Investigative algorithms for disorders affecting plasma transaminases (aspartate transaminase and alanine transaminase)—a narrative review
Introduction
Transaminases may be requested in isolation but more commonly as part of ‘liver function tests’ (LFTs) which include a range of tests, some of which are discussed in companion articles in this series. Local LFT profiles may vary and contain either aspartate transaminase (AST) or alanine transaminase (ALT), or both, but are likely to contain at least one other enzyme released from hepatocytes and also biliary epithelium, either alkaline phosphatase (ALP), or gamma-glutamyl transferase (GGT) (Figure 1). Therefore, if transaminitis is identified ALP or GGT activity may immediately be available to help evaluate the pattern.
The aim of this review is to provide a diagnostic strategy, focussing on laboratory tests, for transaminitis in humans. Detailed discussion of liver function scores, investigation of neonatal jaundice and other not directly related conditions are outside of the scope. It is important to modify recommendations according to local disease prevalence and health care resource although it is hoped the general approach will be useful for all developing local pathways. These pathways are not meant to replace national or international guidelines but provide support to clinicians with an inexplicable result and need suggestions for the next step. We present this article in accordance with the Narrative Review reporting checklist (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-64/rc).
Methods
The narrative literature review was created by searching PubMed/MEDLINE, Google Scholar, OMIM and seminal texts. The diagnostic algorithms were then created using the information gathered for the literature review. The literature was searched over the period September 2022 to September 2023. The language was restricted to English. For further information please see supplementary information (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | September 2022 to September 2023 |
| Databases and other sources searched | PubMed/MEDLINE, Google Scholar, OMIM |
| Search terms used | Transaminitis, ALT, AST, rhabdomyolysis, diagnosis, investigation, causes, aetiology, human |
| Timeframe | From database inception to September 2023 |
| Inclusion criteria | All papers and reviews included were restricted to English |
| Selection process | Both authors conducted initial searches, with refinement by both authors to obtain consensus and agreement |
| Additional considerations | Seminal texts were also searched and the references of important articles and texts were obtained and checked for relevance |
Transaminases
Transaminases, or aminotransferases, catalyse the transfer of an amine for a keto group with the coenzyme pyridoxal phosphate (active form of vitamin B6), and are hence important in the synthesis of amino acids and in protein and carbohydrate metabolism. The commonest measured protein enzymes of this family in human sera are AST and ALT and in this review, “transaminase” will refer to these two enzymes. The term “transaminitis” will refer to elevated activity in one or both enzymes. AST is also called glutamic oxalo-acetic transaminase (GOT) and ALT glutamic pyruvic transaminase (GPT) in the literature.
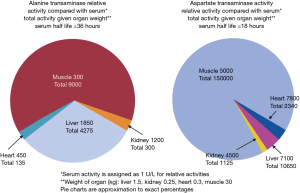
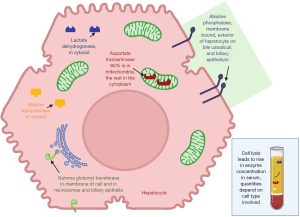
In health the serum transaminase activity is relatively constant representing the balance of release from cell turnover and clearance. The utility of transaminase activity to demonstrate organ pathology depends on the activity of the transaminase in the relevant tissue of the body (see Figure 2) (1-3). As can be seen in Figure 2 the activity of ALT is highest in the cells of the liver (hepatocytes), and AST in both the heart and hepatocytes, therefore hepatocellular disruption can be detected by an increase in either transaminase activity. However, there is more striated muscle by weight in the body so the majority of transaminase activity is actually located in muscle. Renal sources are less likely in the differential diagnosis for a transaminitis as even complete organ infarction will release relatively little enzyme, in comparison with minor liver insults, as organ mass is influential on the actual circulating activity.
Haematocrit is correlated with transaminase activity, for example situations such as smoking or living at altitude will lead to an increase (4,5). Figure 2 demonstrates the organs with the highest tissue transaminase activity but is an incomplete list, for example AST activity (U/g organ) of other tissue includes (note Figure 2 uses kg not g) (6):
- 15 in brain.
- 3 in pancreas.
- 1 in lung.
- 0.8 in erythrocytes.
As can be seen in Figure 2 the AST:ALT ratio in liver is 2.5:1 but often the reference intervals for both enzymes are similar, if not identical, e.g., <40 IU/L. This is due to two main reasons, firstly the much more rapid clearance of AST by the liver, with a half-life of 18 hours compared with 36 hours for ALT (7). Secondly ALT is limited to the cytoplasm of hepatocytes but AST is mostly located in the mitochondria (80%) (Figure 1). Normal enzyme loss is mainly from cytoplasm leakage and it is not until hepatocellular death occurs before the mitochondrial AST is released into the serum (3,7).
Laboratory measurement
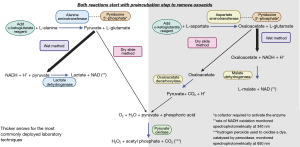
The most common form of analysis involves enzymatic assays [see Figure 3 (8,9)], meaning the activity of the enzyme is measured in the specimen, not the concentration, and therefore cofactor deficiency (pyridoxal phosphate) can affect measured activity resulting in a spuriously lower activity (1). Manufacturers provide enzymatic assays with or without pyridoxal phosphate supplementation. The addition of pyridoxal phosphate can increase enzyme activity in the serum specimen (and the cost of the assay) as it theoretically maximises transaminase measurement in malnourished populations, but data is conflicting, so the cheaper cofactor absent assay formulations are commonly used (1,10-12). Reference intervals therefore depend on assay design as well as the local population and the rate of occult liver disease, e.g., obesity and infection incidence.
Specimen collection
Pneumatic tube systems, for delivery of phlebotomy samples to laboratories, have been shown to cause elevation of lactate dehydrogenases (LDH) but not transaminases (13). Time to analysis, storage temperature and tube type can affect transaminase activity, therefore it is important to follow local laboratory guidelines and to record the time of venesection accurately (14).
Spurious causes
Haemolysis can cause spurious elevation of transaminase activity, particularly AST, if caused by in vitro haemolysis upon venesection which is of no physiological relevance in comparison to in vivo haemolysis (5). The pattern of other measurements may give a clue to in vitro haemolysis for example bilirubin is produced by in vivo metabolism of the haemoglobin (however the bilirubin assay is also affected by the colour change caused by haemolysis so is an imperfect marker) (15).
Macro enzymes are likely under recognised and represent spuriously increased enzyme activity due to reduced clearance of the enzyme rather than increased production (16-18). Serum components adhere to the transaminases e.g., immunoglobulin, and therefore macrotransaminases can be seen in the context of previous organ damage or autoimmune disease (17,18).
Calculations using transaminases
First identified by De Ritis, the ratio of AST to ALT, also known as the De Ritis ratio, may help identify the cause or severity of liver disease (1,7). An isolated elevation of AST indicates a non-liver source, reduced clearance e.g., macroAST, or increased production from red cells (in vivo or in vitro haemolysis) or another organ rich in mitochondria e.g., striated muscle pathology (7). Rhabdomyolysis will result in release of muscle AST and, to a lesser extent, ALT however creatine kinase (CK) is released far in excess of either. It is also worth noting that AST (and CK) may be elevated before ALT and that AST and ALT will persist for longer once the CK has resolved, ALT often remaining elevated once CK and AST normalise (19). The ratio of CK to ALT has been studied e.g., median CK/ALT was 37 in rhabdomyolysis group versus 5 in paracetamol (acetaminophen) overdose (20).
The R ratio, the degree of elevation of one transaminase versus the degree of elevation of ALP activity, has been used to indicate the possible drug causes of hepatitis versus cholestatic liver injury (21). These ratios would have to be validated with local assays and population prior to use but illustrate some of the expected differences in enzyme activity based upon the organ affected and type of pathology. It may also be appropriate to limit the use of both transaminases as first line tests and restrict the less specific AST for example to situations where the ALT activity has already been demonstrated to be abnormal (22).
An isolated elevation of ALT can be indicative of the time course of liver disease as ALT half-life is longer than AST (36 versus 18 h) (7). When the De Ritis ratio was first developed other diagnostic tests were considerably less advanced and other tests, including viral screens and fibrosis markers, have replaced some of the ratio’s functions (1,7). A ratio <1 for example can indicate a viral or obesity-related cause of hepatitis but a raised ratio (>2) can suggest fibrosis or alcohol induced hepatitis (7,23).
Fibrosis scores
Liver biopsies were traditionally used to determine how much a liver has been affected by an underlying pathology but sampling error, complications, and lack of resource has led to the development of many other methods (involved demographic details, blood tests and imaging) (24,25). One of the better known is the FIB4 index developed in those with hepatitis C (HCV) and human immunodeficiency virus (HIV) coinfection to stage the liver disease and avoid liver biopsy (25). FIB4 has since been validated in other populations including those with fatty liver and chronic hepatitis B infection, where it performs better than other scores and is cost efficient (24,26).
Low transaminases
The reference intervals of transaminases can vary between laboratories, but low concentrations can be entirely normal so in the fit and healthy rarely any further investigations need considering. Indeed, regular exercise seems to lower the transaminases (27) as does the oral contraceptive pill and hormone replacement therapy (28). Smoking has been shown to be inversely correlated with transaminase activity in one small study (29) but the opposite was demonstrated in a much larger data set (30). In those who are unwell B6 (pyridoxine) deficiency is associated with low transaminase activity although this is an uncommon vitamin deficiency occurring in those with problem drinking for example (31-33). Chronic kidney disease is associated with low transaminase activity, partly due to low pyridoxine concentration, higher homocysteine concentration and haemodilution (34).
Rare causes
No genetic diseases have yet been associated with an isolated ALT metabolism. There is a rare genetic variation in the Amish population resulting in reduced AST activity, but with no apparent clinical sequalae, due to mutations in the GOT1 gene (gene that produces AST) (35).
High transaminases
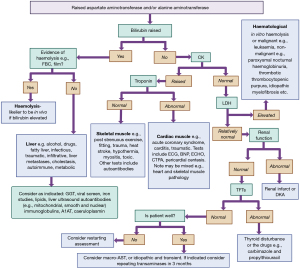
Due to the variety of tissues in which the transaminases are expressed, there are many possible causes of a transaminitis (Figure 2). Investigation will depend on a good history and clinical examination even if apparently asymptomatic (36). Liver pathology is the commonest cause of a transaminitis. One study demonstrated, that after liver causes are excluded from a population of patients with a high transaminase activity, then the next commonest tissue sources are: skeletal muscle (54%), cardiac muscle (39%) and haematological (7%) (37). Liver therefore predominates this and other diagnostic algorithms but the other systems will be touched on and included in a presented algorithm (Figure 4).
Liver
Any disease process that causes liver damage will cause a transaminitis. Common causes include central adiposity, steatotic liver disease (SLD) or metabolic dysfunction associated steatotic liver disease (MASLD) and alcohol, including in children (38,39). Liver fibrosis is a slow process but transient transaminitis is relatively common, therefore in asymptomatic low risk individuals it is suggested to wait 3 months to see if persistent before proceeding with further investigations (23,40). It is important to use this guidance based on local resources, disease incidence and selected reference intervals (which may be set higher to avoid a high incidence of abnormal results) (40,41).
It is recommended that all persistent abnormal LFTs are followed up and investigated and Figure 4 provides a guide on how to approach this (40). It is also important to note that many people may have multiple causes of the liver disease, particularly if progression is unexpectedly rapid, such as obesity, alcohol and chronic infections (42). Although transaminase elevations less than fivefold the upper reference limit are considered mild and above this more likely to represent significant liver injury, very significant liver pathology can exist in those with normal or mildly elevated transaminase activity (23,43).
Cytopenia can be a sign of liver damage. The spleen becomes enlarged secondary to liver disease but hypersplenism (cytopenia and large spleen, the former reversible after splenectomy) can be due to a variety of causes including: (23,44):
- Alcohol;
- Autoimmune; systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis;
- Chronic haemolysis; autoimmune haemolytic anaemia, hereditary spherocytosis, thalassaemia;
- Infection; viral, chronic syphilis or tuberculosis, brucellosis, malaria;
- Malignant; myeloproliferative, involving spleen e.g., leukaemia or metastases;
- Metabolic; Gaucher, Niemann-Pick;
- Portal hypertension.
Liver damage can result in a decrease in its synthetic ability. Albumin is synthesised in the liver but is also a negative acute phase protein and so is unreliable as a marker of liver disease (see companion article in same series) (40). Clotting factors are also synthesised in the liver so prolonged prothrombin times can indicate liver disease. However measurable coagulopathies are a late finding, requiring loss of more than 70% of liver synthetic function before occurring, and could indicate vitamin K deficiency (or medication effects e.g., coumarins) instead (40).
Although both (gamma glutamyl transferase) GGT and ALP are elevated in liver diseases, particularly those affecting the biliary tree, ALP is not such a useful discriminatory test (see companion article in the same series) in children where GGT is a better indicator of involvement of the biliary tree (40). GGT is in fact the more sensitive marker of biliary tract disease compared to ALP (45). However, GGT can be induced by various drugs, e.g., phenytoin, barbiturates and alcohol (45,46).
Excess weight storage as fat in the liver, exacerbated by risk factors, causes SLD which can lead to inflammation, MASLD, fibrosis and cirrhosis (47,48). This common chronic liver disease can be associated with normal or elevated ALT activity (47). The gold standard for diagnosis is a liver biopsy however ultrasound can detect steatosis with exclusion of any other cause of liver disease via assorted biomarkers, alcohol history (alcohol initially causes fatty changes in the liver), plus scores, such as FIB4 mentioned above, or transient elastography used to predict severity (49). The cost effectiveness of identifying cases remains to be proven given the current lack of definitive treatments (40). Obstructive sleep apnoea in patient with the metabolic syndrome cause an elevation of the transaminase activity which is worse in the more hypoxic (50).
Alcohol related liver disease (ALD) is a common cause of liver disease, second only to HCV in the USA, and should require only an alcohol history if patient is forthcoming (42,51). ALD is related to quantity and duration of alcohol intake as well as various risk factors such as female sex, smoking, genes, haemochromatosis and HCV (51). Excess alcohol intake for approximately 5 years or more is usually required for liver disease to develop, but risk factors may precipitate significant disease earlier or at lower intake (42,51). Other features that may indicate alcohol excess when history is contentious include mean cell volume (MCV) and GGT, which may both be elevated (23).
A multitude of drugs have been associated with liver disease, however many associations lack robust evidence and due to the frequency of transient transaminitis, obesity, and alcohol use for example it may be that many drugs are only a co-factor at best. For example, there is increasing evidence that methotrexate does not commonly cause liver fibrosis (instead causes a transient and self-resolving acute hepatitis and transaminitis) and hence resulting in calls to change practice in regard to monitoring liver health (48). Therefore the practice to measure amino terminal type III procollagen peptide (P3NP) has become historic and replaced by the more sensitive and specific test for the cause of liver disease in people with psoriasis (MASLD or alcohol) such as FIB4 or enhanced liver fibrosis (ELF) test which have considerably higher areas under the receiver operator curve (AUROC) (48).
A transmembrane copper ATPase expressed in the liver, ATP7b, transports copper into the bile for excretion or for synthesis of caeruloplasmin (52). Most serum copper is contained within caeruloplasmin (holocaeruloplasmin, 6 atoms of copper per molecule; Figure 5) and copper is also a vital cofactor for many other enzymes (52). A rare cause of transaminitis is accumulation of copper in organs due to mutations in the ATP7b gene, Wilson disease, which affects approximately 1 in 30,000 (52). Presentation is non-specific, with a wide range of symptoms, possible multi-organ involvement (52) and the classical clinical finding of Kayser-Fleischer rings, which are only visible on slit lamp investigation (and only present in 50% of those with a liver presentation) (53). Most people will present between the ages of 5–35, not as neonates, however first presentations of Wilson disease have been very rarely reported up to the eighth decade (40,53). Copper is transported primarily in caeruloplasmin therefore despite being a condition of copper overload serum total copper and caeruloplasmin concentrations are low, but serum free, liver, and urine copper concentrations are high (Figure 5). Wilson disease can cause a Coomb test negative haemolytic anaemia, rhabdomyolysis and renal failure, as well as encephalopathy and coagulopathy (52). The biochemical pattern can show a markedly raised bilirubin concentration but only marginally raised transaminase activity with a normal or low ALP activity (52).
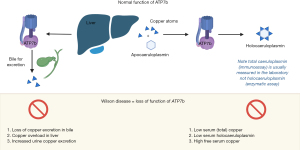
The Leipzig criteria were developed in 2001 as a score combining clinical and laboratory criteria in order to help identify and diagnose Wilson (see Table 2) (54). There are however many limitations of the biochemical tests (53). Immunoassay quantification of caeruloplasmin does not distinguish between apo- and holo-caeruloplasmin (no copper bound and copper bound respectively) and therefore can overestimate caeruloplasmin whereas enzymatic assays only detect holocaeruloplasmin (55). There is also non-commutability and lack of a single pure reference standard for caeruloplasmin (56). The acute phase, including acute liver presentations, causes an increase in caeruloplasmin concentration which can mean the caeruloplasmin may be spuriously normal, also low caeruloplasmin can occur in other conditions such as malnutrition and acaeruloplasminaemia (53). Ranges need to be validated for specific populations and assay methods (57). Urinary copper ranges need to be specific for age and are at risk of false negative (asymptomatic cases) and false positive (cholestasis and nephrotic syndrome) results and diagnostic thresholds are only validated in children (53,57).
Table 2
| Test | Criteria for awarding the points |
|---|---|
| Caeruloplasmin from serum | 1 point <0.2 g/L |
| 2 points <0.1 g/L | |
| Liver copper from liver biopsy (cholestasis absent) | −1 point <50 µg/g dry weight (<0.8 µmol/g) |
| 1 point 50–249 µg/g dry weight (0.8–4 µmol/g) | |
| 2 points >250 µg/g dry weight (>4 µmol/g) | |
| Urine copper (acute hepatitis absent) | 1 point 1–2 times the upper limit of normal |
| 2 points >2 times the upper limit of normal | |
| 2 points normal but >5 times the upper limit of normal after d-penicillamine | |
| Mutations detected | 1 point heterozygote |
| 4 points homozygote/compound heterozygote |
3 points indicates Wilson disease is possible; 4 or more consider Wilson disease as confirmed.
Non-caeruloplasmin bound copper has been suggested as an alternative test for Wilson diagnosis (high in Wilson disease), also known as the free copper index (55). However the majority of laboratories do not have analytical methods robust enough to be able to interpret the results or even calculate it (if caeruloplasmin is below the lower limit of detection), nor is there good agreement on the threshold to indicate Wilson disease presence (55). Direct measurement of free copper may be preferred but is not routinely available (58). Standard LFTs have been shown to be better than copper and caeruloplasmin at identifying Wilson disease in acute liver failure in a small study with no detail on how the LFTs were measured (59). An ALP (IU/L):total bilirubin (mg/dL) ratio <4 with an AST:ALT ratio >2.2 has a near perfect sensitivity and sensitivity (100%) for Wilson disease in those with acute liver disease (59). In summary, testing should be reserved until suspicion is high as there are multiple causes of false negative and positive results and reserved for those older than three years of age (40). At point of testing no single screening test is diagnostic and results should be combined (57).
Alpha-1-antitrypsin (AAT) is a protein synthesised and released by the liver, deficiency of which results in emphysema, liver disease and rarely panniculitis (60). The two common deficiency alleles of this protease inhibitor are PI S and PI Z, mutations in the SERPINA1 gene encoding AAT, causing the rare autosomal recessive condition alpha-1-antitrypsin deficiency (AATD) (60). Primarily the PIZZ homozygous state is associated with the liver sequelae as not only is plasma AAT deficient but the mutation results in accumulation of AAT in the liver, which is pathogenic (60). The liver disease is much rarer than the lung complications presenting either in neonates or in those >50 years old, therefore it is recommended as part of prolonged jaundice or abnormal bleeding screens in neonates or in unexpected cirrhosis in older adults rather than in all patients (40,60-63).
AAT is an acute phase protein which makes isolated concentrations prone to spuriously normal results (64). Once AAT deficiency has been detected, or is suspected despite borderline AAT concentration, then isoelectic focussing to identify the phenotype or genetic testing is warranted (60). The Z allele appears to have originated from southern Scandinavia therefore the highest prevalence occurs in those of northwestern European descent (65). There is low penetrance at birth meaning asymptomatic neonates do not require screening who have parents with known PI Z status (60). Due to the age of presentation of disease, and risk of spurious results, A1ATD should not be screened for as part of first line testing outside of infancy but reserved for specialist testing and it may be less relevant in populations with low disease frequency (40).
Haemochromatosis is a genetic disorder of iron overload (particularly in Northern European populations), and the excess storage in the liver can cause pathology and transaminitis. Ferritin, an iron storage protein, can be used as an indicator of iron metabolism problems (along with transferrin and saturation studies) but note that ferritin is an acute phase protein so can be elevated in a great many different situations (23,40). Guidelines exist for those who need genetic testing for haemochromotosis but could be considered in situations such as either a high ferritin >1,000 µg/L (or in a male with ferritin >300 µg/L, >200 µg/L in a female) or a transferrin saturation >45%; or in a and transferrin saturation >30% (66,67).
When screening, in the UK, antinuclear (ANA), anti-smooth muscle, and mitochondrial, with or without liver-kidney microsomal-1 antibodies and immunoglobulins are recommended when screening for autoimmune hepatitis as infectious diseases are less common (23,40). These immunological investigations should also be considered in those with elevated transaminases in the setting of a pre-existing autoimmune disease, particularly inflammatory disease, as risk of autoimmune hepatitis is higher in these groups. Table 3 shows a simplified diagnostic criteria for the diagnosis of autoimmune hepatitis which has a sensitivity >80% and specificity >95% (68,69). Coeliac disease is recommended to be excluded in paediatric populations, as well as liver kidney microsomal antibodies, but remain important tests to also consider in adults (40). In the presence of cholestatic picture of liver tests ANCA can also be added into the immunology screen (40). A general approach to distinguish autoimmune liver diagnoses are:
- Primary biliary cholangitis has a more cholestatic picture of liver tests plus elevated antimitochondrial antibodies (70);
- whilst autoimmune hepatitis has a hepatitic picture of liver tests and the presence of anti-smooth, liver and kidney antibodies (71);
Table 3
| Variable | Cut off | Points |
|---|---|---|
| Anti-nuclear antibodies or smooth muscle antibodies | ≥1:40 | 1* |
| Anti-nuclear antibodies or smooth muscle antibodies | ≥1:80 | 2* |
| Or liver-kidney microsomal antibodies | ≥1:40 | 2* |
| Or soluble liver antibodies | Positive | 2* |
| IgG | > upper normal limit | 1 |
| >1.1 times upper normal limit | 2 | |
| Liver histology (evidence of hepatitis is a necessary condition) | Compatible with AIH | 1 |
| Typical AIH | 2 | |
| Absence of viral hepatitis | Yes | 2 |
Score of 6 is probably autoimmune hepatitis; a score of ≥7 is definite. *, a maximum of 2 only for any of the antibody results. AIH, autoimmune hepatitis.
and primary sclerosing cholangitis has a cholestatic picture and an elevated IgM and ANCA positivity (72). When screening for a viral cause then HIV, hepatitis B surface antigen and hepatitis C antibody, with reflex polymerase chain reaction (PCR) confirmation, are recommended for the UK population (23,40). Table 4 includes some of the many other infections that can affect the liver and the Centre for Disease Control has good resources for working out which infectious agents need to be considered depending on where you practice, but of course local expertise and guidelines will be good starting places. Infection should always be high on the list for patients who are immunosuppressed or compromised.
Table 4
| Infection | Test |
|---|---|
| Human immunodeficiency virus | Antibody, antibody/antigen tests, nucleic acid test |
| Malaria | Blood smears, antigens, serology, and PCR |
| Mycobacter: tuberculosis | Skin antigen tests, interferon gamma release assay, culture of tissue/fluid samples |
| Herpe family e.g., Epstein Barr | Antigen, antibody, PCR |
| Fungus | Tissue samples for microscopy and culture |
| Parasites | Usually radiologically e.g., ultrasound, CT or MRI scans, or microscopy/PCR of tissue samples (73) |
| Bacteria e.g., abscesses, acute hepatitis or granulomas, including Neisseria sp, Brucella, Treponema and enteric bacteria | Serology, tissue samples, ultrasound or other radiological techniques, blood cultures, antigens, PCR (74) |
PCR, polymerase chain reaction; CT, computed tomography; MRI, magnetic resonance imaging.
Other causes of liver pathology include vascular disease. Cardiac causes of liver disease include heart failure, ischaemia and cardiac valve disease. Investigations required might include imaging [ultrasound scans (USS), Dopplers, echocardiography] or brain natriuretic peptide (BNP) bearing in mind that the cardiac muscle damage itself might also be the source of any transaminitis (see later). Infiltrative processes in the liver will also cause a transaminitis e.g., sarcoid or amyloid whilst traumatic damage to the liver should be diagnosed from history plus or minus examination findings.
Malignancy is a common cause of liver pathology—either primary liver tumour or metastases from a different primary source. Ideally history and examination might provide the clues, but diagnosis often relies on imaging techniques e.g., USS and then biopsy. Biochemical tests are less useful in this situation, with tumour markers more appropriate as disease activity markers rather than diagnostic aids.
Skeletal muscle
A clinical history might elicit symptoms of muscle pain or weakness and dark urine, pointing towards skeletal muscle pathology but diseases such as muscular dystrophy can be pain free. Therefore, there is a rationale for considering CK in children with mild transaminitis. It would be more unusual to find asymptomatic disease in adults. One study showed that 20% of children admitted to hospital with a transaminitis had raised CK indicated a muscle cause (75). Of note CK is a clear and reliable biomarker pointing towards muscle breakdown but does not distinguish between cardiac or skeletal muscle damage, however skeletal muscle has a much higher tissue mass and so rhabdomyolysis leads to much higher CK concentrations compared to myocardial tissue destruction. AST and ALT elevations are part of diagnostic criteria for inflammatory myositis (juvenile or adult) (76,77). Any type of rhabdomyolysis, including infarct, will cause a transaminitis (78-80). Up to 24 hours after a marathon AST can rise up to 4 times its normal value (81). Rhabdomyolysis causes include the list below and there are various guidelines on diagnosis (82-85):
- Excessive muscle contractions e.g., extreme exercise (86) or seizures including neuroleptic malignant syndrome or epilepsy.
- Trauma e.g., crush or burns.
- Ischaemia including compartment syndrome.
- Temperature extremes e.g., heat stroke or hypothermia.
- Toxic e.g., drugs, toxins and venoms (alcohol, benzodiazepines, statins very rarely and associated with SCLO1B1 mutations and snake bites) (87).
- Metabolic and endocrine e.g., hypothyroid, hypokalaemia, hyponatraemia, and diabetic ketoacidosis.
- Infectious e.g., virus, bacteria, parasites (88).
- Autoimmune e.g., polymyositis, dermatomyositis (37).
Myocardium
A predominantly high AST may represent myocardial pathology such as ischaemia, infarction and heart failure, hypertension, myocarditis and Kawasaki (44,89-95) etc. A higher ratio of AST:ALT may therefore lead one to investigate possible cardiac causes of a transaminitis. Further tests like BNP, electrocardiogram (ECG) and echocardiography may all help to identify cardiac causes of a transaminitis (96). One study demonstrated that after an ST elevation myocardial infarction 86% of patients had an elevated AST and 48% had an elevated ALT (97). A list to consider when diagnosing possible cardiac sources of a transaminitis is:
- Acute coronary syndrome including angina.
- Inflammatory e.g., endocarditis, pericarditis, myocarditis.
- Post-operative injury e.g., after coronary artery bypass (37).
Haematological
Haemolysis can cause an elevation in AST, although LDH is elevated to a much greater degree, with LDH:AST ratio of 30 typical in an episode of haemolysis (98). Tumour lysis syndrome is much more associated with other biochemical markers and the transaminases do not form part of the typical biomarkers used in the diagnosis of this condition. Haematological problems may affect the liver and so distinguishing the exact tissue source of a transaminitis might be difficult but a list to consider is:
- Malignant e.g., leukaemia, lymphoma, myeloma.
- Non-malignant e.g., paroxysmal nocturnal haemoglobinuria, thrombotic thrombocytopenic purpura, idiopathic myelofibrosis, myelodysplastic syndrome, haemophagocytic lymphohistiocytosis (37).
Endocrine
Thyroid disturbances might lead to a transaminitis, but it is usually through exacerbating liver pathology or affecting skeletal muscle (99-101). Drugs used in the treatment of hyperthyroidism, e.g., propylthiouracil and carbimazole, can cause a transaminitis due to liver side effects (102). Diabetic comas and ketoacidosis are also situations where transaminitis is a common finding, and higher elevations are associated with a worse prognosis perhaps reflecting tissue ischaemia in multiple organs (103).
Kidneys
Despites kidney tissue containing transaminases renal pathology is rarely a cause of a transaminitis. Indeed patients with renal failure have low transaminase activity as discussed earlier (104). However renal infarction can elevate transaminases (105,106). Acute inflammatory or toxic renal diseases can often overlap with liver diseases and so the tissue source of the transaminases is difficult to separate, and perhaps unnecessary to distinguish as other markers can be used to assess organ damage and treatment response.
Conclusions
Transaminitis is common but may be mild and transient. Presented in this article is a diagnostic algorithm to help steer clinical staff through investigations to help identify the cause of a persistent transaminitis without obvious clues from history and examination. It is important to bear in mind that there might not be a single cause, partly because, for example, multiple liver pathologies are common and processes that damage the liver can also damage other organs too. These algorithms cannot replace specialist knowledge, experience and local guidelines. Instead, they should act as a diagnostic aid when assessing patients with transaminitis or help guide investigations when a cause is not obvious. Low transaminase is rarely encountered clinically.
Acknowledgments
All figures were created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-64/rc
Peer Review File: Available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-64/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jlpm.amegroups.org/article/view/10.21037/jlpm-23-64/coif). The series “Investigative Algorithms in Laboratory Medicine II: Focus on Bone and Liver” was commissioned by the editorial office without any funding or sponsorship. K.E.S. served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of the Journal of Laboratory and Precision Medicine from September 2022 to August 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta 1957;2:70-4. [Crossref] [PubMed]
- King J. Practical clinical enzymology. London: D. Van Nostrand Limited; 1965.
- Rej R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem 1978;24:1971-9. [Crossref] [PubMed]
- Hu Y, Snitker S, Ryan KA, et al. Serum alanine aminotransferase is correlated with hematocrit in healthy human subjects. Scand J Clin Lab Invest 2012;72:258-64. [Crossref] [PubMed]
- Robinson Y, Cristancho E, Böning D. Erythrocyte aspartate aminotransferase activity as a possible indirect marker for stimulated erythropoiesis in male and female athletes. Lab Hematol 2007;13:49-55. [Crossref] [PubMed]
- Schmidt E, Schmidt FW. Guide to Practical Enzyme Diagnosis. Mannheim, Germany: Boehringer Mannheim GmbH; 1976.
- Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev 2013;34:117-30. [PubMed]
- Huang XJ, Choi YK, Im HS, et al. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors (Basel) 2006;6:756-82. [Crossref]
- Hsueh CJ, Wang JH, Dai L, et al. Determination of alanine aminotransferase with an electrochemical nano ir-C biosensor for the screening of liver diseases. Biosensors (Basel) 2011;1:107-17. [Crossref] [PubMed]
- Férard G, Imbert-Bismut F, Messous D, et al. Influence of pyridoxal phosphate in measuring aminotransferases activities in patients with viral hepatitis. Ann Biol Clin (Paris) 2004;62:717-20. [PubMed]
- Ning M, Baker H, Leevy CM. Reduction of glutamic pyruvic transaminase in pyridoxine deficiency in liver disease. Proc Soc Exp Biol Med 1966;121:27-30. [Crossref] [PubMed]
- Matloff DS, Selinger MJ, Kaplan MM. Hepatic transaminase activity in alocholic liver disease. Gastroenterology 1980;78:1389-92. [Crossref] [PubMed]
- Ding X, Wen X, Wang L, et al. Effects of a pneumatic tube system on the hemolysis of blood samples: a PRISMA-compliant meta-analysis. Scand J Clin Lab Invest 2021;81:343-52. [Crossref] [PubMed]
- Hedayati M, Razavi SA, Boroomand S, et al. The impact of pre-analytical variations on biochemical analytes stability: A systematic review. J Clin Lab Anal 2020;34:e23551. [Crossref] [PubMed]
- Liu S, Li J, Ning L, et al. Assessing the influence of true hemolysis occurring in patient samples on emergency clinical biochemistry tests results using the VITROS(®) 5600 Integrated system. Biomed Rep 2021;15:91. [Crossref] [PubMed]
- González Raya A, Coca Zúñiga R, Martín Salido E. Isolated elevation of aspartate aminotransferase (AST) in an asymptomatic patient due to macro-AST. J Clin Lab Anal 2019;33:e22690. [Crossref] [PubMed]
- Šimac M, Šimac DV, Bilić-Zulle L. Prevalence of Aminotransferase Macroenzymes in Rheumatoid Arthritis Patients and Impact on Their Management. EJIFCC 2021;32:280-5. [PubMed]
- Turecky L. Macroenzymes and their clinical significance. Bratisl Lek Listy 2004;105:260-3. [PubMed]
- Lim AK. Abnormal liver function tests associated with severe rhabdomyolysis. World J Gastroenterol 2020;26:1020-8. [Crossref] [PubMed]
- Radke JB, Algren DA, Chenoweth JA, et al. Transaminase and Creatine Kinase Ratios for Differentiating Delayed Acetaminophen Overdose from Rhabdomyolysis. West J Emerg Med 2018;19:731-6. [Crossref] [PubMed]
- Andrade RJ, Chalasani N, Björnsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers 2019;5:58. [Crossref] [PubMed]
- Ivica J, Hill S. The potential of reducing AST testing in hospital settings. Clin Biochem 2019;64:57-9. [Crossref] [PubMed]
- Cobbold JF, Anstee QM, Thomas HC. Investigating mildly abnormal serum aminotransferase values. BMJ 2010;341:c4039. [Crossref] [PubMed]
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104-12. [Crossref] [PubMed]
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. [Crossref] [PubMed]
- Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546-53. [Crossref] [PubMed]
- Fragala MS, Bi C, Chaump M, et al. Associations of aerobic and strength exercise with clinical laboratory test values. PLoS One 2017;12:e0180840. [Crossref] [PubMed]
- McKenzie J, Fisher BM, Jaap AJ, et al. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40-4. [Crossref] [PubMed]
- Harada PH, Cook NR, Cohen DE, et al. Relation of Alanine Aminotransferase Levels to Cardiovascular Events and Statin Efficacy. Am J Cardiol 2016;118:49-55. [Crossref] [PubMed]
- Park EY, Lim MK, Oh JK, et al. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS One 2013;8:e63439. [Crossref] [PubMed]
- Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol 1995;43:405-8. [PubMed]
- Diehl AM, Potter J, Boitnott J, et al. Relationship between pyridoxal 5'-phosphate deficiency and aminotransferase levels in alcoholic hepatitis. Gastroenterology 1984;86:632-6. [Crossref] [PubMed]
- Brown MJ, Ameer MA, Daley SF, et al. Vitamin B6 Deficiency. Treasure Island (FL): StatPearls Publishing; 2023.
- Sette LH, Lopes EP. The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 2015;70:346-9. [Crossref] [PubMed]
- Shen H, Damcott C, Shuldiner SR, et al. Genome-wide association study identifies genetic variants in GOT1 determining serum aspartate aminotransferase levels. J Hum Genet 2011;56:801-5. [Crossref] [PubMed]
- Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266-71. [Crossref] [PubMed]
- Han JH, Kwak JY, Lee SS, et al. Markedly Elevated Aspartate Aminotransferase from Non-Hepatic Causes. J Clin Med 2022;12:310. [Crossref] [PubMed]
- Asayama K, Hayashi K, Hayashibe H, et al. Relationships between an index of body fat distribution (based on waist and hip circumferences) and stature, and biochemical complications in obese children. Int J Obes Relat Metab Disord 1998;22:1209-16. [Crossref] [PubMed]
- Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023;78:1966-86. [Crossref] [PubMed]
- Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut 2018;67:6-19. [Crossref] [PubMed]
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology 1998;27:1213-9. [Crossref] [PubMed]
- Singal AK, Anand BS. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis (Hoboken) 2013;2:53-6. [Crossref] [PubMed]
- Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002;123:1367-84. [Crossref] [PubMed]
- Lv Y, Lau WY, Li Y, et al. Hypersplenism: History and current status. Exp Ther Med 2016;12:2377-82. [Crossref] [PubMed]
- Whitfield JB, Pounder RE, Neale G, et al. Serum -glytamyl transpeptidase activity in liver disease. Gut 1972;13:702-8. [Crossref] [PubMed]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263-355. [Crossref] [PubMed]
- Amarapurkar DN, Patel ND. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Trop Gastroenterol 2004;25:130-4. [PubMed]
- Raahimi MM, Livesey A, Hamilton J, et al. Liver fibrosis for the dermatologist: a review. Clin Exp Dermatol 2023;48:303-9. [Crossref] [PubMed]
- Papatheodoridi M, Cholongitas E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr Pharm Des 2018;24:4574-86. [Crossref] [PubMed]
- Norman D, Bardwell WA, Arosemena F, et al. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep 2008;31:121-6. [Crossref] [PubMed]
- Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol 2014;20:14652-9. [Crossref] [PubMed]
- Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]
- EASL Clinical Practice Guidelines. Wilson's disease. J Hepatol 2012;56:671-85. [PubMed]
- Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int 2003;23:139-42. [Crossref] [PubMed]
- Duncan A, Yacoubian C, Beetham R, et al. The role of calculated non-caeruloplasmin-bound copper in Wilson's disease. Ann Clin Biochem 2017;54:649-54. [Crossref] [PubMed]
- Zegers I, Beetham R, Keller T, et al. The importance of commutability of reference materials used as calibrators: the example of ceruloplasmin. Clin Chem 2013;59:1322-9. [Crossref] [PubMed]
- Ryan A, Nevitt SJ, Tuohy O, et al. Biomarkers for diagnosis of Wilson's disease. Cochrane Database Syst Rev 2019;2019:CD012267. [Crossref] [PubMed]
- McMillin GA, Travis JJ, Hunt JW. Direct measurement of free copper in serum or plasma ultrafiltrate. Am J Clin Pathol 2009;131:160-5. [Crossref] [PubMed]
- Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology 2008;48:1167-74. [Crossref] [PubMed]
- Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis 2008;3:16. [Crossref] [PubMed]
- Sveger T, Thelin T, McNeil TF. Young adults with alpha 1-antitrypsin deficiency identified neonatally: their health, knowledge about and adaptation to the high-risk condition. Acta Paediatr 1997;86:37-40. [Crossref] [PubMed]
- Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand 1978;204:345-51. [Crossref] [PubMed]
- Hiller AM, Piitulainen E, Tanash H. The Clinical Course of Severe Alpha-1-Antitrypsin Deficiency in Patients Identified by Screening. Int J Chron Obstruct Pulmon Dis 2022;17:43-52. [Crossref] [PubMed]
- Fagerhol MK, Laurell CB. The polymorphism of "prealbumins" and alpha-1-antitrypsin in human sera. Clin Chim Acta 1967;16:199-203. [Crossref] [PubMed]
- Hutchison DC. Alpha 1-antitrypsin deficiency in Europe: geographical distribution of Pi types S and Z. Respir Med 1998;92:367-77. [Crossref] [PubMed]
- Fitzsimons EJ, Cullis JO, Thomas DW, et al. Diagnosis and therapy of genetic haemochromatosis (review and 2017 update). Br J Haematol 2018;181:293-303. [Crossref] [PubMed]
- Porto G, Brissot P, Swinkels DW, et al. EMQN best practice guidelines for the molecular genetic diagnosis of hereditary hemochromatosis (HH). Eur J Hum Genet 2016;24:479-95. [Crossref] [PubMed]
- Wisplinghoff H, Appleton DL. Bacterial infections of the liver. In: Weber O, Protzer U. editors. Comparative hepatitis: Springer; 2008:143-60.
- Yeoman AD, Westbrook RH, Al-Chalabi T, et al. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology 2009;50:538-45. [Crossref] [PubMed]
- Hirschfield GM, Dyson JK, Alexander GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018;67:1568-94. [Crossref] [PubMed]
- Linzay CD, Sharma B, Pandit S. Autoimmune Hepatitis. Treasure Island (FL): StatPearls Publishing; 2023.
- Rawla P, Samant H. Primary Sclerosing Cholangitis. Treasure Island (FL): StatPearls Publishing; 2023.
- Peters L, Burkert S, Grüner B. Parasites of the liver - epidemiology, diagnosis and clinical management in the European context. J Hepatol 2021;75:202-18. [Crossref] [PubMed]
- Wisplinghoff H, Appleton DL. Bacterial infections of the liver. In: Weber O, Protzer U, editors. Comparative Hepatitis. 1st ed. Birkhäuser Advances in Infectious Diseases. Basel. Birkhäuser; 2008:143-60.
- Lin YC, Lee WT, Huang SF, et al. Persistent hypertransaminasemia as the presenting findings of muscular dystrophy in childhood. Acta Paediatr Taiwan 1999;40:424-9. [PubMed]
- Rider LG, Aggarwal R, Pistorio A, et al. 2016 American College of Rheumatology/European League Against Rheumatism Criteria for Minimal, Moderate, and Major Clinical Response in Juvenile Dermatomyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol 2017;69:911-23. [Crossref] [PubMed]
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol 2017;69:2271-82. [Crossref] [PubMed]
- Majeed R, Jaleel A, Siddiqui IA, et al. Comparison of troponin T and enzyme levels in acute myocardial infarction and skeletal muscle injury. J Ayub Med Coll Abbottabad 2002;14:5-7. [PubMed]
- Lim AKH, Arumugananthan C, Lau Hing Yim C, et al. A Cross-Sectional Study of the Relationship between Serum Creatine Kinase and Liver Biochemistry in Patients with Rhabdomyolysis. J Clin Med 2019;9:81. [Crossref] [PubMed]
- Lott JA, Stang JM. Serum enzymes and isoenzymes in the diagnosis and differential diagnosis of myocardial ischemia and necrosis. Clin Chem 1980;26:1241-50. [Crossref] [PubMed]
- Young A. Plasma creatine kinase after the marathon--a diagnostic dilemma. Br J Sports Med 1984;18:269-72. [Crossref] [PubMed]
- Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010;48:749-56. [Crossref] [PubMed]
- Kim EJ, Wierzbicki AS. Investigating raised creatine kinase. BMJ 2021;373:n1486. [Crossref] [PubMed]
- Kyriakides T, Angelini C, Schaefer J, et al. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol 2010;17:767-73. [Crossref] [PubMed]
- Gemelli C, Traverso M, Trevisan L, et al. An integrated approach to the evaluation of patients with asymptomatic or minimally symptomatic hyperCKemia. Muscle Nerve 2022;65:96-104. [Crossref] [PubMed]
- Lippi G, Schena F, Salvagno GL, et al. Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus 2012;10:377-83. [PubMed]
- Floyd JS, Bloch KM, Brody JA, et al. Pharmacogenomics of statin-related myopathy: Meta-analysis of rare variants from whole-exome sequencing. PLoS One 2019;14:e0218115. [Crossref] [PubMed]
- Narayanappa G, Nandeesh BN. Infective myositis. Brain Pathol 2021;31:e12950. [Crossref] [PubMed]
- Ren Y, Zhang C, Xu X, et al. A case report of atypical Kawasaki disease presented with severe elevated transaminases and literature review. BMC Infect Dis 2021;21:415. [Crossref] [PubMed]
- Li P, Lei Y, Li Q, et al. Diagnosing Perioperative Cardiovascular Risks in Noncardiac Surgery Patients. J Anal Methods Chem 2019;2019:6097375. [Crossref] [PubMed]
- Lu Z, Ma G, Chen L. De-Ritis Ratio Is Associated with Mortality after Cardiac Arrest. Dis Markers 2020;2020:8826318. [Crossref] [PubMed]
- Ambrosy AP, Gheorghiade M, Bubenek S, et al. The predictive value of transaminases at admission in patients hospitalized for heart failure: findings from the RO-AHFS registry. Eur Heart J Acute Cardiovasc Care 2013;2:99-108. [Crossref] [PubMed]
- Rahman S, Islam S, Haque T, et al. Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults. BMC Cardiovasc Disord 2020;20:128. [Crossref] [PubMed]
- Liu PY, Lin YK, Chen KW, et al. Association of Liver Transaminase Levels and Long-Term Blood Pressure Variability in Military Young Males: The CHIEF Study. Int J Environ Res Public Health 2020;17:6094. [Crossref] [PubMed]
- Havaldar PV, Sankpal MN, Doddannavar RP. Diphtheritic myocarditis: clinical and laboratory parameters of prognosis and fatal outcome. Ann Trop Paediatr 2000;20:209-15. [Crossref] [PubMed]
- Yokoyama M, Watanabe T, Otaki Y, et al. Association of the Aspartate Aminotransferase to Alanine Aminotransferase Ratio with BNP Level and Cardiovascular Mortality in the General Population: The Yamagata Study 10-Year Follow-Up. Dis Markers 2016;2016:4857917. [Crossref] [PubMed]
- Lofthus DM, Stevens SR, Armstrong PW, et al. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis 2012;23:22-30. [Crossref] [PubMed]
- Murakami J, Shimizu Y. Hepatic manifestations in hematological disorders. Int J Hepatol 2013;2013:484903. [Crossref] [PubMed]
- Scappaticcio L, Longo M, Maiorino MI, et al. Abnormal Liver Blood Tests in Patients with Hyperthyroidism: Systematic Review and Meta-Analysis. Thyroid 2021;31:884-94. [Crossref] [PubMed]
- Jiang W, Liu CH, Wu D, et al. Abnormal transaminase and lipid profiles in coexisting diseases in patients with fatty liver: a population study in Sichuan. Biosci Rep 2021;41:BSR20211769. [Crossref] [PubMed]
- Huang MJ, Li KL, Wei JS, et al. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol 1994;89:1071-6. [PubMed]
- Yu W, Wu N, Li L, et al. Side effects of PTU and MMI in the treatment of hyperthyroidism: a systematic review and meta-analysis. Endocr Pract 2020;26:207-17. [Crossref] [PubMed]
- Agarwal A, Yadav A, Gutch M, et al. Prognostic Factors in Patients Hospitalized with Diabetic Ketoacidosis. Endocrinol Metab (Seoul) 2016;31:424-32. [Crossref] [PubMed]
- Sette LH, Almeida Lopes EP. Liver enzymes serum levels in patients with chronic kidney disease on hemodialysis: a comprehensive review. Clinics (Sao Paulo) 2014;69:271-8. [Crossref] [PubMed]
- Gault MH, Steiner G. Serum and urinary enzyme activity after renal infarction. Can Med Assoc J 1965;93:1101-5. [PubMed]
- Sepulveda L, Oliveira M, Oliveira A, et al. Renal Infarction: Three Case Reports and Literature Review. World J Nephrol Urol 2014;3:35-40. [Crossref]
Cite this article as: Shipman AR, Shipman KE. Investigative algorithms for disorders affecting plasma transaminases (aspartate transaminase and alanine transaminase)—a narrative review. J Lab Precis Med 2024;9:14.