To add incremental information: the main problem in cardiovascular risk evaluation
Introduction
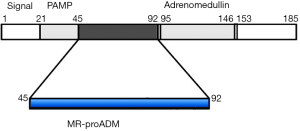
Adrenomedullin (ADM) consists of 52 amino acids with a C-terminal amination and one intra-molecular disulfide bond between residues 16 and 21 (1,2) (Figure 1). This peptide hormone has been first isolated from human pheochromocytoma cells in 1993, hence its name (1). ADM is expressed and secreted by many tissues and organ systems, including cardiovascular, renal, pulmonary, cerebrovascular, gastrointestinal and endocrine tissues (2,3).
After acute administration, ADM shows several important physiological autocrine and paracrine actions, also including natriuretic, vasodilatory, and hypotensive effects, and it also inhibits aldosterone production (2,3). These effects are mediated by the cyclic adenosine monophosphate (cAMP), nitric oxide and renal prostaglandin systems (3). After chronic administration, ADM shows antihypertrophic, anti-apoptotic, antifibrotic, antioxidant and angiogenesis effects (3).
Considering these important physiological effects, ADM was proposed as a potential cardiovascular biomarker (3,4). Indeed, increased ADM circulating levels have been reported in patients with hypertension, chronic renal disease and heart failure (HF) (3,4). However, the accurate measurement of circulating levels of ADM is challenging using immunoassays, due to short half-life in vivo (about 22 min) and rapid degradation in vitro of this peptide. Moreover, the presence in plasma/serum samples of some binding proteins may interfere in the assay (5,6). Finally, other specific analytical aspects (such as preliminary extractions, low circulating levels, absorption on blood tube walls) would make the measurement of ADM by means of immunoassays rather unsuitable for clinical laboratory routine (5,6).
In human tissues, ADM is produced throughout of a post-translational processing from a larger precursor peptide, the preproADM, consisting of 185 amino acids (2,3) (Figure 1). During processing of preproADM, other peptides are generated, including another biologically active peptide (defined pro-ADM N-terminal 20 peptide, PAMP), the midregional part of proADM (MR-proADM 45–92) and the COOH terminus of the molecule (proADM 153–185) (1,2,6) (Figure 1). From an analytical perspective, it is theoretically conceivable that other peptides characterized by higher molecular mass and plasma/serum concentrations than the ADM hormone should be more accurately measured. An immunoradiometric method specific for the MR-proADM peptide has been first set up in 2005 (6) and, more recently, a homogeneous time-resolved fluoro-immunoassay system has been implemented on a fully automated platform (7). The measurement of MR-proADM with these immunoassay systems was shown to accurately reflect those of the active peptide ADM (6,7).
Clinical relevance of MR-proADM measurement
Using fully automated immunoassays (7), several studies have recently evaluated the prognostic relevance of MR-proADM in different clinical settings such as patients with chronic obstructive pulmonary disease (8) and cardiovascular diseases (3), or admitted to emergence department and intensive care units (9). In particular, three recent meta-analyses demonstrated that increased levels of MR-proADM were significantly associated with complications and both short-term and long-term mortality in patients with community-acquired pneumonia (10-12).
As regards cardiovascular diseases, the prognostic accuracy of a large number of biomarkers (over 100) has been evaluated in different populations of patients with HF (13-15). The criteria to evaluate and compare the prognostic efficacy and efficiency of new cardiovascular risk biomarkers have been recently reported and discussed in details (16,17). Innovative risk biomarkers should be evaluated in several phases, including the initial proof of concept, the prospective validation in independent populations, the documentation of incremental diagnostic or prognostic information when added to standard risk markers, the assessment of effects on patient management and outcomes and, ultimately, cost-effectiveness (16,17). Notably, biomarkers not changing disease management are probably unable to significantly affect patient outcome and are thus very seldom cost-effective (as assessed in terms of quality-adjusted life-years gained) (13-17). According to the 2016 guidelines of the European Society of Cardiology (ESC) for management of HF (18), multivariable risk scores may help predicting the risk of death in patients with HF, but they are seemingly less useful for predicting subsequent hospitalizations.
The 2013 guidelines of the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) (19), updated in 2017 (20), recommend the use of natriuretic peptides (in particular the measurement of BNP and NT-proBNP) and cardiac troponin I (cTnI) and T (cTnT) as first line biomarkers for prognostic stratification of HF patients. Accordingly, a new biomarker should demonstrate to provide incremental prognostic information compared to brain natriuretic peptide (BNP)/NT-proBNP and cTn assays, especially in terms of increased risk discrimination (using C-statistics analysis) and reclassification (16,17).
Morbach et al. (21) recently reported that MR-proADM was correlated with global disease burden in 917 patients (68±12 years, 28% females) hospitalized for acute systolic HF and then followed up for 18 months. The results of this study also suggest that MR-proADM is a strong prognostic indicator, capturing incremental risk for both cardiac and non-cardiac death (21). Unlike NT-proBNP, which only predicted cardiac death, MR-proADM was capable of predicting both cardiac and non-cardiac death (21). In addition, the combination of MR-proADM with a clinical prediction model including NT-proBNP showed improved efficiency for risk stratification throughout the entire 18-month observation period. In particular, among 173 patients who subsequently died, 12 (6.9%) were reclassified in a higher risk category, whereas 7 (4.0%) were reclassified to a lower risk category, whilst 89 (12.6%) patients were reclassified to lower risk category and 68 (9.6%) to a higher risk category amongst 705 survivors (21). Morbach et al. (21) reported that MR-proADM outperformed NT-proBNP for predicting all-cause and cardiac mortality and, to a lesser extent, all-cause rehospitalization.
The authors explain the better predictive value of MR-proADM compared to NT-proBNP with the fact that MR-proADM may more efficiently identify patients at high risk of both cardiac and non-cardiac death. Indeed, patients with low NT-proBNP but high MR-proADM experienced non-cardiac death more frequently (21). It is well known that increased MR-proADM levels are associated with a higher risk across various non-cardiac disorders (3,4,8-12). It is hence theoretically conceivable that increased MR-proADM levels in HF patients may not only be associated with worse HF symptoms and cardiac function, but also with non-cardiac comorbidities, which can negatively impact on outcomes by promoting adverse cardiac remodeling and HF progression (18,19). Therefore, data published by Morbach et al. (21) may reflect the risk associated to high MR-proADM levels due to systemic manifestations of HF syndrome, which are not (or poorly) detected by variations of NT-proBNP levels.
Future perspectives
Previous studies which examined the combined use of up to ten biomarkers suggest modest improvements in risk prediction, at best (17). As observed by Wang (17), it is not realistic to expect that any set of biomarkers can substantially improve risk prediction above and beyond traditional risk scores, whilst it is theoretically possible to improve the performance of risk models with a relatively small number of biomarkers, provided that these are weakly or not intercorrelated.
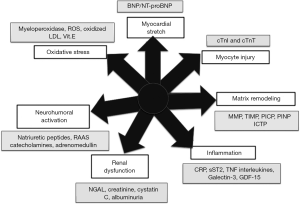
According to Braunwald (22), seven major classes of biomarkers can contribute to set a biomarker profile in HF due to their different pathophysiological mechanisms (Figure 2). We would assume that including biomarkers sharing the same pathophysiological mechanism may not be useful, because they are probably highly intercorrelated and do not add additional information to risk prediction. Conversely, it is conceivable that biomarkers with different pathophysiological mechanisms can significantly improve the statistical analysis, because they can add differential, and so incremental information, to risk prediction, by targeting a number of different biological pathways converging to HF (17).
The results of the study published by Morbach et al. (21) confirm the assumption that biomarkers sharing different pathophysiological mechanisms may significantly improve risk prediction accuracy. Another recent study (23) reported similar results. Jackson et al. (23) measured several biomarkers in 628 patients recently hospitalized with decompensated HF, including MR-proADM, MR-proANP, copeptin, hs-cTnT, suppressor of tumorigenicity 2 (ST2), galectin-3, cystatin C, combined free light chains (cFLC) and high-sensitivity C reactive protein (hsCRP). Using dichotomized cut-points derived from receiver operating characteristics (ROC) curve analysis, MR-proADM, hs-cTnT, cFLC, hsCRP and ST2 remained independent predictors of mortality, and improved model performance (as assessed by C-statistic and net reclassification index). The results of these two studies also confirm that MR-proADM should be considered a valuable predictive biomarker in HF patients. However, large clinical trials are needed to conclusively define that novel biomarkers, such as MR-proADM, may improve management of HF patients and so they may also express a favorable cost/benefit ratio as assessed by accurate methodologies (such as QALY evaluation) (24).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Guo-Ming Zhang (Department of Laboratory Medicine, Shuyang People’s Hospital, Shuyang, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.07.12). Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. Aldo Clerico serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2017 to June 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 1993;192:553-60. [Crossref] [PubMed]
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev 2000;21:138-67. [PubMed]
- Peacock WF. Novel biomarkers in acute heart failure: MR-pro-adrenomedullin. Clin Chem Lab Med 2014;52:1433-5. [Crossref] [PubMed]
- Potocki M, Ziller R, Mueller C. Mid-regional pro-adrenomedullin in acute heart failure: a better biomarker or just another biomarker? Curr Heart Fail Rep 2012;9:244-51. [Crossref] [PubMed]
- Lewis LK, Smith MW, Yandle TG, et al. Adrenomedullin(1-52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem 1998;44:571-7. [PubMed]
- Morgenthaler NG, Struck J, Alonso C, et al. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 2005;51:1823-9. [Crossref] [PubMed]
- Caruhel P, Mazier C, Kunde J, et al. Homogeneous time-resolved fluoroimmunoassay for the measurement of midregional proadrenomedullin in plasma on the fully automated system B.R.A.H.M.S KRYPTOR. Clin Biochem 2009;42:725-8. [Crossref] [PubMed]
- Schuetz P, Marlowe RJ, Mueller B. The prognostic blood biomarker proadrenomedullin for outcome prediction in patients with chronic obstructive pulmonary disease (COPD): a qualitative clinical review. Clin Chem Lab Med 2015;53:521-39. [Crossref] [PubMed]
- Valenzuela-Sánchez F, Valenzuela-Méndez B, Rodríguez-Gutiérrez JF, et al. New role of biomarkers: mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann Transl Med 2016;4:329. [Crossref] [PubMed]
- Cavallazzi R, El-Kersh K, Abu-Atherah E, et al. Midregional proadrenomedullin for prognosis in community-acquired pneumonia: a systematic review. Respir Med 2014;108:1569-80. [Crossref] [PubMed]
- Liu D, Xie L, Zhao H, et al. Prognostic value of mid-regional pro-adrenomedullin (MR-proADM) in patients with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect Dis 2016;16:232. [Crossref] [PubMed]
- Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: A systematic review and meta-analysis. J Infect 2016;72:273-82. [Crossref] [PubMed]
- Vittorini S, Clerico A. Cardiovascular biomarkers: increasing impact of laboratory medicine in cardiology practice. Clin Chem Lab Med 2008;46:748-63. [Crossref] [PubMed]
- Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440-6. [Crossref] [PubMed]
- Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2:429-36. [Crossref] [PubMed]
- Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408-16. [Crossref] [PubMed]
- Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 2011;123:551-65. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776-803. [Crossref] [PubMed]
- Morbach C, Marx A, Kaspar M, et al. Prognostic potential of midregional pro-adrenomedullin following decompensation for systolic heart failure: comparison with cardiac natriuretic peptides. Eur J Heart Fail 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Braunwald E. Heart failure. JACC Heart Fail 2013;1:1-20. [Crossref] [PubMed]
- Jackson CE, Haig C, Welsh P, et al. The incremental prognostic and clinical value of multiple novel biomarkers in heart failure. Eur J Heart Fail 2016;18:1491-8. [Crossref] [PubMed]
- McIntosh E, Clarke PM, Frew EJ, et al. Applied methods of cost-benefit analysis in health care. Oxford: Oxford University Press, 2010.
Cite this article as: Clerico A, Lippi G. To add incremental information: the main problem in cardiovascular risk evaluation. J Lab Precis Med 2017;2:54.