
A holistic approach for the diagnosis of venous thromboembolism
Introduction
Venous thromboembolism (VTE) encompasses two separated, but frequently correlated, pathologies, that are deep vein thrombosis (DVT) and pulmonary embolism (PE), with the latter condition representing the worst complication of the former (1). Unlike what has been occasionally suspected, primary PE does not exist as a single clinical entity, since the onset of pulmonary emboli is always related to embolization of blood clots during a pre-existing episode of DVT. Sometimes investigations are unable to identify DVT in patients with PE because the original thrombus has undergone complete embolization or, equally likely, are simply unable to precisely localize the thrombus; these usually (up to 97% of the time) localize to from veins of the legs, but can also occasionally onset in additional venous districts (e.g., vein of the arms, portal thrombosis, etc.) (1).
According to the most recent statistics (2), the lifetime risk of VTE at age 45 years is approximately 8%, slightly higher in African Americans (around 12%), in patients with obesity (around 11%), and in those bearing thrombophilic abnormalities such as—for example—factor V Leiden mutation (around 17.1%). The 30-day case fatality is approximately 6.5%, whilst the 1-year mortality is about 23%. Notably the 1-year survival rate is close to 50% in patients with underlying cancer, but is as high as 93% in those with unprovoked VTE (2). Importantly, VTE should be considered an almost chronic disease, characterized by episodic recurrence, because nearly 30% of patients develop recurrence within the next 10 years after the original episode in the absence of long-term anticoagulation (2). As concerns the possible complications, the 20-year cumulative incidence of post-thrombotic syndrome is 30%, whilst chronic thromboembolic pulmonary hypertension may affect up to 4% of patients with PE within 2 years from the original thrombotic episode (2).
It is currently estimated that approximately 10 million cases of VTE are diagnosed each year around the world, causing approximately 2.3 million deaths (3). Comparing this data with that of the novel 2019 coronavirus disease (now known as COVID-19), which has affected so far nearly 470,000 people causing about 21,000 deaths (4), one would conclude that the risk of dying for VTE in the general population is still higher than that of dying for COVID-19, while the risk of death in individuals affected by either pathology is over 8-fold higher for VTE than for COVID-19. As regards the future trend, epidemiologic data shows that the number of hospitalizations for PE in the US has more than doubled during the past 20 years, from approximately 75,000 in 1996, up to nearly 180,000 hospitalizations in 2016 (2). The recent data, moreover, does not suggest that this trend will be likely to reverse soon.
The substantial epidemiologic burden of VTE imposes also challenges to acute healthcare resource utilization, whereby the rate of admissions for VTE in the emergency department can be as high as 67 per 100,000 inhabitants (5). Similarly, relevant also is the rate of cumulative hospital admissions for VTE among people aged 60 years or older, which is now as high as 740 per 100,000 inhabitants (6). These important estimates would hence contribute to underscore the essential significance of an appropriate and accurate strategy for both diagnosing or ruling out VTE. It is hence worthwhile to mention here a famous quote attributed to Karl Krause, whereby “one of the most widespread diseases is diagnosis” and, perhaps, this would be especially true when using laboratory tests.
An integrated strategy for diagnosing venous thromboembolism
What has now clearly emerged, becoming virtually incontestable, is that the efficiency of the modern clinical diagnostic reasoning is highly dependent upon the integration of multiple domains, substantially encompassing the combination of clinical judgement with results of diagnostic investigations, that can be laboratory, microbiology or pathology tests, radiology procedures and functional testing (7). The diagnostic approach to VTE makes no exception to this rule, but represents instead a paradigmatic example where clinical interpretation, laboratory medicine and diagnostic imaging contribute to build up the diagnostic reasoning (8,9). Clinical judgement is essential for establishing pre-test probability and driving clinicians towards ordering the most appropriate and efficient investigations, or for safely discharging patients with very low pre-test probability (10). Laboratory diagnostics—through measurement of thrombosis biomarkers—would enable to identify the presence of an ongoing thrombotic process (11). Diagnostic imaging, finally, will be essential to confirm the presence of a thrombotic episode and, even more importantly, to localize the site and the extension of the blood clot (12,13).
The clinical judgement should hence be considered the first essential element of diagnostic reasoning in patients with suspected VTE. This clearly translates into the assessment of the so-called pre-test probability, according to the well-known principle defined by reverend Thomas Bayes, more than 300 years ago (14). Basically, according to the Bayesian interpretation, probability depends on prior knowledge of conditions that might be related to the event. This would help define whether patients could be considered at low, medium or high risk of a certain pathology, such as VTE, for example (15). Risk assessment, and pre-test probability, can be carry out by using some clinical tools, that are specifically defined as score systems for both DVT and PE, and which take into consideration several clinical variables, such as the clinical history (for example the presence of cancer, recent surgery, paralysis or bedridden), which is then combined with some suggestive clinical signs and symptoms such as appearance of physical and respiratory abnormalities (including leg morphology, hemoptysis and so forth) (16). Each of these elements are given a score, which is then averaged to define a final probability of either DVT or PE, in terms of low, moderate or high. The final score will then guide the demand of diagnostics tests, as later discussed. Overall, the most widely used of such systems are the Well’s and the Geneva’s scores. They slightly differ one from the other for the variables included and their relative score, but the clinical significance is almost overlapping, whereby both allow to formulate a final score, which will then guide the requests of diagnostic investigations (16).
Imaging and in vitro diagnostic testing
The diagnostic tests that have been historically proposed for investigating patients with suspected VTE include some notorious milestones such as pulmonary angiography and venography around the 1940s, Doppler ultrasonography in the 1960s, the development of tests for measuring fibrin/fibrinogen degradation products (FDPs) in the 1970s, then followed in the ensuing years by introduction of computed tomography angiography, color Doppler, lung scintigraphy and D-dimer testing in the 1980s-1990s (8). As specifically concerns laboratory diagnostics, a huge number of thrombotic biomarkers have been proposed over the past 3 decades for diagnosing thrombosis (11). These include markers of activation of blood coagulation such as myeloperoxidase (MPO) and fibrin fragment 1 plus 2 (FP1+2), along with marker of thrombin generation such as thrombin-antithrombin (TAT) complexes, activated protein C and protein C inhibitor complexes (APC-PCI), fibrinopeptides A (PFA) and B (FPB), soluble fibrin monomers (sFM), thrombus precursor protein (TpP), as well as biomarkers of both coagulation activation and fibrinolysis such as plasmin and anti-plasmin complexes (PAP), fibrin/fibrinogen degradation products (FDPs) and, last but not least, D-dimer (11). Throughout the past 30 years, an impressive number of papers has been published on the diagnostic efficiency of these biomarkers. What has now emerged now almost clearly, is that no other biomarker than D-dimer combines diagnostic sensitivity and specificity to a comparable diagnostic accuracy (17). To cite some paradigmatic examples, a study carried out by Gibson et al. including has many as 3,306 consecutive patients with clinically suspected PE (18), reported that the diagnostic accuracy of D-dimer, expressed as area under the curve (AUC, 0.82; 95% CI, 0.77–0.87), was substantially higher than that of F1+2 (AUC, 0.69; 95% CI, 0.63–0.76). In another, more recent study, Hasegawa et al. compared the diagnostic accuracy of four different D-dimer immunoassays with that of FDPs (19), concluding that the AUCs of each single D-dimer test (comprised between 0.993–1.000) was always higher than the of FDPs (i.e., 0.812). In particular, despite the diagnostic specificity of each single D-dimer test (comprised between 0.951–0.996) was comparable to that of FDPs (i.e., 0.995), their diagnostic sensitivity (comprised between 0.955–0.977) was consistently better that that of FDPs (0.341). Therefore, the large volume of evidence garnered during the past 20 years supports the conclusion that D-dimer measurement would allow to rapidly rule out a substantial number of patients from other expensive, time-consuming and even more hazardous diagnostic investigations (20).
The role of D-dimer in diagnosing venous thromboembolism
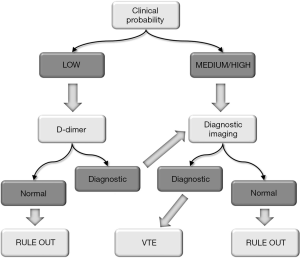
D-dimer can be defined as a mixture of fibrin degradation products originating when breakdown of a stabilized thrombus is catalyzed by plasmin (21). The advantage of D-dimer over other biomarkers, is that whatever increase into the circulation of its concentration not only reflects the activation of the coagulation cascade and the stabilization of the thrombus by factor XIII (FXIII), but also the activation of the fibrinolytic system, thus enabling to unquestionably interpret its increase within the setting of a well defined thrombotic process, rather than attributing its elevation to non-specific degradation of fibrin and/or fibrinogen (21). One additional important aspect is that, unlikely other thrombosis biomarkers, there are already many diagnostic algorithms based on clinical assessment and D-dimer which have been widely validated in a multitude of clinical trials (21). One paradigmatic example comes from a joint document of Italian Societies of Laboratory and Emergency Medicine (22), where D-dimer has been incorporated in algorithms for diagnosing DVT or PE. According to these recommendations, for example, it is clearly emphasized that D-dimer shall not be used as a stand-alone test diagnosing for ruling out VTE, but rather it should be used in the setting of a validated algorithm, which must encompass also the assessment of clinical pre-test probability. Notably, there are several strategies that can be envisaged, where D-dimer could be placed at different places throughout the diagnostic algorithm. For example, D-dimer can be the first test upon patient admission, then followed by diagnostic imaging. Otherwise, D-dimer can be placed after diagnostic imaging. Or, eventually, D-dimer can be placed at the beginning of the diagnostic reasoning, immediately after, or in combination with, clinical judgment (23). Several lines of evidence would now attest that this last strategy is probably the most efficient, but also the safest (21,22). This is hence what is being suggested, for example, by the joint document of Italian Societies of Laboratory and Emergency Medicine (22), whereby results of D-dimer testing shall be interpreted according to the clinical picture. Therefore, when D-dimer is negative and pre-test probability of VTE is low, the patient can be safely discharged, with a residual probability of having thrombosis that is considerably lower than 1%. Nevertheless, when the pre-test probability is moderate or high, diagnostic imaging is then strongly recommended, and results of these investigations will be crucial for ruling in or ruling out both DVT and PE (Figure 1). Interestingly, an almost identical approach is suggested by the UK National Institute for Health and Care Excellence (NICE) (24). Overlapping recommendations have also been released by a joint panel of the European Society of Cardiology (ESC) and the Acute Cardiovascular Care Association (ACCA) (25), as well as by the American College of Chest Physicians (ACCP) (26). It may also be important to mention here that D-dimer is not 100% sensitive and specific for VTE, since there are other obvious reasons that contribute to lower its diagnostic accuracy (21,27). Some of these are biological, other analytical or pathological. Concerning the two former aspects, the diagnostic sensitivity of D-dimer may be lower when the time passed since the onset of the thrombotic event is too short or too long (D-dimer has a half life of nearly 6–8 hours), when fibrinolysis is slow and clot lysis is delayed, when the size of the clot is small, when the thrombus is located in small and peripheral veins (21,28). The diagnostic specificity may also be decreased for the presence of other physiological or pathological conditions associated with enhanced thrombin generation and fibrinolysis, as will be more thoughtfully discussed afterwards, but also because the currently available diagnostic kits for measuring D-dimer are characterized by heterogeneous specificity for the molecular cross-link which is the hallmark of circulating D-dimers (21,28).
The gradual ageing of the organism, altogether with that of blood vessels and coagulation, is another important aspect that may impact the accuracy of D-dimer for diagnosing VTE (29). It has now been clearly demonstrated that normal (i.e., physiological) concentration of D-dimer in plasma increases in parallel with the age of the subject, especially after the age of 50 years. Therefore, the “normal” D-dimer value of a 50 years old man can be nearly 8- to 10-fold lower than that of a 90 years old male subject (30). This evidence has led to a substantial revision of the diagnostic criteria based on the D-dimer diagnostic cutoffs, leading the way to introducing a formula specifically designed to adjust the diagnostic thresholds according to the age of the subjects and thereby improve the diagnostic specificity without affecting the diagnostic sensitivity. The most widely used formula encompasses the definition of the diagnostic threshold by multiplying the age of the subject for a fix coefficient of 10 after the age of 50 years. Therefore, the diagnostic cutoff of a 75 years old subject would be 75×10, and thus 750 µg/L, whilst that of a 90 years old subject would be 90×10, and thus 900 µg/L (31).
In a previous part of this presentation, the concept of physiological and pathological causes of D-dimer increase beside VTE was already introduced. This is in keeping with a foremost quote, attributed to the English writer and philosopher Aldous Huxley, that “medical science has made such tremendous progress that there is hardly a healthy human left”. This provoking assertion is consistently true for D-dimer, especially for healthcare professional who are almost unaware of its many physiological and pathological determinants. As previously mentioned, D-dimer values increase whenever there is enhanced (i.e., supernormal or excess) thrombin generation combined with activation of fibrinolysis pathway (21). This may happen in a number of physiological condition such as ageing and pregnancy, as physiological consequence of healing after surgery, traumas or other injuries, as well as in patients with disseminated intravascular coagulation (DIC), arterial thrombosis such as acute coronary syndrome, stroke, peripheral occlusive disease, intestinal ischemia and acute aortic dissection, or in those with vascular disorders such as Alzheimer’s disease, pneumonia, other severe infections and sepsis, cancer, HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, liver disease, atrial fibrillation and heart failure, among others (21,27).
In a recent study, which has explored the most frequent reasons of D-dimer elevation in patients admitted to the emergency department, VTE was not found to be the most frequent pathology in these patients (12.1%), but was preceded by infections (15.6%), and closely followed by syncope (9.4%), heart failure (8.9%), trauma (8.2%) and cancer (5.8%) (32). In particular, in subjects with D-dimer values between 500 and 1,000 µg/L, thus already considered diagnostic, but only modestly elevated, VTE was only 11th among the causes of increased values of this biomarker (33).
Some of the most useful preanalytical, analytical and clinical issues related to the use of D-dimer for diagnosing VTE have been summarized in the recent consensus document published by the Italian Societies of Laboratory and Emergency Medicine (22). As concern the more important preanalytical aspects, specific focus is given to the facts that D-dimer should not be used alone, but within validated diagnostic algorithms, entailing the assessment of clinical pre-test probability, diagnostic imaging shall then be used according to the available guidelines, blood for D-dimer measurement shall be drawn using 3.2% citrate blood tubes and venipuncture using straight-needles shall be preferred when drawing blood for D-dimer testing, since this will limit spurious activation of blood coagulation. Among the most relevant analytical aspects, major emphasis is given to the facts that quantitative and certified immunoassays shall be used, that techniques characterized by optimal diagnostic sensitivity and acceptable diagnostic specificity are preferable, that the measuring range and the linearity of the assay shall be broad, preferably between 50 and 5,000 µg/L, that the analytical imprecision shall be lower than 10% at the method-specific diagnostic cutoffs, and that the turnaround time shall always be lower than 60 minutes. As finally regards the main post-analytical aspects, it has been recommended that testing shall not be repeated earlier than 6–8 hours, that final result shall be reported in µg/L of fibrinogen equivalent units (FEU), that a clinically validated cutoff shall always be used under the age of 50 years and that age-adjusted cutoffs shall instead be applied to patients aged 50 years or older, by using a validated formula. It is also advised that additional conditions associated with increased D-dimer concentration shall be considered in the differential diagnosis, and that it shall be avoided to measure D-dimer in patients who are admitted to the emergency department too early or too late after the onset of a thromobotic episode, in those with impaired fibrinolysis, whilst caution is necessary when interpreting test results in patients undergoing anticoagulant therapy.
Conclusions
Although the diagnostic approach to patients with suspected VTE remains somehow challenging, the many progresses made during the past decades have enormously contributed to improve the diagnostic armamentarium and thereby to achieve a more efficient diagnosis (8). A final mention shall then be made to the future perspective of integrated—and thereby holistic—diagnostic approach to VTE. The most promising techniques encompass thrombus-targeted molecular imaging by means of radioiodinated monoclonal antibodies, small molecules with fibrin affinity or nanoparticles, along with infrared thermal imaging. These techniques are still confined to the research setting, but some interesting studies are underway. The main advantage is that diagnostics, through molecular imaging, and therapy, could then be combined into the well-known concept of theranostics (8). One of the most paradigmatic and perhaps promising example is thrombous imaging trough peptide-based fibrin-targeting probes (33). These innovative compounds not only could selectively bind with the fibrin, which essentially constitutes the blood clot and thus enabling its visualization, but may also convey fibrinolytic enzymes such as plasmin, urokinase or tissue-type plasminogen activator (t-PA), which would complement clot imaging with its active dissolution. Basically, fibrin-binding substances, simple such as antibodies, or more complex such as nanoparticles and red blood cells which are also essential elements of a blood clot (34), can be conjugated with a variety of fibrinolytic agents, and could then be delivered at the site of thrombosis, where the clot could be contextually visualized and lysed. It is our hope that reliable studies will soon show that these innovative and disruptive strategies could be considered the next paradigm in diagnosis and management of VTE (35).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2020.03.02). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The opinions in this paper are those of the authors, and not necessarily those of the University of Verona or NSW Health Pathology.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Franchini M. Pathogenesis of venous thromboembolism: when the cup runneth over. Semin Thromb Hemost 2008;34:747-61. [Crossref] [PubMed]
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020; [Epub ahead of print]. [Crossref] [PubMed]
- World Thrombosis Day. Understanding Thrombosis. Available online: https://www.worldthrombosisday.org/issue/vte/. Last accessed: February 29, 2020.
- World Health Organization. Coronavirus disease (COVID-2019) situation reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Last accessed: February 29, 2020.
- Yusuf H, Tsai J, Siddiqi A, et al. Emergency department visits by patients with venous thromboembolism, 1998-2009. J Hosp Adm 2012;1:1-8. [Crossref]
- Yusuf HR, Reyes N, Zhang QC, Okoroh EM, Siddiqi AE, Tsai J. Hospitalizations of adults ≥60 years of age with venous thromboembolism. Clin Appl Thromb Hemost 2014;20:136-42. [Crossref] [PubMed]
- Lippi G, Plebani M. Integrated diagnostics: the future of laboratory medicine? Biochem Med (Zagreb) 2020;30:010501 [Crossref] [PubMed]
- Lippi G, Danese E, Favaloro EJ, et al. Diagnostics in venous thromboembolism: from origin to future prospects. Semin Thromb Hemost 2015;41:374-81. [Crossref] [PubMed]
- Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv 2018;2:3226-56. [Crossref] [PubMed]
- Kristoffersen AH, Ajzner E, Bauça JM, et al. Pre- and post-test probabilities of venous thromboembolism and diagnostic accuracy of D-dimer, estimated by European clinicians working in emergency departments. Thromb Res 2017;159:19-23. [Crossref] [PubMed]
- Lippi G, Cervellin G, Franchini M, et al. Biochemical markers for the diagnosis of venous thromboembolism: the past, present and future. J Thromb Thrombolysis 2010;30:459-71. [Crossref] [PubMed]
- Karande GY, Hedgire SS, Sanchez Y, et al. Advanced imaging in acute and chronic deep vein thrombosis. Cardiovasc Diagn Ther 2016;6:493-507. [Crossref] [PubMed]
- Moore AJE, Wachsmann J, Chamarthy MR, et al. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther 2018;8:225-43. [Crossref] [PubMed]
- Lilford RJ, Braunholtz D. Who's afraid of Thomas Bayes? J Epidemiol Community Health 2000;54:731-9. [Crossref] [PubMed]
- Cervellin G, Borghi L, Lippi G. Do clinicians decide relying primarily on Bayesians principles or on Gestalt perception? Some pearls and pitfalls of Gestalt perception in medicine. Intern Emerg Med 2014;9:513-9. [Crossref] [PubMed]
- Kline JA. Diagnosis and Exclusion of Pulmonary Embolism. Thromb Res 2018;163:207-20. [Crossref] [PubMed]
- Thachil J, Lippi G, Favaloro EJ. D-Dimer Testing: Laboratory Aspects and Current Issues. Methods Mol Biol 2017;1646:91-104. [Crossref] [PubMed]
- Gibson NS, Sohne M, Kruip MJ, et al. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost 2008;99:229-34. [Crossref] [PubMed]
- Hasegawa M, Wada H, Miyazaki S, et al. The Evaluation of Fibrin-Related Markers for Diagnosing or Predicting Acute or Subclinical Venous Thromboembolism in Patients Undergoing Major Orthopedic Surgery. Clin Appl Thromb Hemost 2018;24:107-14. [Crossref] [PubMed]
- Lippi G, Mengoni A, Manzato F. Plasma D-dimer in the diagnosis of deep vein thrombosis. JAMA 1998;280:1828-9. [Crossref] [PubMed]
- Favresse J, Lippi G, Roy PM, et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci 2018;55:548-77. [Crossref] [PubMed]
- Lippi G, Cervellin G, Casagranda I, et al. D-dimer testing for suspected venous thromboembolism in the emergency department. Consensus document of AcEMC, CISMEL, SIBioC, and SIMeL. Clin Chem Lab Med 2014;52:621-8. [Crossref] [PubMed]
- Legnani C, Palareti G, Prisco D. Linee guida sull’impiego clinico del D-Dimero. Riv Med Lab 2004;5:225-39.
- National Institute for Health and Care Excellence. Venous thromboembolism in adults: diagnosis and management. Quality standard [QS29]. Available online: https://www.nice.org.uk/guidance/qs29. Last accessed, February 29, 2020.
- Giannitsis E, Mair J, Christersson C, et al. How to use D-dimer in acute cardiovascular care. Eur Heart J Acute Cardiovasc Care 2017;6:69-80. [Crossref] [PubMed]
- Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e351S-418S.
- Lippi G, Franchini M, Targher G, Favaloro EJ. Help me, Doctor! My D-dimer is raised. Ann Med 2008;40:594-605. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. D-dimer measurement and laboratory feedback. J Emerg Med 2009;37:82-3. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different. Ann Transl Med 2017;5:212. [Crossref] [PubMed]
- Favaloro EJ, Franchini M, Lippi G. Aging hemostasis: changes to laboratory markers of hemostasis as we age - a narrative review. Semin Thromb Hemost 2014;40:621-33. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Cervellin G. A review of the value of D-dimer testing for prediction of recurrent venous thromboembolism with increasing age. Semin Thromb Hemost 2014;40:634-9. [Crossref] [PubMed]
- Lippi G, Bonfanti L, Saccenti C, et al. Causes of elevated D-dimer in patients admitted to a large urban emergency department. Eur J Intern Med 2014;25:45-8. [Crossref] [PubMed]
- Oliveira BL, Caravan P. Peptide-based fibrin-targeting probes for thrombus imaging. Dalton Trans 2017;46:14488-508. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Venous and Arterial Thromboses: Two Sides of the Same Coin? Semin Thromb Hemost 2018;44:239-48. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Hemostasis practice: state-of-the-art. J Lab Precis Med 2018;3:67. [Crossref]
Cite this article as: Lippi G, Favaloro EJ. A holistic approach for the diagnosis of venous thromboembolism. J Lab Precis Med 2020;5:20.


