
Lowering the bar of high-sensitivity cardiac troponin: more than limbo dancing?
To discharge or not to discharge remains one of the most common and contentious questions emergency department (ED) physicians are confronted with in patients presenting with acute chest pain. The success story of contemporary high-sensitivity cardiac troponin (hs-cTn) assays is remarkable and had great impact on this question: Biomarkers for the diagnosis of acute myocardial infarction (AMI) were first introduced in the 1960s with the use of aspartate transaminase (AST) which was also incorporated into the World Health organization (WHO) definition of AMI (1,2). AST, though, is not specific for cardiac damage and by 1970s was replaced by lactate dehydrogenase (LDH), creatine kinase (CK) and a bit later complemented by myoglobin (3). Advancements in electrophoresis allowed the detection of cardiac-specific iso-enzymes of CK and LDH, i.e. CK-MB and LDH 1+2 (4). For two decades, these assays played an important role in the diagnosis of AMI. In the late 1990s, a radioimmunoassay was developed to reliably detect serum cardiac troponin (cTn) (5). Novel 5th generation hs-cTn T assays have further improved clinical practice and sensitivity: troponin at concentrations 10- to 100-fold lower than measurable with conventional assays can now be quantified (3). To put it in a nutshell, hs-cTn assays—in contrast to conventional cTn assays—are able to detect troponin with higher sensitivity and higher precision (in particular at the 99th percentile) at an earlier point of time (6), and allow detection and quantification in 50% (ideally 95%) of healthy individuals (7). Hs-cTn assays should assure a low coefficient of variation (low imprecision) of <10% at their respective 99th percentile to assure reliability around their clinical threshold. Many hospitals in Europe now have replaced conventional cTn assays with hs-cTn assays replacing all other laboratory tests, including CK, CK-MB, and myoglobin for its “absolute” myocardial tissue specificity. After the approval by the Food and Drug Administration (FDA) in January of 2017, it will probably only be a matter of time before the wide-spread use of hs-cTn assays also becomes common in the United States.
Varying biomarkers and predominantly the further development of cTn assays to hs-cTn assays, has significantly shortened the time period necessary for the exclusion of AMI. In general, more than 8 h were needed to rule out AMI by CK/CK-MB, conventional cardiac troponin assays had to be re-measured after 6–12 hours (8,9). The introduction of hs-cTn assays halved this time period to 3 h (3-h rule out protocol) (10). In cases of high pre-test probability for NSTEMI and if chest pain onset >3 h, a 1-h algorithm has been recommended provided that “validated algorithms” are available (10).
Pickering, Than and colleagues (11) in the present collaborative meta-analysis of 11 clinically and geographically diverse cohorts and 9,269 patients have used hs-cTnT results to assess the safety of an early rule-out strategy for AMI. In their meta-analysis it has been scrutinized whether a single hs-cTnT concentration below the limit of detection (LoD <5 ng/L) in combination with a nonischemic electrocardiogram (ECG) may successfully rule out AMI. In most but not all settings, patients investigated for acute coronary syndrome with hs-cTnT below the LoD and a nonischemic ECG—almost a third of all patients (30.5%)—had very low risk for AMI or for Major Adverse Cardiac Events (MACEs) within 30 days (11). The authors have proposed that integrating such an early screening approach into existing investigative strategies may enable patients to be safely discharged to outpatient follow-up earlier than in current practice (11).
In principle, such an approach is only feasible because of the very early high sensitivity of hs-cTn assays and the fact that hs-cTn are able to quantify troponin concentrations in the majority of healthy individuals. Troponin assays are positive when there has been myocardial necrosis (and not necessarily myocardial ischemia or infarction). To maintain high specificity for AMI, it is important to distinguish acute from chronic hs-cTn elevation. This has already been addressed by the various algorithms available using absolute (and relative) changes to rule in/out AMI (12,13). The high sensitivity of the assay with its very high negative predictive value for AMI if < LoD/limit of blank (LoB) predestines it for a rule out test. According to current guidelines, the use of single hs-cTn measurements is already recommended for special circumstances: a patient presenting with symptoms suggestive of AMI and highly abnormal hs-cTn (<5× upper limit of normal) at baseline may already today be ruled in; a patient presenting with chest pain onset >6 h and normal baseline hs-cTn (< upper limit of normal) may already today be ruled out for AMI if Global Registry of Acute Coronary Events score (GRACE) score <140 (Figure 1) (3).
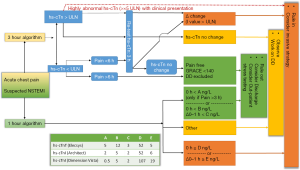
Caveats
High laboratory standards (such as assay calibration, laboratory setup) at individual sites is a sine qua non for the use of hs-cTn assays in general but especially for a potential single measurement approach using very low hs-cTn levels as proposed by this meta-analysis. Many clinicians including cardiologists blindly trust and rely on their clinical laboratories to address the analytical performance of the assays while still being unfamiliar with the laboratory science of troponin testing. Reliable measurements of hs-cTn are made at the 99th percentile with low coefficient of variation of ≤10%. Very low troponin measurements as scrutinized in the present meta-analysis scatter much more around the LoD and LoB (14). Additionally, LoD and LoB are assay specific (hs-cTnT) and even future hs-cTnT assays may have different cut-offs; the authors of the meta-analysis therefore rightly mention that their analysis is assay specific (hs-cTnT).
Diagnostic decision making in patients with chest pain is notoriously treacherous. Although hs-cTn assays have significantly shortened the “troponin-blind interval”, the group of early presenters (chest pain onset within 3 h) might still require serial troponin measurements to rule out AMI even in the context of non-ischemic ECG. From a clinical point of view, physicians should not be tempted to classify patients with very low hs-cTn concentrations and negative ECGs as very low risk for AMI and discharge them instead of working on the various differential diagnoses of acute chest pain. Some clinicians and authors have introduced the term “troponin-negative chest pain” which is not a good term but rather an evasion for identifying the underlying cause (15). Often, the cause of non-AMI chest pain may not be life threating and can be dealt with in the outpatient setting. Nevertheless, even if measured with a hs-cTn assay, patients with “troponin-negative chest pain” may suffer from potentially life-threatening diseases that require prompt further diagnostics and treatment (e.g., pulmonary embolism, aortic rupture or dissection, pneumonia, Boerhaave’s syndrome (esophageal rupture), tension pneumothorax). With EDs crowding and increasing economic pressure on hospitals the market place has influenced clinics and physicians: more and more clinical algorithms are used in EDs in order to free up beds and improve throughput, facilitate quick discharges and process patients. Even the best algorithms cannot substitute clinical acumen to make a comprehensive and correct diagnosis.
Lowering or raising the bar of hs-cTn assays?
The full and long-term prognostic impact of hs-cTn assays remains to be elucidated. The development of more and more sensitive biomarkers of myocardial necrosis and the use of lower cut-offs have led to a much earlier diagnosis and treatment of AMI in many cases. On the other hand, they also seem to have led to a tendency to mandate further evaluation even of patients with little or no clinical evidence of an acute coronary syndrome.
Unlike in most parts of the world, FDA approval of the hs-cTnT assay in the United states specified higher cutoff values (19 vs. 14 ng/L in most other parts of the world), with sex-specific cutoff values as 14 ng/L for women and 22 ng/L for men. Furthermore, the inherent tension between achieving diagnostic certainty and a rapid rule-out has led to an increasing use of diagnostic testing with cardiovascular imaging (16): combining the high-sensitivity of the blood biomarkers tests with imaging techniques that impart specificity for myocardial ischemia and infarction is an increasingly used strategy (17). This approach may raise the bar for acute chest pain evaluation.
Concluding remarks
Regarding the present meta-analysis (11) the authors have to be commended on their work and collaborative skills: One of the major results of this study is that rule out in a significant portion of patients with symptoms suggestive of AMI is likely to be shortened without compromising on patient safety regarding missed AMI. More of such analyses are necessary in order to assess other contemporary hs-cTn assays. Hs-cTn is a marker of myocardial necrosis and not one of myocardial ischemia, which can be tested with cardiovascular imaging techniques. Integrating the results of hs-cTn measurements with algorithms and robust clinical assessment remains the optimal approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chao Xuan (Department of Clinical Laboratory, the Affiliated Hospital of Qingdao University, Qingdao, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.07.08). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ladue JS, Wroblewski F. karmen A. Serum glutamic oxaloacetic transaminase activity in human acute transmural myocardial infarction. Science 1954;120:497-9. [Crossref] [PubMed]
- WHO. Hypertension and coronary heart disease: classification and criteria for epidemiological studies, first report of the Expert Committee on Cardiovascular Diseases and Hypertension. WHO Tech Rep Ser; no. 168.
- Garg P, Morris P, Fazlanie AL, et al. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 2017;12:147-55. [Crossref] [PubMed]
- Dolci A, Panteghini M. The exciting story of cardiac biomarkers: from retrospective detection to gold diagnostic standard for acute myocardial infarction and more. Clin Chim Acta 2006;369:179-87. [Crossref] [PubMed]
- Katus HA, Remppis A, Looser S, et al. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 1989;21:1349-53. [Crossref] [PubMed]
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858-67. [Crossref] [PubMed]
- Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574-81. [Crossref] [PubMed]
- Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28:1598-660. [Crossref] [PubMed]
- Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999-3054. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Pickering JW, Than MP, Cullen L, et al. Rapid Rule-out of Acute Myocardial Infarction With a Single High-Sensitivity Cardiac Troponin T Measurement Below the Limit of Detection: A Collaborative Meta-analysis. Ann Intern Med 2017;166:715-24. [PubMed]
- Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation 2011;124:136-45. [Crossref] [PubMed]
- Haaf P, Drexler B, Reichlin T, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation 2012;126:31-40. [Crossref] [PubMed]
- Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254-61. [Crossref] [PubMed]
- Ali I. “Troponin-negative chest pain”—a diagnostic evasion? BMJ 2012;344:e1682 [Crossref]
- Bhatt DL, Taqueti VR. Out With the Old Rule-Out: Raising the Bar for Acute Chest Pain Evaluation With Randomized Trials of Cardiac Imaging. JACC Cardiovasc Imaging 2017;10:350-3. [Crossref] [PubMed]
- Raff GL, Hoffmann U, Udelson JE. Trials of Imaging Use in the Emergency Department for Acute Chest Pain. JACC Cardiovasc Imaging 2017;10:338-49. [Crossref] [PubMed]
Cite this article as: Haaf P. Lowering the bar of high-sensitivity cardiac troponin: more than limbo dancing? J Lab Precis Med 2017;2:60.


