Laboratory medicine and iron overload: diagnostic and therapeutic aspects
Introduction
Diffuse—or systemic—body iron overload represents an important public health problem, considering both its worldwide frequency and its consequences in terms of morbidity and mortality (1,2). Therapeutic solutions most often exist but are all the more effective as the diagnosis has been made at an early stage. It is therefore essential to manage the diagnostic tools at our disposal in the most efficient way. These tools consist mainly of clinical, biological, and imaging data. The present review will focus on the laboratory input for the diagnostic and therapeutic management of systemic iron overload.
Systemic iron overload spectrum: pathophysiological and nosological aspects
Pathophysiological aspects
Propensity of the human body to develop iron excess
The human body is doubly vulnerable to iron metabolism disturbances (3). On the one hand, it is highly exposed to iron deficiency. Its only source for total body iron storage being dietary iron, every chronic lack of alimentary iron input, meaning dietary insufficiency or iron malabsorption, may therefore be responsible for lack of body iron. On the other hand, the body is not equipped to increase significantly iron elimination in case of excessive iron ingress from the digestive lumen or from parenteral route, which exposes the body to the development of iron overload.
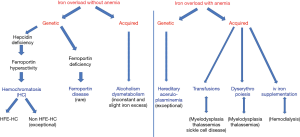
Main mechanisms of iron overload (Figure 1)
Parenteral iron input
Parenteral iron can be introduced in two ways. Transfusions represent the first way knowing that each unit of blood transfusion provides approximately 250 mg of iron corresponding to one-twelfth of the total normal body iron content. This transfusional iron is stored, after degradation of the red blood cells, within the macrophages of the reticuloendothelial system particularly localized primarily in the spleen and, at a lesser degree, in the liver (Kupffer cells). Therefore, in the setting of multiple transfusions, iron overload develops essentially at the splenic level and moderately at the hepatic level. Subsequently, following the progressive release into the plasma of stored iron through ferroportin (the only known cell iron exporter), plasma transferrin saturation increases, leading to the formation of plasma non-transferrin bound iron (NTBI) (4). NTBI, in contrast to transferrin-bound iron which targets mainly the bone marrow, is very quickly taken up by the parenchymal cells, in the first line by the hepatocytes that are much more numerous than Kupffer cells, which leads to major hepatic iron deposition. Moreover, myocardiocytes and pancreatic cells are also targeted by the NTBI. Beside transfusions, excessive injection of iron itself during parenteral iron supplementation represents a risk of iron overload. This injected iron, under macromolecular forms, ends up, like transfusional iron, in the macrophages.
Ferroportin hyperactivity
Ferroportin is especially active in the macrophages and therefore in the spleen but exerts also its cellular iron egress property at the duodenal level. Its activation, essentially due to decreased plasma concentrations of the iron hormone hepcidin but also in rare cases of ferroportin gene mutations leading to a “gain of function” of ferroportin, leads to an increased egress of iron originating from both the spleen and alimentary tract (5). This results in an increase of plasma transferrin saturation, with subsequent appearance of NTBI and hepatocyte iron deposition (whereas the spleen is devoid of iron).
Ferroportin hypoactivity
Decreased ferroportin activity, like increased ferroportin activity, leads to iron overload but by a reverse mechanism. Indeed, the resulting decreased release of iron from cells into the plasma causes iron retention that occurs mainly in the macrophages, and therefore in the spleen, whereas plasma transferrin saturation by iron is normal or low.
Exceptional mechanisms of systemic genetic iron overload
Exceptional mechanisms of systemic genetic iron overload are represented by: (I) atransferrinemia leading, due to the impossibility of plasma iron to interact with transferrin, to iron overload through the formation of NTBI, and (II) aceruloplasminemia, disease in which iron excess is classically attributed to an impairment of cellular iron release; this impairment has been attributed to the fact that ferroportin activity becomes impaired in the wake of the disappearance of ceruloplasmin-related ferroxidase activity (necessary for plasma iron to be taken up by transferrin) (6). However, this mechanism is highly debated in view of the hepatosplenic distribution of iron which resembles that of hepcidin deficiency (hepatic iron overload contrasting with the absence of splenic iron excess) and therefore does not fit with the distribution expected in case of ferroportin impairment (splenic iron overload with absent or moderate hepatic iron excess) (7).
Classification of iron overload diseases
Two main categories of iron overload syndromes can be individualized.
Acquired iron overload
It encompasses: (I) transfusional iron overload as seen in chronic anemias, notably due to myelodysplastic syndromes (8) or to hemoglobinopathies [thalassemias (9), sickle cell disease (10)]. (II) Excessive parenteral iron supplementation, occurring for instance in renal diseases (hemodialysis patients) (11). (III) Dyserythropoiesis occurring in myelodysplasia and thalassemias (especially non-transfusion dependent thalassemia); iron overload develops because of hepcidin deficiency, at least partly as a consequence of an increased bone marrow production of erythroferrone (12). This mechanism explains why iron overload can be present in these diseases prior to any transfusions. (IV) Minor forms of iron overload can develop in chronic alcoholic liver diseases and in the metabolic syndrome (13).
Genetic iron overload
Three main types of diseases: (I) hemochromatosis (1). It corresponds to various entities depending on the mutated genes. One can distinguish HFE-related hemochromatosis, highly prevalent in Caucasian populations (14), and non-HFE-related hemochromatosis which are rare diseases, but with a worldwide distribution (15). HFE-hemochromatosis involves essentially the C282Y gene at the homozygous state (C282Y/C282Y), whereas non-HFE hemochromatosis concerns mutations of the hemojuvelin (HJV) gene, of the hepcidin (HAMP) gene or of the transferrin receptor 2 (TFR2) gene. In very rare cases, gain of function mutations of the ferroportin gene are also involved. These different forms of hemochromatosis share a common mechanism at the origin of iron overload, namely hepcidin deficiency which produces iron overload through an increased ingress of iron, and more precisely of NTBI, into the parenchymal cells (hepatocytes). (II) The ferroportin disease (16). It is due to “loss of function” mutations of the ferroportin (SLC40A1) gene leading to ferroportin activity deficiency which in turn generates iron excess by decreased egress of cellular iron especially in the splenic macrophages. (III) Exceptional cases due to hereditary atransferrinemia (17) or to hereditary aceruloplasminemia (18).
Laboratory medicine and diagnosis of iron overload (Figure 2)

Usual parameters
Plasma transferrin saturation
It corresponds to the ratio, in the plasma, of iron concentration to the number of iron binding sites located on transferrin that is the normal iron carrier in the plasma. This ratio reflects the bioavailability of iron for cells, first and foremost for the bone marrow where iron is used for producing new red blood cells. Physiologically, the number of transferrin binding sites in plasma is in excess compared to the concentration of iron, corresponding to the notion that the total iron binding capacity (TIBC) of transferrin is not fully occupied by iron and that the coefficient of transferrin saturation (CTS) does not normally exceed 45%. The determination of CTS has officially replaced in France, since 2017, the sole assay of plasma iron concentration. Technically, the recommended method is to determine separately plasma iron (usually by a colorimetric method) and transferrin concentrations (for instance by immunonephelometry or immunoturbidimetry). TIBC is calculated using the formula: (transferrin in g/L × 25), and transferrin saturation (%) is calculated as follows: (iron in µmol/L/TIBC) × 100.
Depending on the techniques, the normal range for plasma iron values is approximately 12.5–25 µmol/L and for plasma transferrin 2–4 g/L. An alternative is the indirect measurement of transferrin by the biochemical assay of TIBC or unsaturated iron binding capacity (UIBC); the TIBC and UIBC measurements consist in saturating the iron binding sites through the addition of a known amount of iron to the sample and then to measure the excess of unbound iron. UIBC is the amount corresponding to the difference between added iron and excess iron. TIBC is the sum of UIBC and plasma iron. Using direct measurement of transferrin concentration instead of TIBC or UIBC is justified by the following (19): TIBC may involve other proteins than transferrin as iron carriers leading to the risk of overestimation of TIBC and therefore of underestimation of CTS; moreover, the variability of the indirect methods may reach 35% whereas the introduction of the international CRM 470 standard has significantly reduced interlaboratory variations for transferrin measurements. However, these TIBC or UIBC techniques are less expensive explaining that they may remain useful especially in some countries. Whatever the technique, the interpretation of plasma transferrin saturation needs to be rigorous. There are several possible sampling drawbacks: fasting samples are preferable, given on the one hand the rapid increasing effect of alimentary iron on plasma iron concentration and on the other hand the circadian circle of plasma iron (whose levels can decrease up to 40% between the morning and the evening); a traumatic blood sample or excessive tube shaking should be avoided since it can release hemoglobin (hemolysis) that may overestimate CTS (20); the confirmation of an abnormal result should be obtained on a further sample due to the numerous confounding factors susceptible to impact the result. These possible factors are the following: (I) Inflammation. It decreases plasma iron due to the increasing effect of inflammatory cytokines (especially interleukin-6) on hepcidin production (21); this decreasing effect on iron is more pronounced that the slight decrease of transferrin concentration due to inflammation, so that the ratio of iron to transferrin remains globally decreased. It is therefore important to check the CRP level together with CTS. (II) Hepatic cytolysis. Severely damaged hepatocytes (by alcohol, virus, drug or autoimmunity), release various intracellular components into the plasma, including transaminases but also iron. The resulting hypersideremia, that occurs independently of any increase in cellular iron content, will increase CTS. Plasma transaminases should therefore be checked together with CST. (III) Hepatocellular failure. When the cellular machinery involved in protein synthesis (including especially the granular endoplasmic reticulum) is damaged, the synthesis of transferrin (that is exclusively produced by hepatocytes) is affected and leads to hypotransferrinemia which, in turn, will increase CTS despite the absence of body iron overload; checking the prothrombin time (which reflects the overall quality of hepatocellular synthetic functions) is useful for proper interpretation of CTS. (IV) Massive proteinuria as present in the nephrotic syndrome can also cause a marked CTS increase in the absence of any iron excess, due the urinary loss of transferrin. (V) Finally, individual genetic variations in transferrin synthesis may impact the result of CTS, through various degrees of genetic hypotransferrinemia (22). This review of the possible confounding factors for interpreting CTS illustrates the importance to consider separately the values of the numerator (plasma iron) and of the denominator (plasma transferrin) instead of just reading the global CTS result. The diagnostic interest of CTS in iron overload comes from the fact that its elevation, in case of hepcidin deficiency, that is mainly dependent of the increased plasma iron concentration, represents the earliest sign of abnormal iron metabolism; it precedes hyperferritinemia that reflects organ iron excess and thus is delayed as compared to CTS increase. In practice, hemochromatosis or iron-related dyserythropoiesis can be excluded if CTS is normal.
Plasma ferritin
Plasma ferritin is a key factor. Indeed, there is no total body iron excess without hyperferritinemia. This increase in plasma ferritin levels originates from the release of intracellular ferritin (ferritin being the iron storage protein). Various immunological methods can be used and have comparable accuracy and performance (23). Hyperferritinemia corresponds usually to levels above 300 ng/mL in males and above 200 ng/mL in females. As for CTS, the interpretation of hyperferritinemia requires to be rigorous since several factors can cause an increase in plasma ferritin concentrations independently of body iron overload. These factors can be schematically grouped under the heading of the “MAIL syndrome”: (I) “M”, like metabolic. The metabolic syndrome represents by far, throughout the world, the most frequent cause of increased plasma ferritin (24). It can be observed when one or several of the following signs are present: increased body mass index, increased waist circumference, increased blood pressure, elevated glycemia, hyperlipidemia, hyperuricemia. The ferritin levels are usually less than 1,000 ng/mL and CTS is normal. (II) “A”, like alcoholism. Alcohol increases the synthesis of ferritin; one clue for the clinician is the fluctuation of ferritinemia over time which parallels the periods of excessive alcohol intake. (III) “I”, like inflammation. Ferritin is part of the inflammatory syndrome as an acute phase protein, explaining why CRP should, as for the CTS, be associated with ferritin evaluation (notably, inflammation has a reverse effect on plasma iron parameters: it decreases CTS but increases ferritin). (IV) “L”, like liver. When there is major cytolysis, ferritin is released out of the hepatocytes. Therefore, cytolysis can, on the one hand increase plasma CTS, and on the other hand increase ferritinemia. In practice, provided that “MAIL” components have been excluded, it becomes highly likely that hyperferritinemia reflects body iron overload. It is noteworthy that, for the same amount of cellular iron excess, the corresponding plasma ferritin levels differ according to the type of cellular (and organ) iron localization. When iron deposition concerns mainly the macrophages, such as during transfusions, plasma ferritin levels may be higher than expected as compared to the similar level of iron concentration targeting hepatocytes such as during hemochromatosis or dyserythropoiesis, in relation to hepcidin deficiency (25). This notion is important when considering the threshold values to be adopted for starting, following and resuming the treatment (26). Schematically, in hemochromatosis or dyserythropoiesis, ferritinemia can be considered as moderate under 500 ng/mL, sharp between 500 and 1,000, and major above 1,000.
Liver iron concentration (LIC)
LIC has long been considered as the “gold” standard for assessing total body iron load. It has, for a long time, been based on the measurement of the iron content of a liver biopsy sample by colorimetric assay (27) or element analysis techniques (atomic absorption spectrometry or inductively coupled plasma spectrometry). However, this approach has now largely been replaced by the noninvasive method of magnetic resonance imaging (MRI) (28,29). MRI not only ascertains the presence of iron excess in the whole liver and quantifies it, but also permits to estimate iron load within the spleen. Moreover, by showing the iron distribution between the liver and spleen, MRI specific sequences provide valuable clues to the clinician onto the pathophysiology of iron overload. Thus, in iron overload related to hepcidin deficiency, T2 sequences generate a hepatic “black” hyposignal—contrasting with a spleen “white” normal signal—in contrast, in case of overload due to transfusions or parenteral iron supplementation, “black” spleen together with “white” or “grey” liver may be observed.
Other parameters
Hemogram
The hemogram [with red blood cell count, hemoglobin concentration, mean corpuscular volume (MCV), and reticulocyte count] is essential for indicating the presence or not of anemia (and for orientating its etiology/mechanism). Systemic iron overload without anemia is characteristic of hemochromatosis and, at a lesser degree, of the ferroportin disease, whereas anemia is present in iron overload related to dyserythropoiesis, multiple transfusions or excessive parenteral iron supplementation.
HFE testing
In Caucasians, the very first genetic cause of systemic iron overload is HFE-related hemochromatosis. Genetic testing should consist of the search for the C282Y mutation, its presence at the homozygous state characterizing the genetic predisposition to HFE-hemochromatosis. It is not recommended to search for the H63D mutation since H63D heterozygosity is a frequent polymorphism without pathological “iron” meaning and the same holds true for H63D homozygosity. There is some debate on the interpretation of compound heterozygosity C282Y/H63D: most data converge to conclude that this genetic profile is not susceptible to cause clinically significant body iron overload and that it can just increase CTS (usually less than 75%) without causing significant hyperferritinemia. However, in some cases, it may “boost’ the levels of hyperferritinemia observed in patients presenting another cause of hyperferritinemia (such as the “MAIL” components) (30).
Plasma ceruloplasmin
Ceruloplasminemia is the first line parameter to check when there is a bio-clinical orientation toward the diagnosis of aceruloplasminemia (the trio anemia, hyposideremia with hyperferritinemia, diabetes frequently precedes the development of neurological symptoms). Ceruloplasmin levels are undetectable or very low. Plasma ferroxidase activity, that reflects the ceruloplasmin enzymatic activity, can also be assayed and will be severely affected during aceruloplasminemia. It is only when the ceruloplasmin levels are very low or undetectable that it becomes justified to carry out a specific genetic testing.
Novel iron parameters
Plasma NTBI/labile plasma iron (LPI)
As above mentioned, NTBI is likely to appear in the plasma whenever CTS is over 45% and means that there is an ongoing cellular iron loading process. It can be measured by a fluorescence-based one step assay using fluoresceinated-transferrin. Furthermore, if CTS is over 75–80% (31,32), NTBI can correspond to a novel species of circulating iron, defined by its propensity to produce reactive oxygen species (ROS), and named LPI or reactive plasma iron (RPI). LPI therefore represents the potentially toxic form of plasma iron. The assay is based on the conversion of the non-fluorescent dihydrorhodamine to its fluorescent form by various oxidants (Aferrix Ltd., Rehovot, Israel). This LPI technique is rather tricky so that it should still be reserved for specialized laboratories and essentially for the purpose of clinical research. In practice, pragmatically, the clinician should remember that plasma LPI is highly likely present whenever CTS is over 75–80%.
Plasma hepcidin
Plasma hepcidin, the master systemic iron regulator, can be assayed in a clinical context mainly by enzyme immunoassay or mass spectrometry techniques. Efforts are still necessary to harmonize the assays (33). Despite its major interest for the pathophysiological approach of iron-related diseases, its clinical interest for diagnosing iron overload remains today quite limited. The comparison can be made with the field of glucose-related diseases where the practical interest of assaying insulinemia is also very limited. However, the ongoing clinical research, testing the therapeutic interest of hepcidin supplementation in hemochromatosis or dyserythropoiesis, may open the road to the use of hepcidinemia for selecting and monitoring the patients.
Rare genetic tests
For hemochromatosis
If the HFE test is negative (or is not justified because the patient is not Caucasian, keeping in mind however that populations are increasingly mixed), non-HFE genetic tests can be performed, looking for mutations of the HJV, HAMP or TFR2 genes. These tests require specialized laboratories that, more and more, resort to a next-generation sequencing approach.
For the ferroportin disease
The search for mutations of the ferroportin gene needs also to be performed by reference laboratories.
Laboratory medicine, treatment and follow-up of iron overload
Iron overload without anemia: hemochromatosis and the ferroportin disease
Hemochromatosis
The phlebotomy management can be divided into two main phases (1): (I) The initial or induction phase. It consists of removing the iron excess found at the time of diagnosis, usually by weekly venesections. The biochemical parameters are critical for starting the treatment—high CTS + ferritin ≥300 ng/mL in men and ≥200 ng/mL in women—as well as for following the progressive decrease of iron stores which is paralleled by the diminution of hyperferritinemia. Importantly, the CTS is not useful at this period because it remains elevated until the very end of the induction phase; the final goal of the induction phase is reached as soon as plasma ferritin concentration is ≤50 ng/mL and CTS ≤50% (without anemia). In terms of timing, ferritin should be checked every month until ferritinemia decreases to the upper normal of limits, and every 2 weeks thereafter (and then together with CTS). (II) The second phase is the maintenance therapy that, theoretically, lasts for the whole life and consists of phlebotomies every 2 to 4 months. Its aim is to avoid the reconstitution of iron overload by maintaining ferritinemia around 50 ng/mL. Ferritinemia should be checked before each new phlebotomy. Although not yet officially recommended, it is of good practice to check CTS, for instance twice a year, to ensure that the patient does not belong to the subset of patients whose CTS is permanently elevated (despite ferritinemia around 50 ng/mL) and may be at risk for developing clinical symptoms (such as chronic fatigue and arthropathy) (34). During this depletion treatment, hemoglobin levels should be regularly checked in order to ensure that they remain above 11 g/dL.
Ferroportin disease
The venesection induction schedule should be lighter than for hemochromatosis due to the risk of anemia. Indeed, in ferroportin disease, iron recycling is hampered by the limitation of cellular iron egress. Usually, one phlebotomy every 2 weeks is correctly tolerated and efficient (35). One should keep in mind that, in this disease, CTS is normal or slightly decreased (without basal anemia), and that ferritin levels are higher than in hemochromatosis, due to the macrophagic localization of iron excess. Therefore, in the ferroportin disease, the “hemochromatosis ferritinemia threshold” of 50 ng/mL may represent a risk of anemia, so that the final goal for plasma ferritin should be higher in ferroportin disease than in hemochromatosis.
Iron overload in chronic anemias
Phlebotomies are of course contra-indicated, and the main therapeutic tool is today oral chelation therapy with two main compounds: deferasirox (36) and deferiprone (37). The levels of plasma ferritin and CTS are also critical for starting and adapting the treatment, keeping in mind, for ferritin, that its values should be interpreted and adapted, as above mentioned, according to the type of iron distribution (spleen versus liver iron overload), ideally guided by MRI data.
Biochemical detection of iron overload complications
Iron overload complications, especially during severe HFE hemochromatosis and during juvenile forms, concern essentially the liver, pancreas, and heart.
Hepatic complications
Hepatic iron overload, despite being massive and despite having produced cirrhosis, has usually little consequences on liver functioning, except for a slight increase in plasma transaminase activities (usually less than three-fold the upper normal limits). Importantly, it is admitted that long-lasting ferritinemia levels over 1,000 ng/mL are suggestive of visceral, especially hepatic fibrosis, complications. Moreover, when hepatic cirrhosis has been documented [more and more by combining biochemical tests—“fibrosis tests” (such as the “Fibrotest” and “Fibrometer”), and liver imaging—especially transient elastography (“Fibroscan”)], it becomes critical to detect the development of hepatocellular carcinoma by checking, every 6 months, hepatic ultrasound aspect and plasma alpha-feto-protein levels.
Pancreatic complications
Diabetes may occur during iron overload diseases. Therefore, at least at the diagnosis phase, it is necessary to control glycemia. This is especially recommended during juvenile forms of hemochromatosis and during aceruloplasminemia.
Heart complications
Cardiac failure can be observed in some forms of iron overload including juvenile forms of hemochromatosis and thalassemias. Plasma brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) levels may be helpful for the diagnosis.
Conclusions
Iron overload, either of genetic or acquired origin, represents frequent pathological conditions that require and early and efficient management, considering the major risk of severe complications. Beside clinical and imaging data, the laboratory provides valuable tools for succeeding in this double diagnostic and therapeutic challenge.
Acknowledgments
Funding: The authors wish to thank the valuable support of AFeMERS (Association Fer- Métaux essentiels-Recherche-Santé, AHO (Association Hémochromatose-Ouest), and FFAMH (Fédération Française des Associations de Malades de l'Hémochromatose).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Katell Peoc’h) for the series “Physiology and Pathology of Iron Metabolism” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-2019-im-01). The series “Physiology and Pathology of Iron Metabolism” was commissioned by the editorial office without any funding or sponsorship. OL reports personal fees and other from Diafir, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brissot P, Pietrangelo A, Adams PC, et al. Haemochromatosis. Nat Rev Dis Primers 2018;4:18016. [Crossref] [PubMed]
- Fibach E, Rachmilewitz EA. Iron overload in hematological disorders. Presse Med 2017;46:e296-305. [Crossref] [PubMed]
- Brissot P, Loreal O. Iron metabolism and related genetic diseases: a cleared land, keeping mysteries. J Hepatol 2016;64:505-15. [Crossref] [PubMed]
- Brissot P, Ropert M, Le Lan C, et al. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 2012;1820:403-10. [Crossref] [PubMed]
- Ganz T. The discovery of the iron-regulatory hormone hepcidin. Clin Chem 2019;65:1330-1. [Crossref] [PubMed]
- De Domenico I, Nemeth E, Nelson JM, et al. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab 2008;8:146-56. [Crossref] [PubMed]
- Kenawi M, Rouger E, Island ML, et al. Ceruloplasmin deficiency does not induce macrophagic iron overload: lessons from a new rat model of hereditary aceruloplasminemia. FASEB J 2019;33:13492-502. [Crossref] [PubMed]
- Gattermann N. Iron overload in myelodysplastic syndromes (MDS). Int J Hematol 2018;107:55-63. [Crossref] [PubMed]
- Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet 2018;391:155-67. [Crossref] [PubMed]
- Ware RE, de Montalembert M, Tshilolo L, et al. Sickle cell disease. Lancet 2017;390:311-23. [Crossref] [PubMed]
- Rostoker G, Vaziri ND. Iatrogenic iron overload and its potential consequences in patients on hemodialysis. Presse Med 2017;46:e312-28. [Crossref] [PubMed]
- Gupta R, Musallam KM, Taher AT, et al. Ineffective erythropoiesis: anemia and iron overload. Hematol Oncol Clin North Am 2018;32:213-21. [Crossref] [PubMed]
- Deugnier Y, Bardou-Jacquet E, Laine F. Dysmetabolic iron overload syndrome (DIOS). Presse Med 2017;46:e306-11. [Crossref] [PubMed]
- Pilling LC, Tamosauskaite J, Jones G, et al. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ 2019;364:k5222. [Crossref] [PubMed]
- Wallace DF, Subramaniam VN. The global prevalence of HFE and non-HFE hemochromatosis estimated from analysis of next-generation sequencing data. Genet Med 2016;18:618-26. [Crossref] [PubMed]
- Pietrangelo A. Ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica 2017;102:1972-84. [Crossref] [PubMed]
- Aslan D, Crain K, Beutler E. A new case of human atransferrinemia with a previously undescribed mutation in the transferrin gene. Acta Haematol 2007;118:244-7. [Crossref] [PubMed]
- Piperno A, Alessio M. Aceruloplasminemia: waiting for an efficient therapy. Front Neurosci 2018;12:903. [Crossref] [PubMed]
- Kasvosve I, Delanghe J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med 2002;40:1014-8. [Crossref] [PubMed]
- Koseoglu M, Hur A, Atay A, et al. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med (Zagreb) 2011;21:79-85. [Crossref] [PubMed]
- Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015;15:500-10. [Crossref] [PubMed]
- Hamdi-Rozé H, Beaumont-Epinette MP, Ben Ali Z, et al. Rare HFE variants are the most frequent cause of hemochromatosis in non-c282y homozygous patients with hemochromatosis. Am J Hematol 2016;91:1202-5. [Crossref] [PubMed]
- Garcia-Casal MN, Pena-Rosas JP, Urrechaga E, et al. Performance and comparability of laboratory methods for measuring ferritin concentrations in human serum or plasma: a systematic review and meta-analysis. PLoS One 2018;13:e0196576 [Crossref] [PubMed]
- Chen L, Li Y, Zhang F, et al. Association of serum ferritin levels with metabolic syndrome and insulin resistance in a Chinese population. J Diabetes Complications 2017;31:364-8. [Crossref] [PubMed]
- Musallam KM, Taher AT. Iron-chelating therapy for transfusional iron overload. N Engl J Med 2011;364:1476-author reply 1477. [PubMed]
- Saliba AN, Musallam KM, Cappellini MD, et al. Serum ferritin values between 300 and 800 ng/mL in nontransfusion-dependent thalassemia: a probability curve to guide clinical decision making when MRI is unavailable. Am J Hematol 2017;92:E35-7. [Crossref] [PubMed]
- Barry M. Liver iron concentration, stainable iron, and total body storage iron. Gut 1974;15:411-5. [Crossref] [PubMed]
- Henninger B, Alustiza J, Garbowski M, et al. Practical guide to quantification of hepatic iron with MRI. Eur Radiol 2020;30:383-93. [Crossref] [PubMed]
- Doyle EK, Toy K, Valdez B, et al. Ultra-short echo time images quantify high liver iron. Magn Reson Med 2018;79:1579-85. [Crossref] [PubMed]
- Porto G, Brissot P, Swinkels DW, et al. EMQN best practice guidelines for the molecular genetic diagnosis of hereditary hemochromatosis (HH). Eur J Hum Genet 2016;24:479-95. [Crossref] [PubMed]
- Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003;102:2670-7. [Crossref] [PubMed]
- Le Lan C, Loreal O, Cohen T, et al. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood 2005;105:4527-31. [Crossref] [PubMed]
- Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood 2016;127:2809-13. [Crossref] [PubMed]
- Bardou-Jacquet E, Laine F, Guggenbuhl P, et al. Worse outcomes of patients with HFE hemochromatosis with persistent increases in transferrin saturation during maintenance therapy. Clin Gastroenterol Hepatol 2017;15:1620-7. [Crossref] [PubMed]
- Le Lan C, Mosser A, Ropert M, et al. Sex and acquired cofactors determine phenotypes of ferroportin disease. Gastroenterology 2011;140:1199-207.e1-2.
- Taher AT, Origa R, Perrotta S, et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: results of the randomized, phase II ECLIPSE study. Am J Hematol 2017;92:420-8. [Crossref] [PubMed]
- Hider RC, Hoffbrand AV. The role of deferiprone in iron chelation. N Engl J Med 2018;379:2140-50. [Crossref] [PubMed]
Cite this article as: Brissot P, Brissot E, Loréal O, Ropert M. Laboratory medicine and iron overload: diagnostic and therapeutic aspects. J Lab Precis Med 2020;5:25.