
Pseudothrombocytopenia and leukocyte scattergrams: a case report
Introduction
Multiple causes can cause pseudothrombocytopenia, including platelet clumping, platelet satellitism of neutrophils and, rarely, platelet phagocytosis. Such phenomena can be induced by the anticoagulant ethylene acid-diamino-tetraacetic acid (EDTA) anticoagulant and is immunologically mediated by antiplatelet autoantibodies that cause platelet aggregation in the presence of this anticoagulant (1,2).
The Sysmex XN 20 analyzer (Sysmex, Kobe, Japan uses principles, analytical channels and reagents to measure nucleated red blood cells (NRBC) automatically along with the basic complete blood counts (CBC). For this function, Leukocyte counts [white blood cell (WBC)] and differentials are analyzed by two separate channels, the white cell nucleated (WNR) and the WBC differential (WDF) channels. Also, a dedicated fluorescence optical analysis of platelets (PLT-F) provides accurate platelet counts especially designed for thrombocytopenic samples.
Due to the unexpected thrombocytopenia, the discrepancy of the results for platelet counts generated by the impedance and the fluorescence channels and the abnormal scattergrams of the WDF and WNR channels, a peripheral blood smear was reviewed in order to confirm the decreased platelet counts: platelet clumping, satellitism of neutrophils and phagocytosis explained the spurious count; up to 90% of neutrophils were affected. We present the following case in accordance with the CARE reporting checklist (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-42/rc).
Case presentation
An 85-year-old woman diagnosed with monoclonal gammopathy of uncertain significance IgG λ was treated according to the VTD scheme (Bortezomib-Thalidomide-Dexamethasone). She went to the Hematology Department for follow up. CBC was performed on a Sysmex XN 20 analyzer, the following results were obtained: WBC 3.81×109/L, differential neutrophils 78%, lymphocytes 14% monocytes 8%; red blood cell (RBC) count 3.19×1012/L, hemoglobin (Hb) 126 g/L, platelet count 18×109/L (impedance method).
No flag to alert for platelets clumps was triggered, so a reflex test to perform a re-analysis on the fluorescence platelets channel (PLT-F) was automatically generated, rendering count 52×109/L.
Abnormal scattergrams were observed in the erythroblast/basophil channel (WNR), with the presence of an additional cluster; in the differential leukocyte channel (WDF), an abnormal pattern of the neutrophil cluster was evident (Figure 1).
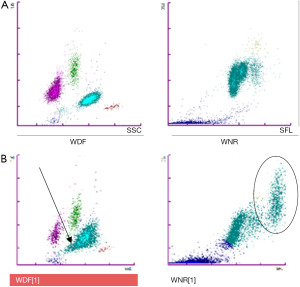
A peripheral blood smear for manual review was requested in order to confirm the decrease in platelet count observed with respect to previous CBC and the discrepancy in the results obtained with both analytical techniques.
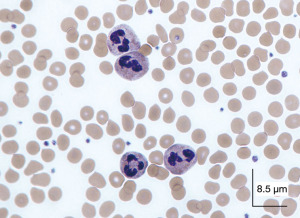
Peripheral blood smear (May-Grünwald’s-Giemsa stain) revealed neutrophil satellitism and the presence of numerous cytoplasmic vacuoles containing phagocytosed platelets (Figure 2).
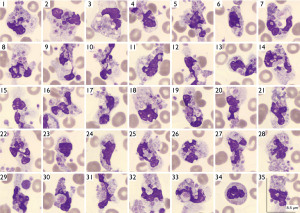
A second sample was drawn in sodium citrate anticoagulant: with the use of this anticoagulant the morphological abnormalities disappeared, the analyzer did not generate alarms, the scatterplots were normal and the platelet count was automatically corrected, 201×109/L, and the patient continued her treatment (Figure 3).
The publication of this case report was approved by our Institutional Ethic Research Committee (OSI Barrualde Comité de Etica en Investigación, reference No. 10548/21).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The unexpected decrease in platelet counts was found to be spurious when platelet satellitism and phagocytosis were evident on the blood smear (3-6).
Pseudothrombocytopenia is an in vitro phenomenon, which is defined as a false decrease in the number of platelets. The incidence ranges from 0.07% to 0.2% and in hospitalized patients it can be up to 0.1% to 2% (7).
The clinical data of those patients is not related to thrombocytopenia and no alterations are found in the coagulation tests. The thrombocytopenia induced by anticoagulant EDTA should always be considered in cases of low platelet counts, in addition to the presence of platelet clumps, in order to avoid performing unnecessary therapeutic actions with potential adverse effects (1,8).
The anticoagulant EDTA can induces platelet aggregation and satellitism, among others less frequent, such as the phagocytosis seen in the present case report. Platelet satellitism consists of the adhesion of platelets to other circulating cells, mainly neutrophils. It is a phenomenon immunologically mediated by antiplatelet autoantibodies that cause platelet aggregation in the presence of the anticoagulant (9).
Adhesion is mediated by the GPIIb-IIIa receptors of platelets; the GPIIb fraction, normally hidden in the platelet membrane, is exposed in the presence of EDTA and can react with autoantibodies (IgG or IgM type and rarely IgA), which recognize these antigens in the membrane of platelets modified by the action of the anticoagulant. These events trigger several pathways of signaling and cellular activation, producing aggregation, satellitism and more rarely phagocytosis (10).
Platelet phagocytosis by neutrophils is an extremely rare finding in smears. However, polymorphonuclear cells remove activated platelets from the circulation, this is a mechanism to avoid thrombosis. So not only this phenomenon can occur “in vitro”, cases of platelet phagocytosis have also been reported in different clinical conditions such as trauma, thrombotic events, heart disease, inflammation, especially vascular inflammation and infection, use of immunosuppressive drugs, autoimmune diseases, viral and bacterial infections, cardiovascular disorders and cardiac surgery, among others (11,12).
The WDF channel reports the leukocyte differential of 5 populations, while WNR is used for the total count of leukocytes, erythroblasts and basophils. The lysing agent is different in both channels, being more acidic in the WNR channel.
In the WDF scattergram the granularity of cytoplasm is plotted along the X axis and Y axis the nucleic acid content; in our case, the cluster of neutrophils is abnormal shaped, due to the platelets within.
In the WNR channel, the X axis represents fluorescence (nucleic acid content) and the light scattered related to cell volume (forward scatter) is plotted along the Y axis. An additional cluster in the WNR scatterplot is due to distortion of neutrophil morphology and increased RNA content due to the additional presence of platelets in the cytoplasm.
In addition, XN 20 analyzers have a fluorescence platelet count channel (PLT-F) designed to report an accurate and reliable count in cases of thrombocytopenia. The reagent is an oxacin-derived fluorescent marker that binds specifically to platelets, minimizing potential interference in the count; this method presents a good correlation with the reference method recommended by the International Council for Standardization in Hematology (ICSH) (13).
The alarms generated by automated counters are very useful for detecting pseudothrombocytopenia. In our case, the absence of flagging was highly misleading. Likewise, the careful study of the scattergrams can also be useful, since the disruption in the morphology of the neutrophils produced by the interaction with platelets is evidenced by anomalous patterns.
The phagocytosis that occurs after satellitism is due to the interaction with the anticoagulant EDTA, so these phenomena do not appear in specimens taken with other anticoagulants: the sample drawn in sodium citrate rendered a platelet count of 201×109/L, which confirms that the phenomenon is an artifact unrelated to the clinical situation of the patient.
The possibility of EDTA-induced thrombocytopenia should always be considered in cases of low platelet counts, in addition to the presence of platelet clumps.
Observation and interpretation of scattergrams can be very useful in validating test results. The altered morphology and the higher nucleic acid content of neutrophils in this case produced abnormal scatterplots, that can alert the need for revision of the blood smear (9,14,15).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-42/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.com/article/view/10.21037/jlpm-22-42/coif). EU serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from October 2019 to November 2023. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The publication of this case report was approved by our Institutional Ethic Research Committee (OSI Barrualde Comité de Etica en Investigación, reference No. 10548/21). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zandecki M, Genevieve F, Gerard J, et al. Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol 2007;29:4-20. [PubMed]
- Baccini V, Geneviève F, Jacqmin H, et al. Platelet Counting: Ugly Traps and Good Advice. Proposals from the French-Speaking Cellular Hematology Group (GFHC). J Clin Med 2020;9:808. [Crossref] [PubMed]
- Campbell V, Fosbury E, Bain BJ. Platelet phagocytosis as a cause of pseudothrombocytopenia. Am J Hematol 2009;84:362. [Crossref] [PubMed]
- Senzel L, Chang C. Platelet phagocytosis by neutrophils. Blood 2013;122:1543. [Crossref] [PubMed]
- Sousa SM, Sousa TM, Silva CF, et al. Pseudothrombocytopenia: a case of platelet satellitism and phagocytosis by neutrophils. Platelets 2020;31:541-3. [Crossref] [PubMed]
- Amouroux I, Lesesve JF. Neutrophilic thrombophagocytosis. Morphologie 2020;104:73-5. [Crossref] [PubMed]
- George JN. Platelets. Lancet 2000;355:1531-9. [Crossref] [PubMed]
- Bizzaro N. EDTA-dependent pseudothrombocytopenia: a clinical and epidemiological study of 112 cases, with 10-year follow-up. Am J Hematol 1995;50:103-9. [Crossref] [PubMed]
- Nagler M, Keller P, Siegrist D, et al. A case of EDTA-dependent pseudothrombocytopenia: simple recognition of an underdiagnosed and misleading phenomenon. BMC Clin Pathol 2014;14:19. [Crossref] [PubMed]
- Criswell KA, Breider MA, Bleavins MR. EDTA-dependent platelet phagocytosis. A cytochemical, ultrastructural, and functional characterization. Am J Clin Pathol 2001;115:376-84. [Crossref] [PubMed]
- Manfredi AA, Rovere-Querini P, Maugeri N. Dangerous connections: neutrophils and the phagocytic clearance of activated platelets. Curr Opin Hematol 2010;17:3-8. [Crossref] [PubMed]
- Maugeri N, Malato S, Femia EA, et al. Clearance of circulating activated platelets in polycythemia vera and essential thrombocythemia. Blood 2011;118:3359-66. [Crossref] [PubMed]
- Harrison P, Ault KA, Chapman S, et al. An interlaboratory study of a candidate reference method for platelet counting. Am J Clin Pathol 2001;115:448-59. [Crossref] [PubMed]
- Wu W, Guo Y, Zhang L, et al. Clinical utility of automated platelet clump count in the screening for ethylene diamine tetraacetic acid dependent pseudothrombocytopenia. Chin Med J 2011;124:3353-7. [PubMed]
- Paul P, Kaur M, Kakkar N, et al. Co-existent Platelet Phagocytosis, Satellitism and Clumping Causing Spurious Thrombocytopenia. Indian J Hematol Blood Transfus 2013;29:158-60. [Crossref] [PubMed]
Cite this article as: Urrechaga E, Fernández M. Pseudothrombocytopenia and leukocyte scattergrams: a case report. J Lab Precis Med 2022;7:35.


