Lifetime risks factors and assessment of cardiovascular disease
Background
Cardiovascular disease (CVD) is a chronic disease that develops from youth or even childhood. A number of genetically determined disorders predispose patients to increased CVD risk, while behavioral risk factors and acquired pathological conditions confer additional risk. Together, these factors increase an individual’s likelihood of developing CVD. Multiple models and/or formulas have been published to guide the assessment of long-term CVD risk, and guidelines are available to modify lifetime risks.
CVD in pediatric population
Recently, the American Heart Association (AHA) published a scientific statement on cardiovascular risk reduction in pediatric patients (1). Childhood conditions that have lifelong impact on an individual’s risk of developing CVD include both familial inherited conditions [e.g., homozygous or heterozygous familial hypercholesterolemia (FH), hypertension, and severe obesity], and acquired medical conditions that increase future risk of CVD [such as chronic renal disease, and chronic inflammatory conditions such as juvenile inflammatory arthritis]. These conditions confer varying degrees of increased future CVD risk, and Table 1 presents disease stratification by risk category.
Table 1
| Category | Condition |
|---|---|
| High risk | Homozygous FH, T2DM, end-stage renal disease, T1DM, Kawasaki disease with persistent aneurysms, solid-organ transplant vasculopathy, childhood cancer survivor (stem cell recipient) |
| Moderate risk | Severe obesity, heterozygous FH, confirmed hypertension, coarctation, Lp(a), predialysis CKD, AS, childhood cancer survivor (chest radiation) |
| At risk | Obesity, insulin resistance with comorbidities (dyslipidemia, NAFLD, PCOS), white-coat hypertension, HCM and other cardiomyopathies, pulmonary hypertension, chronic inflammatory conditions (JIA, SLE, IBD, HIV), s/p coronary artery translocation for anomalous coronary arteries or transposition of the great arteries, childhood cancer (cardiotoxic chemotherapy only), Kawasaki disease with regressed aneurysms (zMax ≥5) |
AS, aortic stenosis; CKD, chronic kidney disease; FH, familial hypercholesterolemia; HCM, hypertrophic cardiomyopathy; IBD, inflammatory bowel disease; JIA, juvenile rheumatoid arthritis; Lp(a), lipoprotein (a); NAFLD, nonalcoholic fatty liver disease; PCOS, polycystic ovarian syndrome; SLE, systemic lupus erythematosus; s/p, status post; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; and zMax, maximum z score at any time during the course of illness (1).
Childhood conditions contributing to CVD risk
Familial hypercholesterolemia (FH)
FH is an autosomal dominant genetically inherited disorder of cholesterol metabolism (caused by LDL receptor defects) that affects about 1 in 250 children in its heterozygous form. FH is characterized by very high levels of LDL cholesterol (LDL-C), resulting in atherosclerosis and early CVD morbidity and mortality, and is directly related to lifetime exposure to LDL-C. Unfortunately, most FH remains undiagnosed, as it is typically asymptomatic prior to onset of CVD events. As per pediatric cholesterol guidelines, children with a family history of premature CVD or significant hypercholesterolemia should be screened for FH using a fasting lipid profile beginning at 2 years of age, and then every 3 to 5 years through adulthood, even if previous profiles are within normal ranges. The pediatric clinical diagnosis of a heterozygous FH patient can be suspected in the presence of an LDL-C level ≥160 mg/dL (4.0 mmol/L) associated with a family history of elevated LDL-C or premature CVD in first- or second-degree relatives. The diagnosis of FH can be considered confirmed if there is a positive genetic testing for an LDL-C–raising gene defect [LDL receptor, apolipoprotein B, or proprotein convertase subtilisin/kexin type 9 (PCSK9)] in a first-degree relative. As with all cases of hypercholesterolemia, secondary causes of hypercholesterolemia should be excluded, including hypothyroidism, nephrotic syndrome, or liver diseases (2-4).
Homozygous FH is characterized by an untreated LDL-C >400 mg/dL (10.0 mmol/L) and one or both parents having clinically diagnosed FH, xanthomata in childhood, supravalvar aortic stenosis, positive genetic testing for an LDL-C–raising genetic mutation, or autosomal-recessive FH (5).
Elevated lipoprotein (a) [Lp(a)]
Lp(a) is a pathological lipoprotein containing LDL-C and apo B linked with apo (a). Apo (a) shares some homology with plasminogen and is thought to be responsible for the prothrombotic activity of Lp(a). Genome-wide association and Mendelian randomization studies indicate that Lp(a) is a causal, independent, and highly heritable risk factor for CVD. Measurement of Lp(a) could thus be useful in identifying children with FH that present with high risk of premature CVD. Elevated Lp(a) has also been associated with thromboembolic events in youth (6-8).
Childhood obesity
Childhood obesity and overweight have been recognized as risk factors for future CVD. The definitions are as follows: body mass index (BMI) greater than or equal to the 95th percentile for age and sex is categorized as obesity, and the 85th to 94th percentile, is grouped as overweight. A recent study of almost 2.3 million individuals followed up for over 40 years found the risk of CVD mortality was 2- to 3-fold higher if an individual’s BMI as an adolescent had been in the overweight category (hazard ratio, 2.25; 95% confidence interval, 1.96–2.58) or obese (hazard ratio, 3.46; 95% confidence interval, 2.93–4.10) compared with adolescents with normal weight (9).
Youth with severe obesity (BMI ≥120% of the 95th percentile or absolute BMI ≥35 kg/m2) are generally viewed as being at the highest level of risk because of the high number and magnitude of CVD risk factors, the presence of endothelial dysfunction and subclinical atherosclerosis, and the strong tracking of adiposity from childhood into adulthood. This represents a significant potential disease burden from a public health perspective, as 6% of all youth 2 to 19 years old (equating to >4,000,000 children and adolescents) have severe obesity in the United States (10).
Annual assessment for obesity is recommended by the American Academy of Pediatrics and others via calculation of BMI and plotting the results on CDC growth charts. Associated cardiovascular risk factors should also be assessed. Waist measurement is an additional good indicator of adiposity-related CVD risk, even before BMI criteria are met, especially in the setting of central obesity (11,12).
Childhood diabetes
Diabetes mellitus, either caused by a lack of insulin [type 1 diabetes (T1DM)] or a lack of response to insulin [type 2 diabetes (T2DM)], is characterized by hyperglycemia associated with the development of CVD. Microvascular complications of diabetes such as retinopathy, nephropathy have been demonstrated in youth with both T1DM and T2DM, including increased carotid intimal medial thickness, worsened arterial stiffness, increased left ventricular mass, and diastolic dysfunction. The differences in pathophysiology between T1DM and T2DM suggest CVD risk should differ, and indeed, the risk was previously thought to be higher for children with T1DM. However, emerging evidence suggests risks are also high for youth diagnosed with T2DM. In subjects with onset of T2DM between age 15 and 30 years, cardiovascular mortality was significantly higher than in subjects with T1DM even if there was shorter duration of disease (13-16). In the Search for Diabetes in Youth study, a national surveillance effort to identify diabetes mellitus in youth, ≈18 000 children and adolescents were diagnosed with T1DM and >5,000 were diagnosed with T2DM from 2008 to 2009. The prevalence of T2DM in youth is increasing at a rate more rapid than T1DM, likely because of high rates of obesity, with a 30.5% increase in incidence rate between 2001 and 2009; T2DM now constitutes almost half of all childhood diabetes mellitus (17).
Diabetes generally presents as polyuria, and polydipsia, and in T1DM, children often present with diabetic ketoacidosis. T2DM has a less acute onset, and is often detected by screening of youth with obesity and a family history of T2DM. Once diagnosed, youth with diabetes mellitus should be screened yearly for additional CVD risk factors to enable initiation of early interventions (18).
Hypertension
Hypertension, whether primary or secondary, is a major contributor to adult CVD and a known risk factor for developing atherosclerosis in youth and CVD events in adulthood. Two longitudinal cohort studies demonstrated that elevated blood pressure in childhood predicts increased central large artery stiffness in adulthood, denoting a worsening of arterial function (19). A study from Pima and Tohono O’odham children of the American Southwest demonstrated that the presence of physician-diagnosed hypertension in childhood significantly increased the risk of mortality before age 55 years (20). The National Heart, Lung, and Blood Institute guidelines include hypertension as a risk condition, with the severity of the risk category determining the use of pharmacotherapy (1). In addition to being a known cardiovascular risk condition itself, hypertension is a feature of other high-risk disease states such as chronic renal disease, inflammatory conditions like systemic lupus erythematosus (SLE), T2DM, obesity, and polycystic ovarian syndrome (21).
The American Academy of Pediatrics “Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents”, endorsed by the AHA and others, recommends measuring blood pressure at all routine healthcare visits beginning at age 3 years, and earlier in higher-risk individuals (22).
Chronic kidney disease
End-stage renal disease is the last stage of CKD, which requires treatment with dialysis or kidney transplantation. There are about 10,000 children in the United States treated with maintenance dialysis, and about 1,000 children undergoing kidney transplant annually. CVD is the most common cause of death, accounting for 25% to 35% of all deaths, and is the biggest obstacle to long-term survival of children and adolescents with CKD. CKD is a vasculopathic state (23) characterized by the accumulation of numerous traditional risk factors (hypertension, dyslipidemia, and obesity) and nontraditional, CKD-related risk factors (abnormal mineral metabolism, anemia, chronic inflammation) which act synergistically to result in early abnormalities of vascular (increased cIMT and stiffness, coronary artery calcification) and cardiac [left ventricular hypertrophy (LVH) and left ventricular dysfunction] structure and function (24). These cardiovascular risk factors and subclinical cardiovascular abnormalities are already evident in the early, predialysis stages of pediatric CKD, become increasingly common during maintenance dialysis, and persist after kidney transplantation (24).
Children with end-stage renal disease should be screened at least yearly for traditional risk factors, including hypertension, dyslipidemia, and obesity. Kidney Disease Improving Global Outcomes (KDIGO) has developed recommendations for the management of most common CVD risk factors based on the available pediatric evidence (25-27).
Congenital heart disease
The prevalence of congenital heart disease (CHD) is estimated at 9 per 1,000 live births (17) with 3 per 1,000 requiring catheter- based or surgical intervention early in life (28). Advances in surgical, percutaneous, and medical management mean that ≥90% of children with CHD survive into adulthood, yielding an estimated 1.0 to 2.9 million adults living with CHD in the United States. However, they are more vulnerable to accelerated atherosclerosis and premature CVD. This risk of premature atherosclerotic CVD in patients with congenital heart defects is based on the following 3 conditions: (I) obstructive lesions of the left ventricle and aorta, (II) cyanotic congenital heart defects leading to Eisenmenger syndrome, and (III) lesions with coronary artery abnormalities (29). In these patients, screening for cardiovascular risk factors and attempts to modify these risks earlier in life could improve their long-term outcomes.
Kawasaki disease (KD)
Kawasaki disease affects 30 per 100,000 children in the United States and is a leading cause of acquired heart disease. Early in the illness, the lipid profile shows high triglycerides in the acute illness and low HDL in the years after (30-32). Therefore, children with KD are at increased CVD risk, and should have cardiovascular assessment and lipid screening as per the recommendations for healthy children (33).
Classification of cardiovascular health status
The AHA formed the Goals and Metrics Committee of the Strategic Planning Task Force in 2010 with the Impact Goal to improve the cardiovascular health of all Americans by 20% while reducing deaths from CVD and stroke by 20% by 2020. The Committee defined a new concept, cardiovascular health, based on seven risk factors (Life’s Simple 7) that can be improved through lifestyle changes (34). Ideal cardiovascular health consists of the simultaneous presence of 4 ideal health behaviors including nonsmoking, body mass index (BMI) <25 kg/m2, physical activity at goal level, diet consistent with current guideline recommendations, and 3 ideal health factors: untreated total cholesterol <200 mg/dL, untreated blood pressure <120/<80 mmHg, and untreated fasting glucose <100 mg/dL. Cardiovascular health status for the general population was categorized as poor, intermediate, or ideal. These metrics are to be followed to determine cardiovascular health status that reflects the achievement of the Impact Goal. The classification based on these cardiovascular health factors are shown in Table 2.
Table 2
| Risk Factors | Ideal | Intermediate | Poor |
|---|---|---|---|
| Smoking | Non-smokers | current smokers | |
| Body mass index (kg/m2) | <22.9 | 23–26.9 | ≥27 |
| Physical Activity | ≥150 min/week moderate or ≥75 min/week vigorous or ≥150 min/week moderate + vigorous activity | 1–149 min/week moderate or 1–74 min/week vigorous or 1–149 min/week moderate + vigorous activity | 0 min/week of moderate or vigorous activity |
| Diet | Meeting thresholds for fruit and vegetable (≥4.5 cups/day of fruits and vegetables), fish (≥3.5 oz servings/week of fish), whole grains (≥three 10z servings a day), sugar sweetened beverage (<4 glasses a week) and sodium (<1,500 mg/day) consumption | ||
| Untreated blood pressure (mmHg) | <120/80 | 120–139/80–88 | ≥140/90 |
| Untreated glucose (mg/dL) | <100 | 100–125 | ≥126 |
| Untreated cholesterol (mg/dL) | <200 |
Lifetime risk factors for CVD
Lifetime cardiovascular risk factors can be categorized into behavioral and health factors.
Behavioral factors
Smoking
According to a 2018 CDC survey, 13.7% of U.S. adults aged 18 years and over (34.2 million people) currently smoke cigarettes, which represents a decline from 20.9% in 2005. Cigarette smoking has long been recognized to have cardiovascular health consequences. Numerous data support that cessation of smoking contributes to the ideal cardiovascular health (35).
BMI
Increase in the prevalence of overweight and obesity has been striking in recent years, and has become a major issue in cardiovascular health. Based on the estimation from the US National Center for Health Statistics in 2015–2016, 31.8% of adults aged 20 or older were overweight, 39.8% were obese, and among them, 7.6% were severely obese. The prevalence of overweight and obesity has increased to similar degrees in all race/ethnicity groups in the US. A number of studies support a significant beneficial effect of maintaining BMI <22 kg/m2, which was associated with fewer CVD events, longer disease-free life, and overall longer life expectancy, but the AHA committee uses a less conservative cut point of 24.9 kg/m2 to define BMI for poor cardiovascular health (36).
Physical activity
Since the first Physical Activity Guidelines released by the US Department of Health and Human Services in 2008, there has been accumulating evidence that any physical activity is better than none. Most health benefits are achieved by 150 minutes a week of moderate-intensity physical activity, while marginal benefit of additional physical activity is little. Therefore, the AHA committee recommends at least 150 minutes per week of moderate-intensity physical activity, or 75 minutes per week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activities for cardiovascular health benefits (36,37).
Diet
Characterization of the optimal diet is complex, and the Nutrition Committee of AHA has been working to develop ideal diet goals for cardiovascular health (38). The Nutrition Committee recommendation is to follow a diet that is appropriate in energy balance, with an overall dietary pattern based on the dietary approaches to stop hypertension (DASH) diet. This diet recommends inclusion of ≥4.5 cup fruits and vegetables per day, ≥ two 3.5-oz servings fish per week (preferably oily fish), ≥3 1-oz-equivalent servings fiber-rich whole grain per day (with ≥1.1 g of fiber per 10 g of carbohydrate), <1,500 mg sodium per day, and ≤450 kcal (36 oz) of sugar-sweetened beverages per week.
Several other factors were recognized by the committee as contributors to a healthy dietary pattern, such as avoiding trans-fat, saturated fat, and processed meats, and meeting other nutrient needs like calcium, potassium, magnesium, and dietary fiber.
Health factors
Cholesterol, blood pressure, and blood glucose
Despite the fact that medications targeting hypercholesterolemia, hyperglycemia and hypertension have lowered CVD risk for patients with these adverse conditions, metabolic control by pharmacotherapy is far less favorable relative to maintenance of ideal levels of cholesterol, glucose and blood pressure without pharmaceutical treatment. The cutoff levels of cholesterol, glucose and hypertension are in agreement with other major clinical practice guidelines. For example, total cholesterol <200 mg/dL is consistent with the 2018 AHA/ACC guideline. The goal of blood pressure <120/<80 mmHg and fasting blood glucose <100 mg/dL are consistent with the optimal levels in the Seventh Joint National Committee of the National High Blood Pressure Education Program and the American Diabetes Association (39-41).
CVD risk assessment tools
In order to reduce the CVD burden in the entire population, besides encouraging healthy lifestyle through local and national initiatives, it is also critical to identify individuals at risk. To effectively reduce CVD-related mortality, identification and assessment of risk factors compromising cardiovascular health must be consistently implemented. A set of traditional risk factors was identified in 1961 in the Framingham Heart study, and includes age (≥45 for male and ≥55 for females), sex, hypertension, dyslipidemia, smoking and diabetes mellitus. More recently, premature history of atherosclerotic cardiovascular disease (ASCVD) (<55 for males and <65 for females) was added as an additional risk factor (42).
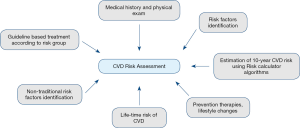
A general approach to CVD risk assessment (Figure 1) includes reviewing a patient’s medical history, physical examination, and determining whether traditional risk factors (such as hypertension, cigarette smoking, diabetes mellitus, premature family history of CVD, chronic kidney disease, or obesity) are present. In addition to this, non-traditional risk factors and biomarkers should be considered, such as metabolic syndrome, chronic inflammatory conditions, extensive comorbidities, and psychological and social determinants, as their presence can significantly alter risk in a subset of patients (43). After the relevant risk factors have been identified, all patients from 40 to 79 years of age should have their CVD risk estimated using a validated CVD risk calculator (44). Based on the risk score result, primary prevention therapies and lifestyle changes can be initiated.
The estimation of the risk score is a critical part of the CVD risk assessment and aids in identifying patients who would benefit from lifestyle changes and pharmacological therapies. There are several CVD risk calculators in widespread use, with new algorithms being developed on a regular basis and adopted by regional organizations and societies that focus on CVD prevention.
The current multifactorial risk models/calculators assess the risk of initial CVD in individuals, prognosticate the risk and provide guidance for initiation or intensification of preventive strategies. Multivariate risk algorithms for the estimation of CVD risk include factors recognized as increasing the incidence of CVD (45). These models are derived from large population-based studies and allow to assess the risk of an individual patient. Table 3 provides a summary of available risk score calculators.
Table 3
| Risk Algorithm | Variables | Endpoints assessed |
|---|---|---|
| ACC/AHA Pooled cohort Risk calculator | Age, gender, race, TC, HDL-C, DBP, DM, smoking, treatment of HTN | CHD death, nonfatal MI, fatal stroke, nonfatal stroke |
| MESA risk score (2015) | Age, gender, ethnicity, TC, HDL-C, SBP, DM, treatment of HTN, lipid lowering treatment, smoking, family history of MI at any age, CAC score | CHD death, nonfatal MI, resuscitated cardiac arrest, coronary revascularization in patient with angina |
| Reynolds Risk Score (RRS) | Age, TC, HDL-C, SBP, DM, smoking, family history of MI before age 60 years, hs-CRP | Cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization |
| QRISK and QRISK2 | Age, gender, cholesterol/HDL-C ratio, DM, SBP, BMI, treatment of HTN, smoking, family history of CVD, CKD, AF, RA | CHD death, nonfatal MI, coronary insufficiency or angina, coronary revascularization, stroke, transient ischemic attack, intermittent claudication |
| European Systematic Coronary Risk Evaluation (SCORE) | Age, gender, TC, HDL-C, SBP, smoking, region of Europe (low vs. high risk), | Assesses fetal CVD risk over 10 years |
| JBS3 risk score (2014) | Age, gender, ethnicity, TC, HDL-C, SBP, DM, treatment of HTN, DM, smoking, family history of CVD, CKD, AF, RA, BMI, Region of United Kingdom | CHD death, nonfatal MI, coronary insufficiency or angina, coronary revascularization, stroke, transient ischemic attack, intermittent claudication |
| China-PAR risk predictor | Age, gender, TC, HDL-C, treatment of HTN, Lipid lowering treatment, SBP, DM, smoking, waist circumference, region of China, urbanization (in males), family history of ASCVD (in males) | CHD death, nonfatal MI, stroke |
| Framingham general CVD risk score (2008) | Age, gender, TC, HDL-C, SBP, treatment of HTN, DM, smoking | CHD death, nonfatal MI, coronary insufficiency or angina, stroke, heart failure, transient ischemic attack, intermittent claudication |
TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; SBP, systolic blood pressure; DBP; diastolic blood pressure; DM diabetes mellitus; HTN, hypertension; CAC, coronary artery calcium; CKD, chronic kidney disease; AF, atrial fibrillation, RA, rheumatoid arthritis; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; MI, myocardial infraction; hs-CRP, high-sensitivity C-reactive protein; CHD, coronary heart disease.
Each model has its advantages and disadvantages and should not be universally applied for all patients. The choice of the risk score evaluator should be patient specific.
It is expected that physicians become familiar with the algorithm validated for the local population. Some regional CVD risk algorithms include the ACC/AHA pooled cohort equation CV risk calculator revised in 2018; in Canada, the Cardiovascular life expectancy model or Framingham risk score; in the UK, the JBS3, in Western Europe, SCORE; or in China, China-PAR (46-49).
Key features of the most commonly used risk calculators include ease of use, applicability to the patient population, and professional society recommendations for use.
There are several limitations to current CVD prediction models. These include a lack of efficiently predicting or identifying risk factors for low risk people with marginal abnormalities (50,51). Many available algorithms, including the 2013 ACC/AHA guideline, do not offer an estimate of lifetime risk which might be significantly higher than the 10-year risk, and also tend to overestimate the 10-year risk (44). The importance of calculating lifetime risk has been increasingly recognized. Another disadvantage is that age is the main component in current score models, leading to underestimation of the risk score in younger individuals. One of the most important limitations of many available calculators is the fact that these risk scores were derived from older cohorts (52,53), which may result in overestimation of risk due to the change in prevalence of risk factors and suboptimal validation in contemporary cohorts.
To address these deficiencies, current risk score calculators are constantly being updated. More recent algorithms like the Multi-Ethnic Study of Atherosclerosis (MESA) offer an optional incorporation of coronary artery calcium (CAC), which is considered a valuable indirect measurement of cumulative exposure to risk factors (54). Recently, Jaspers et al. introduced LIFE-CVD, a new risk predicting tool that allows for treatment benefit estimation (55). The authors used a modern North American cohort, the same as the one validated for the Multi-Ethnic Study of Atherosclerosis (MESA). LIFE-CVD prognosticates both the 10-year and lifetime CVD risk and estimates the potential increase in CVD-free life expectancy with preventive interventions, including initiation or intensifying statin therapy, lowering systolic blood pressure, initiating aspirin or equivalent antithrombotic therapy and smoking cessation. The LIFE-CVD risk score is validated for individuals between 45–80 years old. This tool is available as an online risk calculator.
While the LIFE-CVD risk score is an improved CVD risk prediction tool, continued effort is still required to create comprehensive risk estimators. LIFE-CVD, just like other available tools, does not consider the duration and severity of exposure to risk factors such as smoking, hypertension, diabetes and hyperlipidemia. It also does not include various lifestyle preventive changes, such as weight loss, dietary modifications and exercise, ignoring protective benefits of these changes, as well as overlooking risk enhancing factors that are reported to increase CVD risk such as inflammatory diseases and chronic kidney disease.
2019 ACC/AHA guidelines for primary prevention of cardiovascular disease
The American College of Cardiology and American Heart Association published an updated Clinical Practice Guideline in 2019 regarding primary prevention of ASCVD (56). The 2019 guidelines emphasize the importance of an integrated approach to promoting a healthy lifestyle in ASCVD prevention, incorporating social factors, lifestyle factors, and the management of exacerbating health conditions such as type 2 diabetes mellitus, hypertension, hypercholesterolemia, and overweight or obesity.
The 2019 ACC/AHA guidelines on primary prevention reiterate some important points from previous clinical practice guidelines. The top 10 take-home messages in the new guidelines include a recommendation for all adults aged 40 to 75 to undergo 10-year ASCVD risk estimation, which should be completed prior to starting pharmacological therapy and may be aided by assessment of risk-enhancing factors, such as coronary artery calcium scanning (57). Statin therapy is recommended for diabetics aged 40–75 regardless of estimated 10-year ASCVD risk, and for other adults with LDLc ≥190 mg/dL or with intermediate 10-year ASCVD risk (58). The guidelines also address hypertension and elevated blood pressure, recommending both nonpharmacological intervention and a goal blood pressure of <130/80 mmHg to minimize ASCVD risk (28,59).
The guidelines also recommend that all adults adhere to a diet that emphasizes plants and lean proteins and minimizes processed meats and refined carbohydrates, and that overweight and obese adults are supported in weight loss efforts via counseling and caloric restriction. For adults with type 2 diabetes mellitus, the new guidelines emphasize the importance of diet and exercise interventions, and recommend metformin as first-line therapy for minimization of risk.
New recommendations in the 2019 ACC/AHA clinical practice guidelines address aspects of patient-centered, integrated care in the prevention of ASCVD. These guidelines make strong recommendations for use of team-based care approaches, involving healthcare providers from multiple subspecialties, the patient, and family members, and recommend that decisions regarding individual preventative care should be collaborative rather than prescriptive. Further, these guidelines highlight the relationship between socioeconomic status and ASCVD risk, and recommend that social factors influencing a patient’s individual risk or ability to comply with lifestyle intervention recommendations are evaluated and addressed.
The 2019 guidelines also make new recommendations regarding exercise and physical activity as interventions for primary prevention of ASVCD. Clinicians are recommended to counsel all patients to maintain a physically active lifestyle, and for adults to target at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity exercise per week. For patients unable to meet these targets, the new guidelines indicate that any amount of moderate- or vigorous-intensity exercise is likely to confer some benefit with respect to ASCVD risk reduction, although efforts should be made to encourage meeting the recommended goals.
Low-dose aspirin therapy (75–100 mg daily), while historically widely used as a preventative pharmacotherapy for ASCVD, is not recommended for routine use in primary prevention by the 2019 guidelines. Aspirin is recommended for primary prevention only in patients aged 40–70 years who are at higher ASCVD risk but who do not have increased bleeding risk. However, the strength of this recommendation in the 2019 guidelines is weak (class IIb), as recent studies have not demonstrated clear benefits with respect to bleeding risk conferred by aspirin therapy. Additionally, the new guidelines recommend against aspirin therapy in patients older than 70 or in any patients with increased risk of bleeding due to risk of harm.
The 2019 guidelines specifically address tobacco use as a modifiable risk factor for ASCVD, and urges that tobacco users be counseled to quit and assisted in that pursuit. The guidelines make specific recommendations for monitoring tobacco use and implementation of both behavioral and pharmacological interventions to drive cessation of tobacco use.
Summary
Prevention of CVD requires an integrated approach to reduction of lifetime risk factors. The most recent AHA/ACC guideline on preventative strategies for CVD provides a framework to approach necessary changes, and particularly emphasizes the importance of managing lifestyle factors to reduce the risk of CVD. A focus on prevention of CVD is essential to effectively reduce CVD burden.
Acknowledgments
Funding: Both JJ and EG were funded through the Ching Nan Ou Fellowship in Clinical Chemistry.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Cardiovascular Precision Medicine”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-2019-cpm-04). The series “Cardiovascular Precision Medicine” was commissioned by the editorial office without any funding or sponsorship. SD served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from August 2018 to July 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019;139:e603-34. [Crossref] [PubMed]
- Gidding SS, Sood E. Preventing cardiovascular disease: going beyond conventional risk assessment. Circulation 2015;131:230-1. [Crossref] [PubMed]
- Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478-90a. [Crossref] [PubMed]
- Perak AM, Ning H, de Ferranti SD, et al. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults With the Familial Hypercholesterolemia Phenotype. Circulation 2016;134:9-19. [Crossref] [PubMed]
- Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146-57. [Crossref] [PubMed]
- Kivimäki M, Ferrie JE. Epidemiology of healthy ageing and the idea of more refined outcome measures. Int J Epidemiol 2011;40:845-7. [Crossref] [PubMed]
- Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med 2013;273:6-30. [Crossref] [PubMed]
- McNeal CJ. Lipoprotein(a): Its relevance to the pediatric population. J Clin Lipidol 2015;9:S57-66. [Crossref] [PubMed]
- Twig G, Tirosh A, Leiba A, et al. BMI at Age 17 Years and Diabetes Mortality in Midlife: A Nationwide Cohort of 2.3 Million Adolescents. Diabetes Care 2016;39:1996-2003. [Crossref] [PubMed]
- Skinner AC, Perrin EM, Moss LA, et al. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med 2015;373:1307-17. [Crossref] [PubMed]
- Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120:S164-92. [Crossref] [PubMed]
- Mokha JS, Srinivasan SR, Dasmahapatra P, et al. Utility of waist-to-height ratio in assessing the status of central obesity and related cardiometabolic risk profile among normal weight and overweight/obese children: the Bogalusa Heart Study. BMC Pediatr 2010;10:73. [Crossref] [PubMed]
- Stanković SM, Zivic SR, Saranac L, et al. Determinants of atherosclerosis in children and adolescents with diabetes type 1. Endokrynol Pol 2012;63:414-9. [PubMed]
- Shah AS, Wadwa RP, Dabelea D, et al. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes 2015;16:367-74. [Crossref] [PubMed]
- Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863-9. [Crossref] [PubMed]
- Maahs DM, Daniels SR, de Ferranti SD, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2014;130:1532-58. [Crossref] [PubMed]
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67-e492. [Crossref] [PubMed]
- Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes 2017;9:320-4. [Crossref] [PubMed]
- Juonala M, Jarvisalo MJ, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2005;112:1486-93. [Crossref] [PubMed]
- Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485-93. [Crossref] [PubMed]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128:S213-56. [Crossref] [PubMed]
- Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017;140:e20171904 [Crossref] [PubMed]
- Luke RG. Chronic renal failure--a vasculopathic state. N Engl J Med 1998;339:841-3. [Crossref] [PubMed]
- Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 2012;23:578-85. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9:S1-155. [Crossref]
- Vanbelleghem H, Vanholder R, Levin NW, et al. The Kidney Disease: improving Global Outcomes website: comparison of guidelines as a tool for harmonization. Kidney Int 2007;71:1054-61. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;S1-130. [PubMed]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Tutarel O. Acquired heart conditions in adults with congenital heart disease: a growing problem. Heart 2014;100:1317-21. [Crossref] [PubMed]
- Cheung YF, Yung TC, Tam SC, et al. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol 2004;43:120-4. [Crossref] [PubMed]
- Gupta-Malhotra M, Rao PS. Current perspectives on Kawasaki disease. Indian J Pediatr 2005;72:621-9. [Crossref] [PubMed]
- Ou CY, Tseng YF, Lee CL, et al. Significant relationship between serum high-sensitivity C-reactive protein, high-density lipoprotein cholesterol levels and children with Kawasaki disease and coronary artery lesions. J Formos Med Assoc 2009;108:719-24. [Crossref] [PubMed]
- Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379-85. [Crossref] [PubMed]
- Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586-613. [Crossref] [PubMed]
- (US) OotSG, (US) OoSaH. The Health Consequences of Smoking: A Report of the Surgeon General. 2004.
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res 1998;6:51S-209S. [PubMed]
- 2008 Physical Activity Guidelines for Americans. Washington, DC, 2008.
- Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011-20. [Crossref] [PubMed]
- Delahoy PJ, Magliano DJ, Webb K, et al. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther 2009;31:236-44. [Crossref] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62-9. [Crossref] [PubMed]
- Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003;289:2534-44. [Crossref] [PubMed]
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med 1961;55:33-50. [Crossref] [PubMed]
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849-58. [Crossref] [PubMed]
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935-59. [Crossref] [PubMed]
- Jackson R, Lawes CM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet 2005;365:434-41. [Crossref] [PubMed]
- Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611-9. [Crossref] [PubMed]
- Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136. [Crossref] [PubMed]
- Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987-1003. [Crossref] [PubMed]
- Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation 2010;121:1768-77. [Crossref] [PubMed]
- Vasan RS, Sullivan LM, Wilson PW, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Intern Med 2005;142:393-402. [Crossref] [PubMed]
- Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791-8. [Crossref] [PubMed]
- Bazo-Alvarez JC, Quispe R, Peralta F, et al. Agreement Between Cardiovascular Disease Risk Scores in Resource-Limited Settings: Evidence from 5 Peruvian Sites. Crit Pathw Cardiol 2015;14:74-80. [Crossref] [PubMed]
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266-75. [Crossref] [PubMed]
- McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643-53. [Crossref] [PubMed]
- Jaspers NEM, Blaha MJ, Matsushita K, et al. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J 2020;41:1190-9. [Crossref] [PubMed]
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177-232. [Crossref] [PubMed]
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935-59. [Crossref] [PubMed]
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285-350. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127-248. [Crossref] [PubMed]
Cite this article as: Jung J, Garnett E, Cao J, Devaraj S. Lifetime risks factors and assessment of cardiovascular disease. J Lab Precis Med 2020;5:23.