
Accuracy of Sysmex XN immature granulocyte percentage compared to manual microscopy
Introduction
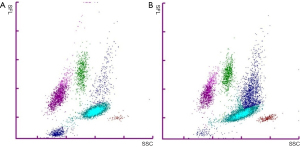
Immature granulocytes (IG) are neutrophilic cells produced by bone marrow in response to inner or outer stimuli, e.g., infection, inflammation or physiological stress (1). Metamyelocytes, myelocytes and promyelocytes are classified as IG, while segmented and band form neutrophils are considered mature. Blast cells are not included in the IG class. Neither are eosinophilic or basophilic immature cells classified as IG. Under normal physiological conditions IG% is low, less than 1% (2). Higher IG% is linked to several medical conditions needing urgent treatment, such as bacterial sepsis, pancreatitis and appendicitis (3-8). Modern haematology analysers are able to classify IG based on the size, granularity and nucleic acid content of the cells (9). For white blood cell (WBC) analysis Sysmex XN analysers lyse red blood cells and use flow cytometry with a semiconductor laser (633 nm) to detect cells based on forward-scattered (FSC), side-scattered (SSC), and side-fluorescent (SFL) light. Lysis reagent causes morphological changes in the cells and a fluorescent stain reacts based on individual properties of the WBC types enabling their classification based on scatter grams. Basophils are detected and enumerated, together with possible nucleated red blood cells, using white cell nucleated (WNR) channel with SFL vs. FSC. The analysers use the WNR channel also to enumerate total WBC. White blood cell differential (WDF) channel with SSC vs. SFL gram is used to differentiate and enumerate neutrophils, eosinophils, lymphocytes, and monocytes, and to detect atypical lymphocytes and abnormal cells. IG form a cluster of events above the mature neutrophil cell cloud in the WDF channel (9) (Figure 1).
Most research regarding the usefulness of IG% as a marker of inflammation relies on analyser data, not on manual microscopy. Some studies have found an automated haematology analyser (Sysmex XE-2100) to be more precise than manual microscopy in enumerating IG (10). However, Sysmex XN is also described to systematically give higher IG percentage compared to manual microscopy (11). Sysmex XN analyser algorithms may produce interpretive program messages (flagging) based on scatter grams, particle size, and numerical data. Samples marked with flagging should be reviewed by the user, while samples without any flagging may be autovalidated. Sysmex XN manufacturer, as well as certain studies, have recommended to use IG 3% as the cut-off for analyser flagging and microscopy review (11). Some studies have set the limit to 5% and even showed that automated analysers should be used as the reference method instead of manual microscopy (10,12). Still manual microscopy remains the gold standard for WDF, in spite of high inter-individual variation and rather low reproducibility (13). Digital microscopy has improved the work flow with the samples requiring review after analyser flagging. Despite of this, the review process can cause delay in patient care, reducing the value of IG as an inflammation marker.
The data regarding the reliability of Sysmex XN IG remains somewhat controversial, while this parameter is increasingly used as a clinical inflammation marker. In this study we investigated the reliability of Sysmex XN IG% in comparison to manual microscopy. We evaluated whether the IG% cut-off for microscopy review could be raised from the recommended 3% in order to reduce the amount of manual work, and compared the data from Sysmex XN to manual microscopy at different IG%, absolute neutrophil count (ANC) and WBC levels. For the reference, we used samples with a special request for manual microscopy WBC differential, but with no analyser flagging and IG <3%. We present the following article in accordance with the MDAR reporting checklist (available at https://dx.doi.org/10.21037/jlpm-21-33).
Methods
Samples
The samples in this retrospective study were anonymised routine analysis whole blood samples collected in K2EDTA tubes and stored at room temperature at the Turku University Hospital Laboratory, Turku, Finland. The samples were analysed and the blood films were prepared within 8 hours after sample taking. The study comprised of 557 samples, 242 (43%) from female, overall median age 67 years (IQR with 25th–75th percentile 54–74 years) with a request for WBC differential, collected Dec 21, 2018 to Jul 31, 2019. The reference samples, 245 with 81 (33%) females, were from babies (<1 year old) with a special request for manual microscopy WBC differential, collected in Apr 3, 2018 to Feb 27, 2019. Without the special manual microscopy review request, these samples would have been autovalidated. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No patient permission or evaluation from the ethics committee were needed for this retrospective study with anonymised samples.
Laboratory tests
All samples were analysed with Sysmex XN-10 or Sysmex XN-20 (Sysmex Co. Kobe, Japan) analysers with WNR and WDF channels. The total leukocyte count (WNR channel), IG percentage (WDF channel) and ANC (WDF channel) were recorded. The analyser flagging for IG was set to 3%. No samples with other or additional analyser flagging, i.e., notifications regarding other WBC abnormalities, were included. No samples with interference in WBC count or IG measurement leading to an unreliable WBC enumeration or WBC identification were included in the study. The reference samples had IG <3% and no analyser flagging. All samples were single analysis according to routine protocol. The precision was 7% (at IG 0.74%) for intra-assay and 4% (at IG 11%) for inter-assay IG analysis, the WBC reportable range was (0–440) ×109/L, and the WBC reference range (3.4–8.2) ×109/L for adults and (6.0–17) ×109/L for babies <1 years.
The blood films were made with Sysmex SP-10 (Sysmex Co., Kobe, Japan) and dyed with May-Grünwald-Giemsa –dye (MGG, RAL Diagnostics, Martillac, France) or prepared manually and dyed with MGG (Reagena, Toivala, Finland) according to established protocols (14,15). With manual microscopy 200 WBC were evaluated. Promyelocytes, myelocytes, and metamyelocytes were classified as IG, and band form and segmented neutrophils as mature (15). The inter-individual variation for manual microscopy IG has varied up to CV 77% at IG% <3 and occasionally exceeded the accuracy limits (13), while the CV for manual mature neutrophil percentage has been <5% in our laboratory.
Statistical analysis
To evaluate the differences in the analyser and microscopy IG% we compared the methods in samples with IG <3% (N=245) and >3% (N=557). With all the samples combined (N=802), we divided the samples into nine subgroups with IG% <1 (N=90), 1–1.9 (N=109), 2–2.9 (N=46), 3–3.9 (N=165), 4–4.9 (N=126), 5–5.9 (N=68), 6–6.9 (N=72), 7–7.9 (N=36) and >8 (n=90) (see Figure 2). We further compared the differences in IG% based on total WBC <3.4×109/L (N=53), (3.4–8.2) ×109/L (N=255), and >8.2 ×109/L (N=494), and ANC <1.5×109/L (N=48), (1.5–6.7) ×109/L (N=404), and >6.7×109/L (N=350) (see Table 1). MedCalc 19.7 with nonparametric Shapiro-Wilk test, Regression model with 95% confidence and 95% prediction intervals for the regression line, and Kruskall-Wallis H test with Dunn post-hoc method were used due to not normally distributed data.
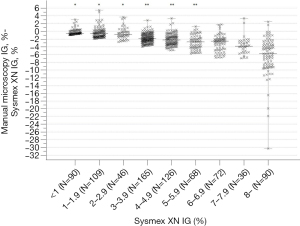
Table 1
| Group | N | Manual microscopy-Sysmex XN IG%, median (IQR) | Sysmex XN IG%, median (IQR) |
|---|---|---|---|
| ANC | |||
| <1.5×109/L | 48 | −1.4 (−3.4 to −0.25) | 3.9 (1.2–4.8) |
| (1.5–6.7) ×109/L | 404 | −1.8 (−3.5 to −0.60) | 4.0 (2.2–6.1) |
| >6.7×109/L | 350 | −1.7 (−3.1 to −0.60) | 3.8 (2.0–5.3) |
| WBC | |||
| <3.4×109/L | 53 | −2.9* (−5.5 to −1.375) | 5.3 (3.9–7.4) |
| (3.4–8.2) ×109/L | 255 | −2.2 (−3.8 to −0.33) | 4.6 (3.3–6.7) |
| >8.2×109/L | 494 | −1.5 (−2.8 to −0.60) | 3.5 (1.4–5.1) |
The method difference was slightly higher in samples with low white blood cell (WBC) count (*, P<0.001) compared to normal or high WBC samples. IQR, interquartile range.
Results
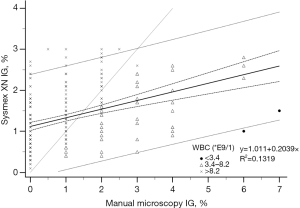
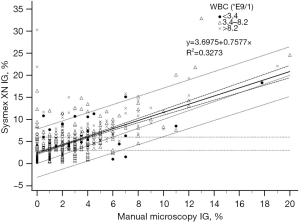
The analyser IG% and manual microscopy IG% fitted only moderately in the regression model both in the reference samples with IG <3% (R2=0.1319, Figure 3) and in samples with IG above 3% (R2=0.3273, Figure 4). The deviation increased with higher analyser IG%, Sysmex XN giving positive error.
We compared the degree of difference between analyser IG% a manual microscopy IG% at different Sysmex XN IG thresholds (Figure 2). In the subgroups above 3%, the median positive error for the analyser IG% was higher than in IG <3% (P<0.001). The positive error and the degree of difference between the methods further increased above IG 6%. When the subgroups were further compared, within subgroups IG <1, 1–1.9, and 2–2.9%, and again within subgroups IG 3–3.9, 4–4.9, and 5–5.9%, there was no statistically significant difference, but subgroups with IG >6% did differ statistically significantly from the other groups (P<0.001).
Neutropenia or neutrophilia, compared to samples with normal ANC, did not affect the degree of difference in IG% between Sysmex XN and manual microscopy (P=0.296). In samples with low WBC, the IG% difference between the two analysis methods was slightly higher (P<0.001) compared to samples with normal or high WBC (Table 1).
Conclusions
Manual microscopy is the gold standard and the worldwide confirmatory method for WBC differential after an automated haematology analyser, such as Sysmex XN. In general, Sysmex XN analyser WBC differential correlates well with manual microscopy results in samples with no abnormal cells (8,9), but analyser specific cut-off limits have been debated and further studied (16-18). The recommendation for Sysmex XN haematology analysers is to review WBC differential samples with IG above 3% with manual microscopy to achieve higher accuracy. Samples with IG <3% may be autovalidated. Some studies have showed that IG 5% for Sysmex XN analysers is an adequately accurate cut-off instead of 3% (12,19). Possible variations between an automated haematology analyser and manual microscopy result are often explained by inaccuracy of the microscopy review with only 200 WBC. However, increasing the WBC amount counted in microscopy review, even with digital microscopy, is practically impossible in hospital laboratories due to time and labour demand.
In this study, Sysmex XN gave positive error for IG% at every IG level, even in autovalidated samples (IG <3%). Also occasional lower IG% compared to manual microscopy were recorded. As seen with these data, we would accept the differences exceeding the widely adopted limits from Rümke (13) in the autovalidated Sysmex XN results below IG 3%, were they reviewed by microscopy. Similar deviation as with IG <3%, but with slightly higher median positive error, was seen in samples with IG up to 6%. There was no statistically or clinically significant difference between the methods with IG 5% compared to IG 6% in this study. It could also be claimed that the difference in IG% results between Sysmex XN and manual microscopy was not clinically significant up to IG 7%, even when the IG subgroup 6-7% did differ statistically significantly from the lower IG% subgroups. Deviation of single results, however, increased with IG above 6% and there were samples with a high analyser IG% and no, or miniscule, amount of IG with manual microscopy.
In the samples with Sysmex XN IG above 15% and manual microscopy IG% very low, the majority of the WBC were neutrophils (84–96%) and Sysmex XN showed a distinct IG cloud above mature neutrophils. In these cases, it is possible that the deviation between the methods is caused by incorrect microscopy review, although this cannot be confirmed retrospectively. Possible explanations are high amounts of ghost cells on the blood film causing deviation in the cell classes or morphologically challenging samples with, for example, abnormal neutrophil granulation. It is also possible that the analyser data is erroneous if algorithms misclassify IG among highly granulated neutrophils in neutrophilic samples or if there is merging of neutrophil and IG cell clouds confusing algorithm-based identification (see Figure 1B). It is important to note that the inter-individual CV% of IG in manual microscopy in our accredited laboratory has been up to 77% (at IG <3%) while for neutrophils it has been remarkably lower. For the Sysmex XN intra- and inter-assay CV has been under 10% with low and higher IG%, putting the value of the manual microscopy IG as the reference method under doubt in accordance to some previous studies (10).
In the samples with low WBC, the IG% difference was slightly higher than in samples with normal or high WBC. Human error might explain deviation in low WBC samples due to low amount of representative WBC evaluated in manual microscopy. In comparison, ANC did not affect the accuracy of the Sysmex XN compared to manual microscopy IG%, nor did it explain the differences between the analysis methods. IG% can thereafter be reported in neutropenic samples according to the same criteria as normal ANC samples. In general, it can be claimed that automated haematology analysers enumerating thousands of cells are more accurate compared to manual microscopy. It is possible that IG% measured with Sysmex XN reflects a more truthful physiological situation in cases with still rather low circulating IG. This hypothesis should be verified with consecutive samples, e.g., after leukocyte growth factor administration.
In this study, there were samples with Sysmex XN IG 3–6% and manual microscopy IG 0%. Similarly, there were samples with Sysmex XN IG <1% and manual microscopy IG 6%. The trueness of microscopy IG% cannot be verified based on these data. The clinical significance of these differences can, however, be considered minimal. A positive error in IG measured with Sysmex XN could lead to suspicion of infection or inflammation, but in patient care this can be confirmed with consequent samples and other clinical chemistry parameters such as C-reactive protein or procalcitonin, were not investigated in this study. Falsely low IG, on the other hand, might lead to missed acute inflammatory conditions. If the IG is reported as a WBC parameter (six-part WBC differential), we suggest that the cut-off IG 6% for manual microscopy is adequate when clinical significance of the possible inaccuracy is concerned. If the cut-off is used only for directing the samples for microscopy review and the IG parameter is not reported as such, the focus is on reporting the left shift of neutrophils in general and the IG cut-off 3% is more adequate. Reporting immature neutrophil forms with manual microscopy or with an automated haematology analyser is of clinical significance and this would be missed with an IG cut-off above 3% and five-part differential only. Based on these data we suggest to consider actions described in Figure 5 when analysing IG% with Sysmex XN.
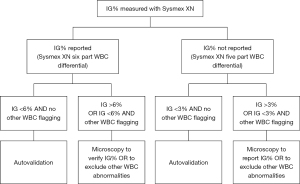
In our university hospital laboratory, IG cut-off 3% causes about 30% of all Sysmex XN interpretive program WBC messages, or flagging, leading to manual microscopy. In this study, 65% of the routine analysis samples had IG under 6%. No clinically significant deviation from manual microscopy IG was seen in this study doubting the need of sample review below this IG cut-off. Reporting Sysmex XN IG% and rising the cut-off to 6% would reduce the need for WBC microscopy review by 20%. This can be considered a significant reduction on monthly and yearly basis. Faster reporting of the analyser IG would benefit patient care and possibly promote using IG%, or absolute IG amount not evaluated in this study, as an inflammatory marker. The data presented here still requires further confirmation, but is of value for laboratories considering to evaluate the IG% cut-off for Sysmex XN.
Acknowledgments
We thank the staff of Turku University Hospital laboratory for the invaluable help in analysing the samples. We thank MD, PhD Annika Kouki and BM Riku Pihlaja for the help in data processing.
Funding: This work was supported by the Finnish Society of Clinical Chemistry (23/2019 to AM Linko-Parvinen).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/jlpm-21-33
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jlpm-21-33
Peer Review File: Available at https://dx.doi.org/10.21037/jlpm-21-33
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jlpm-21-33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No patient permission or evaluation from the ethics committee were needed for this retrospective study with anonymised samples.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amulic B, Cazalet C, Hayes GL, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012;30:459-89. [Crossref] [PubMed]
- Monteiro WO, Bó SD, Farias MG, et al. Definition of Reference Range for the Immature Granulocytes Parameter Provided by a Hematology Analyzer. Clin Lab 2021;67: [Crossref] [PubMed]
- Wiland EL, Sandhaus LM, Georgievskaya Z, et al. Adult and child automated immature granulocyte norms are inappropriate for evaluating early-onset sepsis in newborns. Acta Paediatr 2014;103:494-7. [Crossref] [PubMed]
- Mathews EK, Griffin RL, Mortellaro V, et al. Utility of immature granulocyte percentage in pediatric appendicitis. J Surg Res 2014;190:230-4. [Crossref] [PubMed]
- Karakulak S, Narcı H, Ayrık C, et al. The prognostic value of immature granulocyte in patients with acute pancreatitis. Am J Emerg Med 2021;44:203-7. [Crossref] [PubMed]
- Güngör A, Göktuğ A, Tekeli A, et al. Evaluation of the accuracy of immature granulocyte percentage in predicting pediatric serious bacterial infection. Int J Lab Hematol 2021;43:632-7. [Crossref] [PubMed]
- Bedel C, Korkut M, Selvi F. New markers in predicting the severity of acute pancreatitis in the emergency department: Immature granulocyte count and percentage. J Postgrad Med 2021;67:7-11. [Crossref] [PubMed]
- Buoro S, Mecca T, Azzarà G, et al. Extended leukocyte differential count and C-reactive protein in septic patients with liver impairment: diagnostic approach to evaluate sepsis in intensive care unit. Ann Transl Med 2015;3:244. [PubMed]
- Briggs C, Longair I, Kumar P, et al. Performance evaluation of the Sysmex haematology XN modular system. J Clin Pathol 2012;65:1024-30. [Crossref] [PubMed]
- Fernandes B, Hamaguchi Y. Automated enumeration of immature granulocytes. Am J Clin Pathol 2007;128:454-63. [Crossref] [PubMed]
- Maenhout TM, Marcelis L. Immature granulocyte count in peripheral blood by the Sysmex haematology XN series compared to microscopic differentiation. J Clin Pathol 2014;67:648-50. [Crossref] [PubMed]
- . Abstracts of the XXV International Symposium on Technological Innovations in Laboratory Hematology. May 21-24, 2012. Nice, France. Int J Lab Hematol 2012;34:1-180. [PubMed]
- Rümke CL, Bezemer PD, Kuik DJ. Normal values and least significant differences for differential leukocyte counts. J Chronic Dis 1975;28:661-8. [Crossref] [PubMed]
- Briggs C, Bain BJ. Basic Haematological Techniques. In: Bain BJ, Bates I, Laffan MA, et al. editors. Dacie and Lewis Practical Haematology. London, UK: Churchill Livingstone Elsevier; 2012: 23-56.
- Palmer L, Briggs C, McFadden S, et al. ICSH recommendations for the standardization of nomenclature and grading of peripheral blood cell morphological features. Int J Lab Hematol 2015;37:287-303. [Crossref] [PubMed]
- Barnes PW, McFadden SL, Machin SJ, et al. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol 2005;11:83-90. [Crossref] [PubMed]
- Ronez E, Geara C, Coito S, et al. Usefulness of thresholds for smear review of neutropenic samples analyzed with a Sysmex XN-10 analyzer. Scand J Clin Lab Invest 2017;77:406-9. [Crossref] [PubMed]
- Buoro S, Mecca T, Seghezzi M, et al. Analytical comparison between two hematological analyzer systems: CAL-8000 vs. XN-9000. Int J Lab Hematol 2017;39:147-62. [Crossref] [PubMed]
- Hotton J, Broothaers J, Swaelens C, et al. Performance and abnormal cell flagging comparisons of three automated blood cell counters: Cell-Dyn Sapphire, DxH-800, and XN-2000. Am J Clin Pathol 2013;140:845-52. [Crossref] [PubMed]
Cite this article as: Linko-Parvinen AM, Kurvinen K, Tienhaara A. Accuracy of Sysmex XN immature granulocyte percentage compared to manual microscopy. J Lab Precis Med 2021;6:27.


