Cardiac troponins in diagnostics of equine myocardial injury
Clinical use of cardiac troponins
The measurement of cardiac biomarkers, namely cardiac troponin I (cTnI) and cardiac troponin T (cTnT), has now become the cornerstone for diagnosing cardiac injury, regardless of its ischemic, infective, traumatic, toxic, inflammatory or autoimmune origin (1,2). The introduction of these biomarkers shall be seen as a real breakthrough in the diagnostics of heart diseases, especially when assayed with the novel high-sensitivity immunoassays, which allow detecting even physiological concentrations of both cTnI and cTnI in the vast majority of healthy subjects (3,4).
It is now well-established that different forms of physical exercises are associated with substantial biochemical abnormalities (5), including a temporary and almost completely reversible increase of cTnI and cTnT concentrations in humans practicing endurance exercise (6), which appears directly proportional to the amount of cardiac tissue temporarily injured by the increased biological and physical stress placed on cardiomyocytes (7). Although the relative post-exercise variation of both cTnI and cTnT depends on running intensity and race distance, in the vast majority of people practicing recreational running (i.e., between 5–20 km at low-to-mild intensity) such increase is typically modest (e.g., between 2- and 6-fold from pre-exercise) and persists for less than 12–24 h (8). It is hence reasonable that the regular assessment of these cardiac biomarkers in physically active subjects shall be seen as a reliable and suitable opportunity as preparticipation athletic evaluation, but also for longitudinal monitoring athlete’s health as well as for predicting the risk of cardiovascular events and death in regular endurance practitioners (9).
Although this notion is now straightforward and virtually incontestable, many doubts remain as to whether the measurement of cardiac troponins, which have been for long used for diagnosing human cardiac diseases, would also be reliable and clinically informative in veterinary medicine. This holds especially true for horses, one of the animal species which has mostly impacted human history for its stamina and speed (10). Although the use of horses for transportation has significantly declined over the last decades, these beautiful and faithful animals are still largely employed for their most recognized qualities, i.e., power and strength in riding, thus including equestrian performance sports (11). Proactive integration of equine care shall hence be seen as an important strategy for avoiding or mitigating trainer effect and risk of injuries, including that of severe cardiac pathologies, up to sudden death (12). In this perspective, the measurement of either cTnI or cTnT in horses is a potentially valuable perspective, whose leading clinical opportunities and potential drawbacks will be analyzed in the following parts of this article.
Biochemistry and measurement of cardiac troponins in horses
The heart size increases in parallel with body size across most mammalian species, so that horses have a heart that is usually ~10-fold bigger (i.e., 3.5 vs. 0.35 kg) than humans. Despite the difference in size, the biochemical, biological, anatomic, physiological, physiopathological (i.e., athlete’s heart is commonplace in racehorses) and even pathological characteristics of the heart are almost unvaried among different mammalian species (13), thus leading the way to identify many overlapping areas between human and veterinary cardiology.
The essential premise for using human diagnostic tests in veterinary medicine is that these techniques shall be both analytically and clinically validated in the target animal species, as recently endorsed by the American Society for Veterinary Clinical Pathology (ASVCP) (14), and cardiac biomarkers would make no exception to this rule. Concerning the biochemical structure of cardiac troponins, Rishniw and Simpson sequenced the cTnI gene in equines and compared it with the equivalent genes in other species, including humans (15). Although it could be observed that the cTnI epitope region identified by most commercial cTnI methods was highly conserved among different animal species (i.e., humans, horses, dogs, and cats), eight amino acid differences could be found. Even more importantly, equine cTnI was found to be deprived of 6 amino acids in the N-terminal region, whilst displaying 3 highly horse-specific polymorphisms which may ultimately affect the affinity of monoclonal antibodies for the target protein moiety. This hypothesis has been recently confirmed by Rossi et al., who published the results of an interesting study aimed at validating the use of some cTnI immunoassays in horses (16). A comprehensive analytical protocol was defined and completed by the authors, who finally concluded that two out of the six methods tested should be considered unsuitable in horses due to problems in accurately detecting equine cTnI. Significant heterogeneity was also found in reference ranges and diagnostic thresholds among the different cTnI immunoassays so that these methods would require comprehensive local validation before being introduced into clinical veterinary practice. Unfortunately, no clear information has been published on the structure of the gene encoding for cTnT across mammalian species, except data retrievable from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/gene/7139), highlighting that the human (Homo Sapiens TNNT2—18,667 bp DNA) and horse (Equus caballus TNNT2—14,880 bp DNA) cTnT genes encode for a homologous protein, which displays some important differences between these two species. As for cTnI, a thoughtful analytical validation protocol was defined and completed in horses by Shields and co-authors (17), who finally concluded that the commercial high-sensitivity cTnT immunoassay can be reliably used for equine cardiac diagnostics. This is mostly due to the fact that the epitopes identified by the human cTnT method are also present (with perfect homology) in horses (18). Interestingly, Shields et al. also found that the 99th percentile upper limit of the reference range URL) of cTnT was nearly 40% higher in racing thoroughbreds than in a population of non-competition horses (i.e., 23.2 vs. 16.2 ng/L) (17).
In another interesting study, including as many as 586 healthy horses aged between 2–14 years, Slack et al. reported that cTnI concentration is not apparently influenced by sex and gait, whilst a weak positive correlation could be found with age (P=0.03) and some potentially significant differences could also be observed across different horse breeds (19). Unlike what could naturally envisaged from human and equine anatomy (i.e., horse’s heart being nearly 10-time bigger than the human counterpart) (13), the circulating values at rest of both cardiac troponins appears very similar between these two species, since the 99th URL of cTnI is ~30 ng/L in horses and ~15 ng/L in humans, whilst the 99th URL of cTnT is ~14 ng/L in humans and ~23 ng/L in horses, respectively. This can be explained by the fact that these reference ranges have been calculated in animals that are nearly 5-time younger than humans (i.e., 2–8 vs. 20–80 years), so that the effect of the age may contribute—at least partially—to attenuate the difference.
As specifically regards the use of commercial human cardiac troponin immunoassays in equine cardiac diagnostics, several studies have now allowed to definitely establish that enhanced (i.e., diagnostic) values of these biomarkers are found in serum or plasma of animals with various forms of cardiomyopathies (i.e., ischemic, inflammatory or toxic), structural heart disease (e.g., ventricular septal defects, valvular heart disease or aortopulmonary fistula), toxic myocardial injury and even arrhythmias (18,20,21).
Taken together, these findings would lead us to conclude that once an analytical and clinical validation of both cTnI and cTnT immunoassays has been completed, these methods can be clinically used in equine diagnostics. Moreover, as recently confirmed by Van Der Vekens et al. (20), and in keeping with previous findings in humans (22), both cTnI or cTnT can be independently used for detecting and diagnosing myocardial injury in horses.
Post-exercise increase of cardiac troponins in horses
Since horses are mostly born to run, and their anatomic and biologic apparatus has evolved accordingly (23), the identification and appropriate interpretation of exercise-related variation of both cTnI and cTnT is essential in equine like in human medicine.
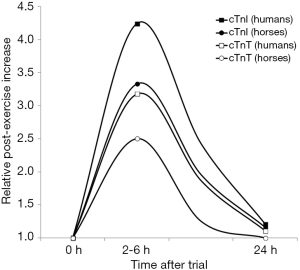
An interesting study was published by Rossi et al. (24), who measured cTnI with a high-sensitivity immunoassay in 5 healthy racehorses, which carried out a warm-up of 28.8±8.4 min, and then run a mile at near-race intensity, with a mean time of 2:09 min (range, 2:02–2:23 min), thus averaging an overall ~30 min endurance exercise. Blood samples were drawn immediately before and after exercise, up to 24 h afterwards. The baseline mean concentration of cTnI (1.38±0.6 ng/L) increased by up to 3.3-fold between 2 to 6 h after the trial and returned to values similar to the baseline after a mean period of 9.0±1.15 h post-exercise. A similar study was carried out by Shields et al. (25), who instead measured cTnT with a high-sensitivity immunoassay in 38 healthy racehorses, which completed a 5/8th mile Chuckwagon race. Despite the race distance was completed in a relatively short time (i.e., 72.7 seconds), the concentration of cTnT increased by approximately 2-fold between 2 and 6 hours after the end of the trial (i.e., from 4.0 to 8.0 ng/L), and returned to baseline values nearly 12 h afterwards.
The comparison of both cTnI and cTnT variation after exercise in horse and humans is shown in Figure 1. Although the race distance is different, and thereby the relative increase cannot be straightforwardly compared among species, the pattern is virtually identical, with a significant post-exercise increase, which peaks in both humans and horses between 2–6 h post-exercise, and values returning to the baseline level almost within 12–24 h after exercise (26,27). Importantly, neither in humans nor in horses the post-exercise increase of both cTnI and cTnT seemingly exceeds the species-specific 99th URL. This evidence would lead us to conclude that the assessment of either cardiac troponin retains identical clinical significance in humans and horses, so that the basic concepts underlying cardiac troponin usage in human cardiac pathology may be straightforwardly translated to equine diagnostics.
The way forward
In ancient times entire economies have been dependent on equines, whereby these powerful and intelligent animals have been—and still are in certain parts of the modern world—essential for many human activities. Due to their anatomy and physiology, horses are not only suited to canter, trot, and gallop, reaching speeds approaching 90 km/h, thus being the second-fastest land-animal, only preceded by the Cheetah (28), but can also perform other working- and entertainment-related tasks. The innate equine inclination to run has been for long exploited by humans, whereby horses have been first domesticated around the 3500 BC, and have then been used for transportation, agricultural work, and warfare, always privileging—and even refining through selection—their extraordinary running performance (10). Like every other living organism, horses are also vulnerable to a kaleidoscope of pathologies, including heart injuries, for which early and accurate diagnosis is always advisable.
Nearly 40 years after their first clinical application, both cTnI and cTnT have now virtually become the biochemical gold standard for diagnosing cardiac injury in humans (29). Due to the substantial homology between the human and equine cardiovascular system, which only differ for some minor variations in ventricular septal blood supply (30). the measurement of both cTnI and cTnT using commercial (preferably high-sensitivity) immunoassays can now be seen as a workable opportunity for diagnosing cardiac injuries also in horses. Taken together, the current literature data would lead us to conclude that the clinical significance of measuring these biomarkers in humans and horses appears nearly overlapping, though some important drawbacks must be recognized and overcome (Table 1). First and foremost, the use of commercial methods targeting human cardiac cTnI and cTnT must be preliminarily validated, both analytically and clinically, in horses before they can be introduced into veterinary practice. This would allow preventing the generation of false-negative test results by using kits that inaccurately detect equine proteins, but will also enable an appropriate definition of reference ranges and diagnostic thresholds, which could be largely method-, bread- and age-dependent. Unlike humans, who no longer need to run—neither even to walk—for surviving (31), riding is an almost normal occupation for many horses. Equine heart tends to adapt to endurance exercise, displaying a plastic response to intense athletic training, increasing the size and releasing larger amounts of both cTnI and cTnT during resting conditions. Post-exercise increments of both cTnI and cTnT are hence commonplace in active horses and need to be accurately distinguished from pathological increases, more or less like we need to do in humans (32). This would not be inherently easy since—unlike humans—horses cannot plentifully describe their recent physical activity, nor the owner or the veterinary can always follow the animal lifestyle. Nevertheless, the paradigmatic features of post-exercise variation of cardiac biomarkers (Figure 1) would enable distinguishing between and exercise-related release (almost modest and transitory) and increases following structural cardiac injuries (considerable and persisting for up to 1 week). A final consideration shall be made on the use of diagnostic algorithms. Although these have been largely validated in humans (33), and equine and human cardiology displays many overlapping features (30), clinical validation would always be needed before straightforwardly translating human algorithms into equine diagnostics.
Table 1
| Validation (analytical and clinical) of the specific method in horses |
| Accurate establishment of reference ranges according to: |
| Method |
| Breed |
| Age |
| Training |
| Appropriate interpretation of post-exercise elevations |
| Validation of human diagnostic algorithms in horses |
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-85). GL serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Doorn WP, Vroemen WH, de Boer D, et al. Clinical laboratory practice recommendations for high-sensitivity cardiac troponin testing. J Lab Precis Med 2018;3:30. [Crossref]
- Lippi G, Plebani M. Understanding cardiac troponin biology: all other cardiac biomarkers shall rest in peace? J Lab Precis Med 2019;4:9. [Crossref]
- van der Linden N, Streng AS, Wodzig WK, et al. Better, higher, lower, faster: increasingly rapid clinical decision making using high-sensitivity cardiac troponin assays. J Lab Precis Med 2017;1:14. [Crossref]
- Clerico A, Lippi G. The state-of-the-art of “high-sensitivity” immunoassay for measuring cardiac troponin I and T. J Lab Precis Med 2018;3:53. [Crossref]
- Sanchis-Gomar F, Lippi G. Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 2014;24:68-79. [Crossref] [PubMed]
- Le Goff C, Lennartz L, Vranken L, et al. Comparison of cardiac biomarker dynamics in marathon, semi-marathon and untrained runners: what is the impact on results interpretation? J Lab Precis Med 2019;4:6. [Crossref]
- Lippi G, Cervellin G, Schena F. How much myocardium mass may be injured during endurance physical exercise? Clin Chim Acta 2017;470:29-30. [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Lippi G. Time-dependent results in troponin exercise-induced fluctuations. Int J Cardiol 2019;293:258. [Crossref] [PubMed]
- Aengevaeren VL, Hopman MTE, Thompson PD, et al. Exercise-Induced Cardiac Troponin I Increase and Incident Mortality and Cardiovascular Events. Circulation 2019;140:804-14. [Crossref] [PubMed]
- Librado P, Fages A, Gaunitz C, et al. The Evolutionary Origin and Genetic Makeup of Domestic Horses. Genetics 2016;204:423-34. [Crossref] [PubMed]
- Rogers CW, Bolwell CF, Gee EK. Proactive Management of the Equine Athlete. Animals (Basel) 2012;2:640-55. [Crossref] [PubMed]
- Molesan A, Wang M, Sun Q, et al. Cardiac Pathology and Genomics of Sudden Death in Racehorses From New York and Maryland Racetracks. Vet Pathol 2019;56:576-85. [Crossref] [PubMed]
- Shave R, Howatson G, Dickson D, et al. Exercise-Induced Cardiac Remodeling: Lessons from Humans, Horses, and Dogs. Vet Sci 2017;4:9. [Crossref] [PubMed]
- American Society for Veterinary Clinical Pathology. Principles of Quality Assurance and Standards for Veterinary Clinical Pathology ASfVCP, Madison, WI. Available online: http://www.asvcp.org/pubs/qas/index.cfm. Accessed December 31 2019.
- Rishniw M, Simpson KW. Cloning and sequencing of equine cardiac troponin I and confirmation of its usefulness as a target analyte for commercial troponin I analyzers. J Vet Diagn Invest 2005;17:582-4. [Crossref] [PubMed]
- Rossi TM, Kavsak PA, Maxie MG, et al. Analytical validation of cardiac troponin I assays in horses. J Vet Diagn Invest 2018;30:226-32. [Crossref] [PubMed]
- Shields E, Seiden-Long I, Massie S, et al. Analytical validation and establishment of reference intervals for a 'high-sensitivity' cardiac troponin-T assay in horses. BMC Vet Res 2016;12:104. [Crossref] [PubMed]
- Nath LC, Anderson GA, Hinchcliff KW, et al. Serum cardiac troponin I concentrations in horses with cardiac disease. Aust Vet J 2012;90:351-7. [Crossref] [PubMed]
- Slack J, Boston RC, Soma L, et al. Cardiac troponin I in racing standardbreds. J Vet Intern Med 2012;26:1202-8. [Crossref] [PubMed]
- Van Der Vekens N, Decloedt A, Ven S, et al. Cardiac troponin I as compared to troponin T for the detection of myocardial damage in horses. J Vet Intern Med 2015;29:348-54. [Crossref] [PubMed]
- Divers TJ, Kraus MS, Jesty SA, et al. Clinical findings and serum cardiac troponin I concentrations in horses after intragastric administration of sodium monensin. J Vet Diagn Invest 2009;21:338-43. [Crossref] [PubMed]
- Lippi G, Cervellin G. Is one cardiac troponin better than the other? J Lab Precis Med 2019;4:19. [Crossref]
- Ricard A, Robert C, Blouin C, et al. Endurance Exercise Ability in the Horse: A Trait with Complex Polygenic Determinism. Front Genet 2017;8:89. [Crossref] [PubMed]
- Rossi TM, Kavsak PA, Maxie MG, et al. Post-exercise cardiac troponin I release and clearance in normal Standardbred racehorses. Equine Vet J 2019;51:97-101. [Crossref] [PubMed]
- Shields E, Seiden-Long I, Massie S, et al. 24-Hour Kinetics of Cardiac Troponin-T Using a "High-Sensitivity" Assay in Thoroughbred Chuckwagon Racing Geldings after Race and Associated Clinical Sampling Guidelines. J Vet Intern Med 2018;32:433-40. [Crossref] [PubMed]
- Lippi G, Schena F, Dipalo M, et al. Troponin I measured with a high sensitivity immunoassay is significantly increased after a half marathon run. Scand J Clin Lab Invest 2012;72:467-70. [Crossref] [PubMed]
- Danese E, Benati M, Sanchis-Gomar F, et al. Influence of middle-distance running on muscular micro RNAs. Scand J Clin Lab Invest 2018;78:165-70. [Crossref] [PubMed]
- Bertram JE, Gutmann A. Motions of the running horse and cheetah revisited: fundamental mechanics of the transverse and rotary gallop. J R Soc Interface 2009;6:549-59. [Crossref] [PubMed]
- Lippi G. The origin of some laboratory medicine milestones. J Lab Precis Med 2019;4:14. [Crossref]
- Van Der Vekens N, Hunter I, Goetze JP, et al. Human and equine cardiovascular endocrinology: beware to compare. Cardiovasc Endocrinol Metab 2013;2:67-76. [Crossref]
- Mattson MP. Evolutionary aspects of human exercise--born to run purposefully. Ageing Res Rev 2012;11:347-52. [Crossref] [PubMed]
- Lippi G, Cervellin G, Sanchis-Gomar F. Critical appraisal to using relative or absolute cardiac troponins change for diagnosing acute myocardial infarction. J Lab Precis Med 2018;3:43. [Crossref]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
Cite this article as: Lippi G, Sanchis-Gomar F. Cardiac troponins in diagnostics of equine myocardial injury. J Lab Precis Med 2020;5:31.