
Validation of urine citrate and oxalate measurement on the Roche Cobas® c502 chemistry analyzer
Highlight box
Key findings
• We demonstrate that the use of the Roche Cobas® c502 instrument, combined with third-party reagents, can provide accurate, reliable, and stable measurements of urinary citrate and oxalate.
What is known and what is new?
• Many methods have been developed for urine citrate and oxalate measurement, but most are not amenable to routine clinical laboratories.
• We validated third party reagents for measurement of citrate and oxalate on the Roche Cobas® c502, an automated, high-throughput analyzer commonly used in clinical chemistry laboratories.
What is the implication, and what should change now?
• Measurement of urinary citrate and oxalate on a routine clinical chemistry analyzer will facilitate adoption of these assays into more clinical laboratories by improving workflow and efficiency.
Introduction
Citrate and oxalate are important urine markers in the formation of kidney stones. Urine citrate is a major inhibitor of stone formation and hypocitraturia is considered a risk factor for urolithiasis (1). Increased oxalate is a risk factor for stone formation, as oxalate excreted by the kidney can combine with calcium to form calcium oxalate stones (2,3). Measurement of urine citrate and oxalate can help guide treatment as well as urolithiasis prophylaxis (4,5).
Urine citrate and oxalate are not routinely measured in clinical laboratories as most available methods entail multiple manual steps or techniques that are not readily available (6-9).
Various methods that have been published in the literature include capillary zone electrophoresis, three-channel ion chromatography, liquid chromatography tandem mass spectrometry (LC-MS/MS) and nuclear magnetic resonance (NMR) (9-13). These methods often require complex equipment that necessitates skilled personnel and as such, are not routinely available in most clinical laboratories.
Measurement of urine citrate and oxalate is often combined with other analytes such as electrolytes, calcium, and uric acid when determining a patient’s risk for kidney stone formation. These latter analytes are typically measured on large-scale automated chemistry platforms. As such, the availability of urine citrate and oxalate assays on the same equipment could improve workflows, maximize laboratory efficiency, and result in a decreased turn-around time for results to be available.
To the best of our knowledge, there are no published studies that have evaluated citrate and oxalate on an automated clinical chemistry platform. Running these assays on such an instrument could allow adoption of these tests into more clinical laboratories. We therefore sought to validate citrate and oxalate on a routine chemistry analyzer (Roche Cobas® c502 instrument, Roche Diagnostics, Laval, QC, Canada) using third-party reagents that utilize end-point enzymatic spectrophotometric assays. Precision, bias, linearity, functional sensitivity, and both frozen and refrigerated stability were assessed.
Methods
Participants in the current study included patients >18 years who had 24-hour urine samples collected for oxalate and citrate measurement as part of standard of care from January 31, 2021 to March 31, 2022.
Urine specimens for citrate and oxalate measurement were collected without preservative and were kept refrigerated during collection and prior to testing. Samples for oxalate measurement were acidified using 6N HCl within 24 hours of the end of the collection period. Oxalate specimens were further pre-treated prior to testing, where urine was pipetted into sample purifier tubes containing activated charcoal before being mixed then centrifuged (5 minutes, 2,000 rpm) according to the manufacturer’s instructions.
All samples were tested on a Roche Cobas® c502 instrument as part of a Roche Cobas® 8000 automation track using third party reagents (Citrate, Biomedical Enterprises, Milano, Italy; and Oxalate, Trinity Biotech, Jamestown, NY, USA). Briefly, in these assays, citrate is converted to oxalacetate and acetate by the enzyme citrate lyase. A subsequent reaction reduces NADH to NAD+ in the presence of malate dehydrogenase and lactate dehydrogenase. The change in absorbance is determined at 340 nm. For the oxalate assay, oxalate is oxidized to carbon dioxide and hydrogen peroxide by oxalate oxidase. The hydrogen peroxide reacts with two chromogens in the reagent to yield an indamine dye that is absorbed at 590 nm.
We assessed precision by running two levels of vendor-supplied quality control (QC) material twice per day (morning and afternoon) for a total of 20 days. We also assessed precision using two patient specimens—one for citrate and one for oxalate which were run five times per day over a 5-day period. Next, we verified bias of both assays by comparing n=41 (citrate) and n=46 (oxalate) patient specimens on our Roche Cobas® 8000 c502 instrument to results from a referral laboratory that also utilizes an enzymatic method for both analytes (In Common Laboratories, Toronto, ON, Canada). Samples were sent to the referral laboratory refrigerated with next day delivery. Linearity was assessed across the measuring range for both assays using diluted patient specimens that were measured in triplicate. We verified the vendor’s limit of quantification (LoQ) by diluting a patient specimen with saline to 0.05, 0.04, and 0.02 mmol/L for citrate and to 0.3, 0.2, and 0.1 mmol/L for oxalate. Each diluted specimen was measured 10 times in a single run. The LoQ was determined to be the lowest concentration where a coefficient of variation (CV) <20% could be achieved. Lastly, 14-day refrigerated (4 ℃) and frozen (−20 ℃) stability was assessed for both analytes using pooled patient urine. After initial measurement of oxalate/citrate concentration (noted as timepoint 0 hours), aliquots were made and stored either refrigerated or frozen for the 14-day study period. A fresh aliquot from each storage temperature was tested daily and a change less than 10% (based on our laboratory’s total allowable error goal) was deemed acceptable.
All results were analyzed in EP Evaluator (Version 12.3.0.2; Data Innovations LLC, Colchester, VT, USA) and the total allowable error that was used to determine assay acceptability was set to 10% for both analytes. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Board of Horizon Health Network (File #2022-3116). A waiver of consent for secondary use of personal information was approved in accordance with TCPS2 requirements.
Results
Using QC material, we determined that the imprecision of the citrate assay as determined by calculation of the CV was 3.0% and 2.0% at a concentration of 1.18 and 2.07 mmol/L, respectively. The CV of the patient pool was 2.9% at a concentration of 1.07 mmol/L. For oxalate, we observed a CV of 4.0% and 2.4% at a QC concentration of 0.24 and 1.10 mmol/L, respectively. The CV of the patient pool was 4.3% at a concentration of 0.25 mmol/L.
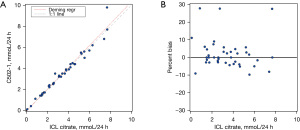
We found minimal bias between results on the Roche Cobas® c502 instrument compared to the enzymatic method used at the referral lab. The average bias was 1.5% for citrate while the slope was 1.043 (95% CI: 0.978 to 1.109), y-intercept was −0.094 (95% CI: −0.352 to 0.164), and correlation coefficient (R) was 0.9810 using Deming regression (Figure 1). Oxalate also compared well against the reference laboratory (Figure 2) with an average bias of −0.467%, slope 0.934 (95% CI: 0.843 to 1.024), y-intercept of 0.023 (95% CI: −0.013 to 0.059) and correlation coefficient of 0.9495.

The citrate assay was determined to be linear over a concentration range of 0.05–4.50 mmol/L while the oxalate assay was linear from 0.11–1.17 mmol/L. The LoQ for citrate was 0.05 mmol/L and the LoQ for oxalate was <0.12 mmol/L (the lowest concentration that was tested which exhibited a CV of 2.9%). Lastly, we assessed both refrigerated and frozen stability for both analytes; both citrate (Figure 3) and oxalate (Figure 4) were stable for 14 days refrigerated and frozen as determined by an average change ≤10% or <0.2 mmol/L from the initial baseline measurement at each time point.
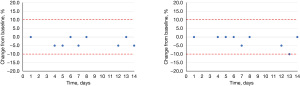
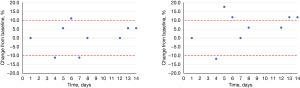
Discussion
Measurement of urine citrate and oxalate are routinely requested by urologists in patients at risk of kidney stone formation. It is often combined with other routine urine tests (calcium, magnesium, uric acid, electrolytes) which are offered in most clinical laboratories. As such, being able to test urine oxalate and citrate along with these other analytes on a single platform can offer significant improvements to clinical service as well as more efficient laboratory services. In this study, we demonstrate that urine citrate and oxalate can be easily adapted onto a routine clinical chemistry analyzer in combination with third-party reagents. Our results showed acceptable precision for both citrate and oxalate assays, as determined by low CVs with both QC material and patient samples. Further, the average bias for citrate and oxalate assays was minimal. Assay linearity across the measuring range was also demonstrated for both citrate and oxalate, as was the functional sensitivity, with acceptable LoQ determined for both analytes. In addition, we also established 14-day stability for both analytes at refrigerated and frozen temperatures.
To the best of our knowledge, this is the first study to demonstrate acceptable performance of an enzymatic citrate assay on a large-scale automated analyzer. Others have evaluated citrate measurement using a variety of other methodologies; however, most of these are not easily amenable to the clinical laboratory. Specifically, Matyus et al. recently developed a high-throughput method for measuring urinary citrate using NMR spectroscopy (12). This assay demonstrated robust performance, with acceptable imprecision and bias, and showed potential for simultaneous multiplexing with other urine metabolite marker assays, including creatinine. However, the instrument used in this study is a stand-alone piece of equipment. Incorporation of citrate on a clinical chemistry automation track allows for improved throughput in a high-volume clinical laboratory.
Simultaneous measurement of citrate and oxalate has also been demonstrated by others using ion chromatography (10) and capillary electrophoresis (9). Both of these studies showed imprecision <8% and percent recoveries of spiked samples ranging from 99% to 102%. While our method has a common goal with these studies of providing validated quantification of urinary markers involved in stone formation, it uniquely leverages the practicality and efficiency of routine chemistry analyzers for broader application.
Others have evaluated use of a similar enzymatic end point spectrophotometric assay to measure urinary oxalate (14). Specifically, Ruiz Santamaria et al. validated an assay that also uses oxalate oxidase and was combined with manual spectrophotometric measurements from urine specimens obtained from 26 healthy individuals (14). The assay showed acceptable imprecision (CV <8.0%) and was linear up to 0.89 mmol/L. Our study builds on these findings by showing that a similar oxalate assay can be easily adapted for use on an automated chemistry analyzer. Furthermore, we also showed that the assay was linear beyond what was reported in this study.
There are a few limitations with our study. Validation was limited to a single vendor’s instrument. It will be important to determine if the results obtained in this study hold true if the third-party reagents are used on another vendor’s instrumentation. In addition, our study was conducted at a single centre, and therefore may not generalize to other labs where standard operating procedures may differ. As well, the comparative analysis of results was performed with a single referral laboratory. Future multi-centre studies as well as comparisons with additional reference laboratories could provide further assurance of the robustness of the assays. Lastly, it would be beneficial to repeat stability experiments at higher concentrations, where risk of crystal formation is higher. We were limited by the patient specimens we received in our laboratory that had sufficient volume to allow for measurement over the 14-day stability experiment.
Conclusions
In conclusion, our results demonstrated that the use of the Roche Cobas® c502 instrument, combined with third-party reagents, can provide accurate, reliable, and stable measurements of urinary citrate and oxalate. The assays studied showed acceptable precision, minimal bias, satisfactory linearity, sensitivity, and stability under refrigerated and frozen conditions. Our study supports the measurement of citrate and oxalate on automated clinical chemistry analyzers which can lead to significant improvements in the service and cost savings of labs performing these assays.
Acknowledgments
The authors thank Claudette Clark (Department of Laboratory Medicine, Saint John Regional Hospital, Horizon Health Network, Saint John, NB, Canada), Christina Quondam Franks (Department of Laboratory Medicine, Saint John Regional Hospital, Horizon Health Network, Saint John, NB, Canada), and Tiffany Rose (Department of Laboratory Medicine, Saint John Regional Hospital, Horizon Health Network, Saint John, NB, Canada) for the technical support they provided.
Funding: None.
Footnote
Data Sharing Statement: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-43/dss
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-43/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Research Ethics Board of Horizon Health Network (File #2022-3116). A waiver of consent for secondary use of personal information was approved in accordance with TCPS2 requirements.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hosking DH, Wilson JW, Liedtke RR, et al. Urinary citrate excretion in normal persons and patients with idiopathic calcium urolithiasis. J Lab Clin Med 1985;106:682-9. [PubMed]
- Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol 2018;2018:3068365. [Crossref] [PubMed]
- Mitchell T, Kumar P, Reddy T, et al. Dietary oxalate and kidney stone formation. Am J Physiol Renal Physiol 2019;316:F409-13. [Crossref] [PubMed]
- Leslie SW, Sajjad H, Bashir K. 24-Hour Urine Testing for Nephrolithiasis: Interpretation Guideline. 2023.
- Fisang C, Anding R, Müller SC, et al. Urolithiasis--an interdisciplinary diagnostic, therapeutic and secondary preventive challenge. Dtsch Arztebl Int 2015;112:83-91. [PubMed]
- Muñoz JA, López-Mesas M, Valiente M. Development and validation of a simple determination of urine metabolites (oxalate, citrate, uric acid and creatinine) by capillary zone electrophoresis. Talanta 2010;81:392-7. [Crossref] [PubMed]
- Holmes RP, Kennedy M. Urinary oxalate and citrate. Methods Mol Med 1999;27:199-202. [PubMed]
- Li MG, Madappally MM. Rapid enzymatic determination of urinary oxalate. Clin Chem 1989;35:2330-3. [Crossref] [PubMed]
- García A, Muros M, Barbas C. Measurement of nephrolithiasis urinary markers by capillary electrophoresis. J Chromatogr B Biomed Sci Appl 2001;755:287-95. [Crossref] [PubMed]
- Li Q, Liu G, Cheng Y, et al. Three-channel ion chromatograph for improved metabolic evaluation of urolithiasis. BMC Urol 2021;21:151. [Crossref] [PubMed]
- Shen Y, Luo X, Li H, et al. Evaluation of a high-performance liquid chromatography method for urinary oxalate determination and investigation regarding the pediatric reference interval of spot urinary oxalate to creatinine ratio for screening of primary hyperoxaluria. J Clin Lab Anal 2021;35:e23870. [Crossref] [PubMed]
- Matyus SP, Wolak-Dinsmore J, Garcia E, et al. Vantera Mediated Quantification of Urine Citrate and Creatinine: A New Technology to Assess Risk of Nephrolithiasis. Diagnostics (Basel) 2022;12:2606. [Crossref] [PubMed]
- Kataoka K, Takada M, Kato Y, et al. Determination of urinary oxalate by high-performance liquid chromatography monitoring with an ultraviolet detector. Urol Res 1990;18:25-8. [Crossref] [PubMed]
- Ruiz Santamaria J, Coll R, Fuentespina E. Comparative study of two commercial enzymatic kits for determining oxalate concentrations in urine. Clin Biochem 1993;26:93-6. [Crossref] [PubMed]
Cite this article as: Sherazi A, Stevens A, Shea JL. Validation of urine citrate and oxalate measurement on the Roche Cobas® c502 chemistry analyzer. J Lab Precis Med 2024;9:1.


