Data-driven laboratory stewardship: an implementation science perspective
Introduction
Background
Evidence indicates substantial inappropriate and inefficient use of resources in Pathology and Laboratory Medicine. For example, a synthesis of 42 studies including over 1.6 million test results found that 16–25% of laboratory tests ordered are not clinically indicated, while 34–56% of tests that are clinically indicated are not ordered (1). Over-use can lead to negative effects for patients, including avoidable phlebotomies, pain, delays in appropriate tests, inaccurate diagnosis due to false-positive results, and a cascade of secondary and potentially more invasive testing with increasing likelihood of over diagnosis and ‘slippery slope’ inappropriate treatments that may have adverse side-effects (2-5). Over-use also represents wasted healthcare resources which could have been better used, and increasingly, the negative impacts of over-use on the environment are being recognized (6-8). Under-use can lead to delays in accurate diagnosis and treatment, with delays potentially worsening the patients’ condition. Therefore, there is an urgent need to improve quality and more effectively manage scarce resources by reducing low-value testing and increasing high-value testing. Activities focused on improving the appropriateness of Pathology and Laboratory Medicine resource use are advocated as a key component of the quality improvement work conducted by clinical laboratories (9,10). Such activities have been labelled as ‘laboratory stewardship’, to emphasize a focus on value, i.e., “the quality of the service provided relative to its cost” (10).
Rationale and knowledge gap
Evaluations of laboratory stewardship initiatives show highly variable results. A systematic review of 119 interventions deployed across primary and secondary healthcare settings found that interventions that include an educational component achieved the highest median relative reduction in test volume at 34% (11). Additionally, interventions involving audit and feedback (providing physicians with a summary of their performance compared to their own previous performance or their peers) or system-based interventions involving one-time, permanent changes to test ordering processes both reduced volumes by 22% on average, with the latter advocated as generally requiring fewer resources to implement and more promising for achieving sustainable change (11). Two included studies achieved a 99% reduction in test use, one which changed laboratory policy such that urine microscopy was done only if specifically requested by the physician (12), and another providing education (distributing guidelines) and feedback (flagging tests orders not in line with guidelines) on ordering lactate dehydrogenase (LD)-isozymes (13). However, many effects were much smaller, and some interventions had opposite than intended effects, resulting in increased test use. Whilst conclusions from this review are limited due to heterogeneity between interventions deployed and issues with study quality, the wide variation in effectiveness indicates that more work needs to be done to fully understand how to maximize the effectiveness of laboratory stewardship interventions. This is further supported by a systematic review of 83 studies which identified modifications to existing computerized physician order entry (CPOE) systems, reflex testing, and multifaceted initiatives as ‘best practice’ approaches (based on the number of studies, study quality ratings, and effect size ratings) (14). No recommendations were made for other initiative types—clinical decision support, education, audit & feedback, test review, and utilization teams—due to insufficient evidence. However, all initiative types worked some of the time, and again the review identified a limited number of good-quality studies. This review also noted that initiatives were not associated with adverse impacts on patient-related outcomes (such as length of stay, morbidity, and mortality).
Whilst many issues may contribute to variations in initiative effectiveness, core issues are likely to be (I) numerous factors drive overuse of testing; (II) the strength of influence of these factors will vary between settings and for different types of tests; and (III) specific initiative types will vary in the extent to which they can adequately address specific influencing factors. An overview of the literature on factors influencing inappropriate testing indicates that influencing factors include intrapersonal issues (such as knowledge, fear of litigation, and cognitive biases); interpersonal issues (such as medical culture, pressure from patients); and issues related to the environment or context (such as protocols, time constraints, and ease of access) (15). It is clear that strategies such as education or system-based interventions would each be better at addressing some of these factors, and likely inadequate for addressing others. This wide range of influencing factors add further challenges to the selection of an appropriate change strategy.
Implementation science approaches can help overcome these challenges. During the emergence of the evidence-based medicine movement in the 1990s when it was argued that practice should be based on current best evidence, it was also acknowledged that effecting practice change is challenging and active efforts would need to be made to encourage the spread and uptake of evidence (16). Implementation science is the scientific study of strategies to facilitate the uptake of best practices into routine, everyday healthcare (17). Implementation strategies can include dissemination of clinical practice guidelines, educational meetings/outreach visits, audit and feedback, reminders, changing physical structure/equipment, local champions who support implementation, and changing incentive/disincentive structures (18,19). Implementation scientists use a range of methodologies, including qualitative methods to investigate barriers to change, randomized controlled trials (RCTs) to investigate the effectiveness of specific implementation strategies, and systematic reviews to synthesize evidence about strategy effectiveness. Behaviour change-informed implementation science approaches conceptualize efforts to close a gap between best evidence and current practice as supporting people in the healthcare system to change their behaviour (20). Ordering laboratory tests is something that healthcare professionals physically do as part of their role: i.e., a behaviour. The purpose of laboratory stewardship efforts can therefore be conceptualized as supporting healthcare professionals—as well as others in the healthcare system where necessary (for example, laboratory team members or clinical/hospital leaders)—in changing their behaviour. Correspondingly, initiatives (such as education, audit & feedback, and system-based interventions) can be conceptualized as behaviour change strategies. Framing laboratory stewardship as a behaviour change endeavour facilitates stewardship teams in drawing on more than 50 years of research on predicting and influencing behaviour in the psychological and behavioural sciences.
Objective
In this article, we build upon our experiences of developing a laboratory stewardship program within a network of hospital-based clinical laboratories [the Eastern Ontario Regional Laboratory Association (EORLA)]. We outline how behaviour change-informed implementation science approaches can be applied to laboratory stewardship problems to enable teams to consider new forms of data concerning (I) the factors influencing over- or under-use of tests and enablers of change/barriers impeding change; and (II) behaviour change strategies that are most suited to disrupting current patterns of test use and capitalizing on enablers of change/breaking down barriers impeding change. This is embedded within an over-arching approach to guide the design, implementation, and evaluation of laboratory stewardship initiatives.
An implementation science-informed approach to laboratory stewardship
Laboratory stewardship definition and project approach
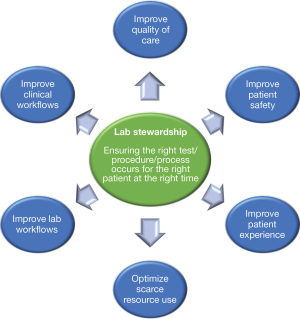
We formed a multi-hospital laboratory stewardship committee to help guide our stewardship work. Individuals in various roles relevant to stewardship issues were invited to join the committee. As such, the committee includes those in the following roles: Laboratory Director, Laboratory Manager, Division Head, Physician, Patient Advisor, Quality Improvement Specialist, Implementation Scientist, and Hospital Senior Management Representative. Our committee defines stewardship activities as those which help ensure that the right test/procedure/process occurs for the right patient at the right time. Stewardship initiatives supported by our committee should target one or more of the (interlinked) outcomes specified in Figure 1. Within this framing, financial perspectives can be incorporated (e.g., as part of optimizing scarce resource use) but should not be the sole or main reason for an initiative, and should not run contrary to quality of care or patient safety. We established an approach, informed by the implementation science literature (21-24), to help guide laboratory stewardship projects after a specific laboratory target is selected (Figure 2). This also includes implementation science/behaviour change tools that can be used to support project activities at specific stages, which are further described below. The first step focuses on establishing the project scope. For this step, we identify the key metrics to be measured at baseline and follow-up to evaluate initiative effectiveness and develop a plan for analyzing these metrics. Key metrics can include test volumes, test costs, blood volumes, blood transfusions, and patient safety outcomes such as hospital length of stay, readmission, morbidity, and mortality. Targets for improvement should be clinically meaningful and realistic. Some sites may end their project participation after baseline analyses are conducted (for example, if they decide that the project is not high-priority based on the findings for their specific site). The next step involves understanding the problem. This requires activities to establish the enablers of improved test use and any barriers to practice change. Based on this information, the third step involves developing solutions, i.e., selecting change strategies/implementation interventions that address these enablers/barriers. The fourth step involves implementing and monitoring those solutions (for example, by assessing fidelity i.e., the extent to which the intervention is deployed as intended). The final step focuses on evaluation, wherein the pre-planned outcomes are analyzed to determine the impact of the solutions implemented. This article focuses primarily on understanding laboratory stewardship problems and developing solutions (steps 2 and 3). A mock case in which a stewardship project team follows these steps is provided in Appendix 1.

Data-driven approaches to understanding the problem
While it is tempting to immediately begin selecting and executing ‘common-sense’ interventions to facilitate practice change once a problem is identified, this approach may results in the selection of strategies that are not best placed to solve the problem (for example, deploying an educational solution when the key driver of test use is structural/organizational, rather than a lack of knowledge). Just as the most appropriate diagnostic tests cannot be ordered until details about patient symptoms have been gathered, the most appropriate implementation intervention cannot be selected until information has been gathered about the factors causing the stewardship problem and potentially impeding change. So, it is important to focus initially on understanding the problem and the contributing factors. To solve healthcare quality problems using a behaviour change-informed approach, the first step involves clearly outlining the behaviour(s) that need to be changed. This amounts to answering the question, ‘Who needs to do what, differently?’ (21). Multiple behaviours conducted by individuals and by teams may play a role in the chain of events leading to the stewardship issue occurring. Knowing this information up-front helps with selecting behaviours for change that are central to bringing about the desired outcomes (25). For example, resident physicians are often responsible for inputting test orders, but their behaviour may not be the only target for change. Residents describe worrying about being perceived as incompetent for asking too many questions about test ordering, have concerns about criticism from senior colleagues at ward rounds, and want to avoid being ‘caught out’ when a test requested by their attending was not ordered, all of which contribute to test over-use (30). In this case, an initiative targeted solely at residents may not have maximal impact: considering the significant social pressures in this context, it may also be beneficial to target their attendings and senior colleagues. This demonstrates the value of considering the multiple actors that may be involved and their behaviours at the outset of initiative planning.
Identifying relevant behaviour(s) for change involves (I) developing a list of the behaviour(s) involved in the care process to be improved; and then (II) selecting one or more to target with a change initiative (25). Activities that stewardship groups can conduct to help develop a list of behaviour(s) involved in the relevant testing-ordering process are outlined in Table 1. The suggested activities vary in terms of resource-intensiveness, as well as relative depth or comprehensiveness of assessment. For example, discussions at existing meetings are less resource-intensive than formal one-on-one interviews or observations of care processes, but may provide a less fulsome picture of the contributing behaviours. However, positive impacts can still be achieved with limited resources.
Table 1
| Activities for developing a list of behaviour(s) |
| • Review local procedures and processes of care (e.g., medical directives) |
| • Review clinical guideline recommendations and relevant literature regarding appropriate behaviours/evidence-practice gaps (either brief or in-depth) |
| • Gather site audit data and compare this with guidelines/evidence |
| • Have discussions with key stakeholders in different professional roles to help identify important behaviours, embedded within existing meetings if time and resources are limited |
| • Conduct observations of care processes, taking notes on who does what |
| • Have informal conversations with those involved in care processes (or conduct more formal, research-based interviews or focus groups), asking them to describe what they do (and potentially what those in other roles do) as it relates to the stewardship problem |
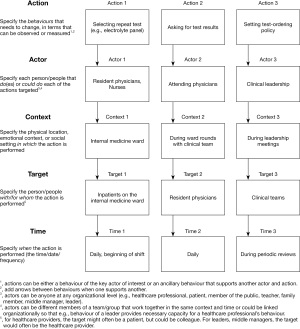
The AACTT framework (26) can be used to specify behaviours in a systematic way, as demonstrated in Figure 3. Using this framework, behaviours are specified in terms of: the relevant “Action” (a discrete observable behaviour); “Actor” (the individual or group of individuals who perform (or should/could perform) the action; “Context” (the physical setting in which the actor performs (or should/could perform) the action; “Target” (the individual or group of individuals for/with whom the actor performs the action; and “Time” (the time period and duration that the actor performs the action in the context with/for the target).
Having specified the behaviour, the next step is to identify what needs to change in the person and/or the environment in order to achieve the desired change in behaviour. Suggestions of activities for identifying enablers of change and/or barriers impeding change are outlined in Table 2. Again, these activities vary in terms of resource-intensiveness and relative depth or comprehensiveness of assessment.
Table 2
| Activities for identifying barriers/enablers |
| • Gather key stakeholders’ thoughts and experiences regarding factors influencing the behaviours to be changed (e.g., at existing meetings) |
| • Have informal conversations (or conduct formal, research-based interviews, focus groups, or questionnaires) with a few of those who will be targeted by the intervention (i.e., your ‘actors’) about what drives what they do, and what would need to change for them to change their behaviour—create a safe environment for people to open up |
| • Conduct structured observations of care processes, taking notes on factors observed to influence behaviour |
| • Review relevant local documents which may provide some insights into factors influencing behaviour (e.g., medical directives) |
| • Review relevant literature on known barriers to change for the specific stewardship problem, and consider applicability to your particular context (either brief or in-depth) |
Lists of barriers/enablers can be assembled from the various sources outlined in Table 2. It can often be difficult to organize the information in a systematic and consistent way. Fortunately, the task of organizing barriers and enablers is facilitated with theory and evidence-based tools that list factors influencing care processes, such as the Theoretical Domains Framework (TDF) (27,31,32). It comprises a set of domains representing modifiable individual, socio-cultural, and environmental factors that can influence behaviour in healthcare contexts (33). The domains can be used to (I) inform targeted questions to ask your stakeholders/actors about barriers and enablers; (II) guide observations or documentation/literature reviews; and/or (III) map any of the information you gather to help identify key influences. Table 3 outlines the 14 domains, with some example barriers relevant to laboratory testing. Choosing Wisely Canada’s ‘Using Blood Wisely’ initiative provides an example of using the TDF to structure questions to identify key barriers to change (34). More detailed guidance on using the TDF is available elsewhere (27).
Table 3
| Example barriers to change | Relevant TDF domain label & description |
|---|---|
| Clinicians are not aware of updated evidence base which demonstrates that the test is low-value | Knowledge: existing procedural knowledge, knowledge about guidelines, knowledge about evidence |
| Clinicians have not been trained in discussing harms and downstream impacts of over-testing with patients | Skills: competence in and abilities to perform the relevant procedural techniques |
| Clinicians have a strong sense of medical obligation to do all they can for the patient, which includes ordering this test | Social/professional role and identity: extent to which the practice is something the individual thinks they are supposed to do as part of their role; boundaries between professional groups |
| Clinicians are less confident in their ability to identify deteriorating patients without ordering the test | Beliefs about capabilities: individuals’ perceptions about their competence and confidence/self-efficacy |
| Clinicians are not hopeful that an initiative to modify testing patterns will be effective | Optimism: the outlook that things will happen for the best or that desired goals will be attained |
| Clinicians believe that the risks of not ordering the test outweigh the benefits | Beliefs about consequences: perceptions about positive and negative outcomes |
| Clinicians missed something in a patient once, now order the test indiscriminately | Reinforcement: the impact of previous experiences |
| Clinicians do not have strong intentions to reduce their ordering of the test | Intentions: a conscious decision to act in a certain way |
| Reducing testing is not a high-priority goal compared to other patient care goals | Goals: mental representations of end states that an individual wants to achieve (priorities, importance) |
| Clinicians order the tests habitually/as part of the clinical routine | Memory, attention and decision processes: ability to retain information, focus selectively and choose between two or more alternatives; extent of forgetting; impact of routines and habits |
| It is logistically very easy to order the test or add it to a previous order | Environmental context and resources: influence of any circumstance of the situation or environment (e.g., organizational, physical, financial) |
| Clinicians order the tests they know a senior supervising colleague will ask for the results of | Social influences: interpersonal processes; external influence from others; views of other professions, patients, families |
| Clinicians feel overwhelmed, ordering the test streamlines care processes | Emotion: influence of feelings, moods, affect (positive or negative) |
| Clinicians do not self-monitor their test ordering patterns or use any strategies to help reduce their testing | Behavioural regulation: strategies that individuals use to self-monitor/manage/change their practice |
Domain labels and descriptions published by Cane et al., 2012 (32). TDF, Theoretical Domains Framework.
Regardless of the approach taken to identifying barriers/enablers, it is typically not possible or feasible to address them all in one initiative. They will likely have to be prioritized. It may be helpful to discuss prioritization within the project team and wider stakeholders as appropriate. Questions to consider which may help with prioritization include (35,36): (I) which barriers/enablers were most commonly reported/identified? (II) Where are there conflicting views (e.g., conflicting comments about which professional groups are responsible for the test ordering)? (III) Which factors seem to be (or are reported as) most strongly enabling change or preventing change? (IV) Which barriers/enablers could potentially be grouped together and targeted with a similar type of change strategy? (V) Which barriers/enablers are easiest or most feasible to address in the current context?
Data-driven approaches to developing solutions
Whilst there has been substantial research into the effectiveness of different implementation strategies, none work all of the time. Taking time to think through and select the strategies which are best placed to capitalize on identified enablers of change and/or break down barriers to change will increase chances of success and help to minimize the waste of resources (time, energy, effort) that can occur using trial-and-error approaches which may not lead to change. Indeed, Rubinstein and colleagues noted in their review of laboratory test utilization initiatives that “A comprehensive LTU approach should evaluate the merit of planned test utilization practice interventions … prior to implementation” (14). Selection of strategies can be informed by a range of existing theory- and evidence-informed resources, including (I) taxonomies of implementation strategies; (II) evidence synthesis for specific implementation strategies; and/or (III) tools which support mapping of identified barriers/enablers to strategies best placed to address them (37). These resources can be used to inform project team discussions that form the basis of solution development.
Taxonomies
Taxonomies are formal classification systems. In recent years, implementation and behavioural scientists have focused on developing taxonomies which list and describe different types of change strategies and provide examples of how they can be used. These can provide novel ideas and support consideration of a broader range of potentially helpful strategies. They also provide a shared language for describing strategies that can improve clarity and support future replications or scale and spread of successful strategies (e.g., to different departments/sites). Two commonly used taxonomies in healthcare change work are: the Cochrane Effective Practice and Organization of Care (EPOC) taxonomy (18) and the Expert Recommendations for Implementing Change (ERIC) taxonomy (19). The Behaviour Change Techniques Taxonomy Version 1 (BCTTv1) (28), whilst operating at a different level of specificity than the EPOC and ERIC taxonomies, is also often used. The BCTTv1 is currently being updated and transformed into an ontology (a classification system that includes relationships between entities) (38). The taxonomies can be used together: generally speaking, the EPOC and ERIC taxonomies contain high-level strategies that are defined more broadly (e.g., continuing education meetings/workshops), whilst the BCTTv1 lists behaviour change techniques (BCTs) that can be embedded within those broader strategies (e.g., instruction on how to perform the behaviour; information about health consequences; demonstration of the behaviour; information about others’ approval; behavioural practice/rehearsal; goal setting; and/or problem solving). However, some ERIC strategies are more granular than BCTs (39). It can be helpful to read over these taxonomies and discuss potentially useful strategies as part of the solution development process.
Evidence syntheses
There are many published evaluations of implementation strategies. Much of this evidence has also been synthesised, to draw broader conclusions about the effectiveness of different types of strategies. These syntheses can also be helpful for informing your strategy selection. Due to the sheer number of evidence syntheses produced and their varying quality, it can be helpful to initially consider the syntheses produced by Cochrane, a global independent network focused on producing high-quality, relevant, up-to-date systematic reviews to inform health decision making (40). The Cochrane Library contains systematic reviews of strategies designed to improve healthcare systems and healthcare practice (41), and can be searched for reviews on specific strategies. Some reviews also identify contextual factors that can enhance the effectiveness of specific implementation strategies. For example, audit & feedback is most effective when the feedback is relayed by a supervisor or colleagues, is provided more than once, is delivered in both verbal and written formats, and when it includes both explicit targets and an action plan (42). Reviews of different types of strategies specifically evaluated in a laboratory stewardship context can also be consulted (11,14). Considering these multiple sources of evidence may help strengthen the rationale for selecting specific approaches: for example, giving feedback repeatedly is also suggested to improve effectiveness of audit & feedback for laboratory test ordering outcomes specifically (14). Where a laboratory stewardship project team has a shortlist of a few different types of potential implementation strategies, consulting the evidence base can help with deciding which ones to take forward, and with designing the selected strategies in such a way to maximize their effectiveness.
Mapping tools
Tools exist which can help with mapping identified enablers of change and/or barriers to change to the specific strategies which are best placed to address them (25,29,43,44). Here we introduce the Theory and Techniques Tool (43), because it specifically links to the framework introduced in the previous section on identifying barriers/enablers (i.e., the TDF) and one of the taxonomies introduced above (BCTTv1). This tool was developed based on a synthesis of results from a literature review and an expert consensus study (29). It comprises a matrix of 74 BCTs and 26 mechanisms of action (which include the 14 TDF domains). The matrix includes green colour-coding to identify techniques which may be more likely to help overcome specific barriers. As an illustrative example, Table 4 outlines example barriers related to over-use of laboratory testing, how they map to TDF domains, potentially helpful BCTs identified from the Theory and Techniques Tool, and an example of how to use the BCT.
Table 4
| Barrier to change | TDF domain | Potentially helpful BCT | Example of how to use the BCT |
|---|---|---|---|
| My colleagues are doing this testing, and so I do the same as them | Social influences | 6.2. Social comparison | Provide audit & feedback reports which compare physicians’ ordering practices with their top-performing colleagues |
| Our clinical leaders have not raised any issues with levels of testing | Social influences | 6.3. Information about others’ approval | Have clinical leaders advocate for and champion the appropriate practice, and have all initiative communications signed by them |
| I order these tests habitually/as part of the clinical routine | Memory, attention and decision processes | 7.1. Prompts/cues | Add a best practice alert in the CPOE which prompts physicians to reconsider orders for specific tests in specific patient groups |
| I don’t consciously order these tests: they are ordered automatically as part of an order set and I can’t de-select them | Environmental context and resources | 12.1. Restructuring the physical environment | Remove the test from the order set |
Choosing between intervention strategies
There are often multiple possible techniques or strategies that can be used to address a barrier/enabler, and multiple ways in which those techniques and strategies can be put to use. In addition, specific laboratory stewardship strategies are more or less feasible to implement in specific contexts depending on aspects such as organizational structures or budgetary considerations (14). The APEASE criteria (Acceptability, Practicability, Effectiveness, Affordability, Side effects, Equity) (25) can be used to help select between multiple options (Table 5). Potential options can be rated against each of these criteria to help with decisions about which to take forward. This can be done with stakeholders using formal ranking/consensus processes such as the Delphi technique, which involves multiple rounds of rating and feedback (45). APEASE can also be integrated into an iterative process whereby initiative teams work out what can be done given potential delivery mechanisms and contexts: for example, the criteria can be used to frame stakeholder discussion about different options.
Table 5
| APEASE criteria | Description |
|---|---|
| Acceptability | How acceptable is it to all key stakeholders? |
| Practicability | Can it be implemented as designed within the intended context, material and human resources? |
| Effectiveness | How effective and cost-effective is it in achieving desired objectives in the target population? |
| Affordability | How far can it be afforded when delivered at the scale intended? |
| Side effects | How far does it lead to unintended adverse or beneficial outcomes? |
| Equity | How far does it increase or decrease differences between advantaged and disadvantaged sectors of society? |
APEASE criteria published by Michie et al., 2014 (25). APEASE, Acceptability, Practicability, Effectiveness, Affordability, Side effects, Equity.
Distinguishing the ‘what’ from the ‘how’ of implementation strategies
A key part of intervention development is deciding exactly how selected strategies will look in the specific context in which they will be deployed. For example, if the aim is to target identified gaps in knowledge, what specific content should be developed to address those gaps, who will deliver it, and when, where, and how will it be delivered? Alternatively, if a prompt will be added to the CPOE, how should it be phrased, and how can it be designed to minimize additional cognitive load (i.e., be appropriately attention-grabbing and facilitate quick decision making without overloading the clinician with too much information)? The results of the barriers/enablers assessment should help with this, since this will have supported the laboratory stewardship project team with identifying very specific issues relevant to the target group that can inform exactly how to put the selected strategies into use. When designing the initiative, it is important to distinguish the ‘what’ and the ‘how’ of change strategies (37). The ‘what’ is the content that is anticipated to lead to change, and the ‘how’ is the mode of delivery of that content. It is advisable to start with the ‘what’, and then consider the best way to package this content. For example, if specific knowledge gaps are identified in the target clinical group during the barriers assessment, content which specifically targets these can be developed (i.e., the ‘what’), which could be delivered via an educational session, or physical educational materials, or embedded within best practice alerts or an audit and feedback report (i.e., the ‘how’). Or, if a specific issue with the ordering process in the CPOE is identified, there may be multiple ways in which the CPOE environment can be changed to target that issue. There are no hard-and-fast rules for working up intervention content, and it is typically a somewhat iterative process involving input from key stakeholders who will impact or be impacted by the change.
Evaluation of interventions
While this article primarily focuses on understanding laboratory stewardship problems and developing solutions (steps 2 and 3 in the laboratory stewardship approach introduced previously), a brief discussion of evaluation methods is warranted. Approaches used in implementation science have much to offer the field of laboratory stewardship in this regard. Laboratory stewardship interventions are typically complex and multifaceted and deployed in real-world environments as opposed to tightly-controlled experimental contexts, which can make evaluation challenging. For example, although RCTs are considered the gold standard evaluation methodology (46), they are not always feasible in quality improvement contexts given the resource and logistical constraints. Where RCTs are used, recently published guidance on designing and undertaking randomized trials of implementation interventions should be informative (47). Where an RCT is not feasible, an interrupted time series (ITS) design serves as a robust, quasi-experimental approach to evaluation (48). With an ITS approach, repeated observations of a particular outcome are collected over time (for example, monthly volumes of electrolyte panels ordered per inpatient day) and divided into two segments: one before the interruption (i.e., the time point at which the intervention was deployed), and one after (49). A segmented regression analysis can then be applied to the data to determine the immediate and longer-term impact of the intervention. ITS models are more robust than other quasi-experimental methods (e.g., before-and-after studies), as they focus on calculation of intervention effects over-and-above any secular trends (i.e., changes in outcomes that would have happened had there been no intervention) (50).
Whilst RCTs and ITS designs focus on establishing whether an intervention works, process evaluation approaches can be used to help understand how or why an intervention works (or does not work). Process evaluations can focus on understanding aspects of the implementation process (e.g., fidelity—to what extent was the intervention deployed as intended?), mechanisms of action (e.g., does an intervention designed to operate via knowledge actually increase knowledge?), and/or the context in which the initiative is deployed (e.g., why does intervention effectiveness vary depending on different ways that clinical teams work together across different wards?) (47,51). A range of methodologies can be used to fulfill process evaluation aims, including surveys, observations, interviews, and documentation analysis (51). Broader guidance is also available for developing and evaluating complex interventions (52). Taken together, the systematic and robust evaluation approaches used in implementation science can be easily transferred to laboratory stewardship contexts and used to provide a more fulsome understanding of stewardship intervention effectiveness.
Strengths and limitations
This article presents current evidence and approaches from implementation science which can be embedded into laboratory stewardship programs to support rigorous development and evaluation of stewardship initiatives. We have focused on behaviour change-informed approaches, which conceptualize efforts to close a gap between best evidence and current practice in a healthcare setting as involving at least one person at some level in the healthcare system doing something—i.e., behaving—differently. It should be noted that there are other theories, models, frameworks, and approaches developed or applied within implementation science not addressed here which may provide different perspectives on quality improvement problems and be informative for this work (53,54).
Discussion
Laboratory stewardship has become an important focus in healthcare. Stewardship consists of identifying areas of over or under use and involves multidisciplinary strategies for implementing interventions that have far ranging implications for patient care, healthcare finances, as well as environmental and health human resource sustainability. Effective laboratory stewardship relies on data-driven methodologies, which are fundamental in the field of implementation science. Implementation science uses evidence-based methods to promote the systematic uptake of research findings and best practices into routine healthcare and public health, with the overall aim of improving quality and effectiveness (17). Implementation science is rooted in using data to (I) understand factors contributing to evidence-practice gaps; (II) identify behaviors that need to be changed; (III) recognize the enablers of change and barriers to change; and (IV) select the appropriate intervention strategies. Thus, implementation science is by nature data-driven and highly relevant to laboratory stewardship, offering many evidence-based tools and approaches to design, guide, and facilitate effective interventions, as described in this article.
In the context of an appropriately resourced and supported stewardship structure, the initial step is to develop in-depth understanding of the problematic behaviour(s). This understanding is achieved by outlining the behaviour(s) that need to be changed, and answering the question, ‘Who needs to do what, differently?’ (21). Different strategies can be used to identify the relevant behaviours, such as reviewing clinical guidelines, local laboratory utilization audits, direct observations of ordering practices and processes, and discussions with key stakeholders. This information can then be organized using the AACTT framework, which systematically categorizes behaviours in terms of Action, Actor, Context, Target, and Time (26).
Upon identifying the target behaviour, the next step involves understanding what needs to change in the person or environment to achieve the desired behaviour. This can include gathering key stakeholders’ thoughts and experiences, conducting structured observations, reviewing relevant procedures, and considering literature on known barriers to change. To assist in organizing the identified barriers and enablers, stewardship programs can use tools such as the TDF. The TDF is a comprehensive framework designed to identify and understand the psychological and environmental factors that influence behaviour (27,31,32). The TDF consists of 14 domains, each representing a key aspect of behaviour, such as knowledge, skills, emotions, beliefs about capabilities, and environmental context and resources. By using such frameworks, stewardship leaders can systematically identify barriers to behaviour change, thereby aiding the design of intervention strategies.
When selecting intervention strategies, it is important to consider various theoretical and evidence-informed resources, including taxonomies of implementation strategies, evidence syntheses, and mapping tools. Taxonomies like the Cochrane EPOC, ERIC, and BCTTv1 can provide a range of potential strategies (18,19,28). Evidence syntheses, particularly those conducted by Cochrane, can provide valuable insights into the effectiveness of various strategies, while mapping tools such as the Theory and Techniques Tool can link identified barriers/enablers to strategies best placed to address them (41,43). To choose among multiple options, the APEASE criteria (Acceptability, Practicability, Effectiveness, Affordability, Side effects, Equity) can be used, involving formal ranking/consensus processes or stakeholder discussions framed using these criteria (25). Ultimately, any intervention requires a holistic and collaborative approach with consideration of site-specific issues and limitations, but the outcomes are more likely to be achieved with adoption of known, evidence-based strategies for behaviour change.
The final step in developing interventions involves deciding how the chosen strategies will be implemented in a specific context. This involves distinguishing between the ‘what’ and the ‘how’ of change strategies, wherein the ‘what’ is the content that leads to change, and the ‘how’ is the mode of delivery of that content. The design process should be iterative, involving key stakeholders who will impact or be impacted by the change.
Whilst the examples provided in this article focus primarily on supporting change in the behaviours of clinicians involved in the test ordering process, it is important to reiterate that the multiple actors that are involved in the testing process more broadly should be identified and their potential role in the initiative considered at the outset of initiative planning. This may include hospital administrators, clinical biochemists, hematologists, microbiologists, laboratory managers, laboratory technologists, and patients. In some instances, these actors may themselves be targets for intervention. In others, they could play a key role in intervention delivery, or could serve on the project team responsible for developing and evaluating the initiative.
While implementation science has to date not been widely used in laboratory stewardship initiatives, there is widespread use and advocacy for quality improvement methodology (55,56). Quality improvement methods, which tend to focus on processes and systems, can be highly useful for helping to close a specific local gap in quality, such as under- or over-use of tests. Quality improvement projects may be augmented with integration of implementation science approaches for specific purposes, including to (I) determine barriers to change using existing literature and locally-gathered data; (II) design an intervention and develop the associated theory of change using evidence-supported theories and associated change techniques; and (III) support the spread of successful projects elsewhere by facilitating fulsome description of all of these elements of the initiative (57). From an evaluation and statistical perspective, integration of ITS analyses offers an opportunity to strengthen the analyses approaches typically used in quality improvement projects (58).
Conclusions
In summary, to effectively implement laboratory stewardship interventions, it is crucial to understand the underlying problem, identify the behavioural changes needed, acknowledge the barriers to these changes, and employ practical, evidence-based intervention strategies. Systematic intervention development in this manner should then be followed by rigorous intervention evaluation. All of these aspects, from problem definition, intervention design, implementation delivery, and evaluation can be well-informed by implementation science principles and driven by associated data.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lee Schroeder) for the series “Data-Driven Laboratory Stewardship” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Peer Review File: https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-46/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-46/coif). The series “Data-Driven Laboratory Stewardship” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhi M, Ding EL, Theisen-Toupal J, et al. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One 2013;8:e78962. [Crossref] [PubMed]
- Thomas RE, Vaska M, Naugler C, et al. Interventions at the laboratory level to reduce laboratory test ordering by family physicians: Systematic review. Clin Biochem 2015;48:1358-65. [Crossref] [PubMed]
- Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem 1996;42:813-6. [Crossref] [PubMed]
- Auditor General of Ontario. Laboratory Services in the Health Sector (Ministry of Health and Long-Term Care). Office of the Auditor General of Ontario. 2017. [Cited 2019 Mar 27]. Available online: http://www.auditor.on.ca/en/content/annualreports/arreports/en17/v1_307en17.pdf
- Naugler C, Wyonch R. What the Doctor Ordered: Improving the Use and Value of Laboratory Testing. C. D. Howe Institute. 2019. [Cited 2019 Oct 1]. Available online: https://www.cdhowe.org/public-policy-research/what-doctor-ordered-improving-use-and-value-laboratory-testing
- Lopez JB, Badrick T. ad hoc IFCC Panel on the Environmental Responsibility of Clinical Laboratories, et al. Proposals for the mitigation of the environmental impact of clinical laboratories. Clin Chem Lab Med 2012;50:1559-64. [Crossref] [PubMed]
- Gordon IO, Sherman JD, Leapman M, et al. Life Cycle Greenhouse Gas Emissions of Gastrointestinal Biopsies in a Surgical Pathology Laboratory. Am J Clin Pathol 2021;156:540-9. [Crossref] [PubMed]
- McAlister S, Barratt AL, Bell KJ, et al. The carbon footprint of pathology testing. Med J Aust 2020;212:377-82. [Crossref] [PubMed]
- Plebani M, Laposata M, Lippi G. Driving the route of laboratory medicine: a manifesto for the future. Intern Emerg Med 2019;14:337-40. [Crossref] [PubMed]
- Dickerson JA, Fletcher AH, Procop G, et al. Transforming Laboratory Utilization Review into Laboratory Stewardship: Guidelines by the PLUGS National Committee for Laboratory Stewardship. J Appl Lab Med 2017;2:259-68. [Crossref] [PubMed]
- Kobewka DM, Ronksley PE, McKay JA, et al. Influence of educational, audit and feedback, system based, and incentive and penalty interventions to reduce laboratory test utilization: a systematic review. Clin Chem Lab Med 2015;53:157-83. [Crossref] [PubMed]
- Froom P, Barak M. Cessation of dipstick urinalysis reflex testing and physician ordering behavior. Am J Clin Pathol 2012;137:486-9. [Crossref] [PubMed]
- Lewandrowski K, Bailey E, Dhanak E, et al. Mandatory laboratory consultation: how one hospital’s program reduced overuse of cardiac tests. Lab Med 1994;25:460-3. [Crossref]
- Rubinstein M, Hirsch R, Bandyopadhyay K, et al. Effectiveness of Practices to Support Appropriate Laboratory Test Utilization: A Laboratory Medicine Best Practices Systematic Review and Meta-Analysis. Am J Clin Pathol 2018;149:197-221. [Crossref] [PubMed]
- Lam JH, Pickles K, Stanaway FF, et al. Why clinicians overtest: development of a thematic framework. BMC Health Serv Res 2020;20:1011. [Crossref] [PubMed]
- Nilsen P, Birken SA. Prologue. 2020. [Cited 2021 Oct 28]. Available online: http://www.elgaronline.com/view/edcoll/9781788975988/9781788975988.00006.xml
- Eccles MP, Mittman BS. Welcome to Implementation Science. Implementation Science 2006;1:1. [Crossref]
- Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy. 2015. [Cited 2021 Jan 8]. Available online: http://epoc.cochrane.org/epoc-taxonomy
- Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [Crossref] [PubMed]
- Patey AM, Fontaine G, Francis JJ, et al. Healthcare professional behaviour: health impact, prevalence of evidence-based behaviours, correlates and interventions. Psychol Health 2023;38:766-94. [Crossref] [PubMed]
- French SD, Green SE, O'Connor DA, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci 2012;7:38. [Crossref] [PubMed]
- Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof 2006;26:13-24. [Crossref] [PubMed]
- Grimshaw JM, Patey AM, Kirkham KR, et al. De-implementing wisely: developing the evidence base to reduce low-value care. BMJ Qual Saf 2020;29:409-17. [Crossref] [PubMed]
- Skolarus TA, Sales AE. Implementation issues: towards a systematic and stepwise approach. In: Complex Interventions in Health: An Overview of Research Methods. London: Routledge; 2015:291-8.
- Michie S, Atkins L, West R. The behaviour change wheel. A guide to designing interventions. 1st ed. Great Britain: Silverback Publishing; 2014.
- Presseau J, McCleary N, Lorencatto F, et al. Action, actor, context, target, time (AACTT): a framework for specifying behaviour. Implement Sci 2019;14:102. [Crossref] [PubMed]
- Atkins L, Francis J, Islam R, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77. [Crossref] [PubMed]
- Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. [Crossref] [PubMed]
- Johnston M, Carey RN, Connell Bohlen LE, et al. Development of an online tool for linking behavior change techniques and mechanisms of action based on triangulation of findings from literature synthesis and expert consensus. Transl Behav Med 2021;11:1049-65. [Crossref] [PubMed]
- Ericksson W, Bothe J, Cheung H, et al. Factors leading to overutilisation of hospital pathology testing: the junior doctor's perspective. Aust Health Rev 2018;42:374-9. [Crossref] [PubMed]
- Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. [Crossref] [PubMed]
- Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7:37. [Crossref] [PubMed]
- Gibson R, D'Annibale M, Oliver N, et al. Exploration of the individual, social and environmental factors influencing dietary behaviour in shift workers with type 2 diabetes working in UK healthcare-The Shift-Diabetes Study: A qualitative study using the theoretical domains framework. Diabet Med 2023; Epub ahead of print. [Crossref] [PubMed]
- Using Blood Wisely. Audit & Intervention. [Cited 2023 Jul 14]. Available online: https://usingbloodwisely.ca/intervention/
- Patey AM, Islam R, Francis JJ, et al. Anesthesiologists' and surgeons' perceptions about routine pre-operative testing in low-risk patients: application of the Theoretical Domains Framework (TDF) to identify factors that influence physicians' decisions to order pre-operative tests. Implement Sci 2012;7:52. [Crossref] [PubMed]
- Patey AM, McCleary N, Presseau J, et al. Chapter 8: Identifying target behaviours and potential barriers to change (Phase 2a). In: Kool T, Patey AM, Van Dulmen S, et al. editors. How to Reduce Overuse in Healthcare: A Practical Guide. 1st ed. Chichester: Wiley Blackwell; 2024.
- Presseau J, McCleary N, Patey AM, et al. Chapter 9: Selecting de-implementation strategies and designing interventions (Phase 2b). In: Kool T, Patey AM, Van Dulmen S, et al. editors. How to Reduce Overuse in Healthcare: A Practical Guide. 1st ed. Chichester: Wiley Blackwell; 2024.
- Marques MM, Wright AJ, Corker E, et al. The behaviour change technique ontology: Transforming the behaviour change technique taxonomy v1. Wellcome Open Res 2023;8:308. [Crossref] [PubMed]
- McHugh S, Presseau J, Luecking CT, et al. Examining the complementarity between the ERIC compilation of implementation strategies and the behaviour change technique taxonomy: a qualitative analysis. Implement Sci 2022;17:56. [Crossref] [PubMed]
- Cochrane. About us. [Cited 2023 Jul 14]. Available online: https://www.cochrane.org/about-us
- Cochrane Library 9174. [Cited 2023 Jul 27]. Available online: https://www.cochranelibrary.com/cdsr/reviews
- Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;CD000259. [Crossref] [PubMed]
- BCT Theory. Welcome to the Theory & Techniques Tool. [Cited 2019 Oct 25]. Available online: https://theoryandtechniquetool.humanbehaviourchange.org/
- Waltz TJ, Powell BJ, Fernández ME, et al. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci 2019;14:42. [Crossref] [PubMed]
- McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38:655-62. [Crossref] [PubMed]
- Eccles M, Grimshaw J, Campbell M, et al. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 2003;12:47-52. [Crossref] [PubMed]
- Wolfenden L, Foy R, Presseau J, et al. Designing and undertaking randomised implementation trials: guide for researchers. BMJ 2021;372:m3721. [Crossref] [PubMed]
- Shadish W, Cook T, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston: Houghton Mifflin; 2002.
- Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13:S38-44. [Crossref] [PubMed]
- Taljaard M, McKenzie JE, Ramsay CR, et al. The use of segmented regression in analysing interrupted time series studies: an example in pre-hospital ambulance care. Implement Sci 2014;9:77. [Crossref] [PubMed]
- Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [Crossref] [PubMed]
- Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. [Crossref] [PubMed]
- Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10:53. [Crossref] [PubMed]
- Birken SA, Bunger AC, Powell BJ, et al. Organizational theory for dissemination and implementation research. Implement Sci 2017;12:62. [Crossref] [PubMed]
- Beriault DR, Gilmour JA, Hicks LK. Overutilization in laboratory medicine: tackling the problem with quality improvement science. Crit Rev Clin Lab Sci 2021;58:430-46. [Crossref] [PubMed]
- Eaton KP, Levy K, Soong C, et al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med 2017;177:1833-9. [Crossref] [PubMed]
- Ovretveit J, Mittman BS, Rubenstein LV, et al. Combining Improvement and Implementation Sciences and Practices for the Post COVID-19 Era. J Gen Intern Med 2021;36:3503-10. [Crossref] [PubMed]
- Fretheim A, Tomic O. Statistical process control and interrupted time series: a golden opportunity for impact evaluation in quality improvement. BMJ Qual Saf 2015;24:748-52. [Crossref] [PubMed]
Cite this article as: McCleary N, Brehaut J, Grimshaw JM, McCudden C. Data-driven laboratory stewardship: an implementation science perspective. J Lab Precis Med 2024;9:8.