce the insulin resistance associated with obesity. Clin Chim Acta 2018;485:126-32. [Crossref] [PubMed]
Cite this article as: Nakajima K, Tokita Y, Tanaka A. Atherogenic postprandial remnant lipoproteins—a causal lipoproteins for the initiation of obesity and atherosclerosis. J Lab Precis Med 2020;5:19.


