
High-sensitivity cardiac troponin I immunoassay reduces the chance of patient misclassification in the emergency department
Introduction
The figures reflected by the current epidemiology of cardiovascular disease still make acute myocardial infarction (AMI) the leading healthcare issue around the world (1). Although many progresses have been made during the past decades, the diagnostic approach to this condition remains somehow challenging (2). The development and recent commercialization of the so-called high-sensitivity (HS) cardiac troponin (cTn) immunoassays has represented another essential step forward toward a better characterization of patients with suspected AMI, especially in short stay units such as the emergency department (ED) (3). The considerably improved analytical sensibility of these techniques now allows measuring so little amounts of cTn that were virtually inconceivable 10 years ago. In clinical practice, this enhanced analytical performance is mirrored by the possibility to identify negligible myocardial injuries (i.e., micrograms of damaged myocardial tissue), such as those often observed after practicing endurance sports (4).
Albeit HS immunoassays should hence be seen as an appealing perspective for improved patient management in the ED, doubts remains as to whether these assays are really cost-effective, or else if their practical advantages will convincingly overwhelm some potential practical drawbacks, which mainly emerge from the challenge of clearly identifying the source of measurable cTn in all patients presenting to the ED with a kaleidoscope of differential diagnoses other than myocardial ischemia (5,6). To overcome this issue, many diagnostic algorithms have been proposed, most of which based on a dichotomous approach, i.e., early rule-out of AMI in patients whose cTn values are below a very low cut-off value (usually corresponding to the limit of detection of the assay), combined with rule-in of AMI in those with 1 to 3 hours cTn kinetics suggestive of ongoing myocardial damage (7,8).
Quite recently, some interesting studies showed that implementation of HS cTn immunoassays for management of patients in the ED was associated with many practical and organizational advantages, including increased discharge rate, decrease length of ED stay, lower exposure to diagnostic radiation and, finally, reduced overall costs of care in the ED (9,10). Whether these benefits, mostly appreciated by clinicians and hospital administrators, will then translate into clinical advantage for patients remains a debated matter, as recently highlighted by Sandoval et al., who showed that both an innovative HS and the former contemporary-sensitive (CS) cardiac troponin I (cTnI) immunoassays are capable to provide efficient rule out of AMI when used within protocols entailing serial testing and electrocardiogram data (11).
Therefore, this study was designed to verify whether the practical advantages of a HS cTnI immunoassay may also be mirrored by a more efficient diagnostic performance, expressed as lower chance of patient misclassification upon ED admission.
Methods
Study population
The study population consisted of 57 consecutive patients admitted to the ED of the University Hospital of Parma (Italy) with suspected AMI. The University Hospital of Parma is a 1,150-bed, tertiary academic referral facility, whose ED averages nearly 90,000 visits per year. A blood sample for cTnI assessment was collected immediately upon patient presentation to the ED, and the diagnosis of AMI was made according to the third universal definition of myocardial infarction (12). The value of cTnI was measured with both a CS immunoassay (Beckman Coulter AccuTnI+3; Beckman Coulter, Brea, Ca, USA) and the novel HS method (Beckman Coulter hsTnI), on Beckman Coulter DxI (Beckman Coulter). The specific analytical characteristics of these assays have been previously described elsewhere (13,14).
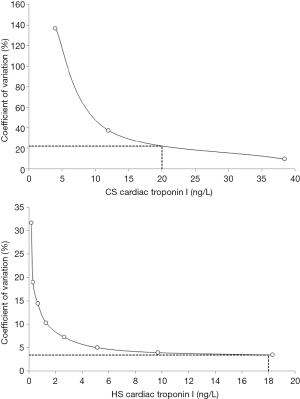
The best cut-off for diagnosing AMI was derived from receiver operating characteristic (ROC) curves, calculation of the area under the curve (AUC), and selection of cTnI values characterized by the best diagnostic sensitivity (i.e., 1.00). The imprecision at the assay-specific diagnostic cut-off was calculated for both the CS and HS cTnI immunoassays by measuring scalar dilutions of a sample with high cTnI value, which was serially diluted with sample buffer until reaching a virtually unmeasurable cTnI value (i.e., below the limit of detection of the methods). All dilutions were tested in 10 consecutive runs and the imprecision was estimated for each dilution as coefficient of variation (CV%). A model fit was then constructed to extrapolate imprecision at the optimal diagnostic cut-off previously selected for both cTnI immunoassays from ROC curve analysis. The potential impact on patient misclassification (i.e., the sum of potential false positive and false negative results, expressed as a percentage of all patients) was finally estimated according to data previously published by Sheehan et al. (15). The study protocol was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008), according to the terms of relevant local legislation, and was cleared by the institutional review board of the University Hospital of Parma. No ethical approval or patient’s consent were deemed necessary, since the study was based on pre-existing samples, no additional measurements were performed, and results did not affect the clinical management of the patients.
Results
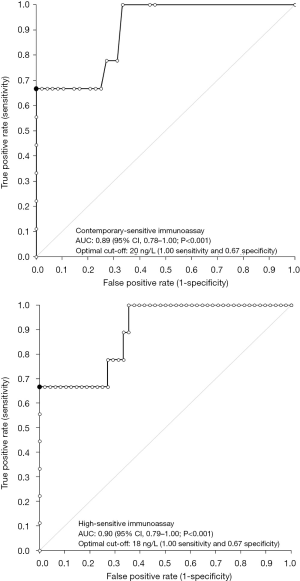
A final diagnosis of AMI according to the third universal definition of myocardial infarction (12) could be made in 9/57 (i.e., 16%) patients. The results of ROC curve analysis are shown in Figure 1. The AUC was not significantly different for cTnI measured with either the CS or the HS immunoassays (0.89 vs. 0.90; P=0.393). The best diagnostic cut-offs were 20 ng/L (1.00 sensitivity and 0.67 specificity) for CS cTnI and 18 ng/L (1.00 sensitivity and 0.67 specificity) for HS cTnI, respectively. The results of assay imprecision at the two diagnostic cut-offs for CS and HS immunoassays is shown in Figure 2, being 22.0% at 20 ng/L for the CS cTnI immunoassay and 3.4% at 18 ng/L for the HS cTnI immunoassay, respectively.
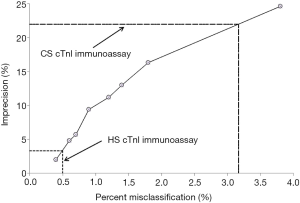
The potential impact on the rate of theoretical patient misclassification attributable to imprecision at the assay-specific diagnostic cut-offs is shown in Figure 3. Briefly, the 22.0% imprecision at the 20 ng/L diagnostic cut-off of the CS cTnI immunoassay was associated with a 3.2% chance of patient misclassification, whilst the 3.4% imprecision at the 18 ng/L diagnostic cut-off of the HS cTnI immunoassay was associated with a 0.5% change of patient misclassification.
Discussion
Although it seems now reasonable to conclude that the use of HS cTn immunoassays may generate a vast array of practical and economic benefits for accurate and timely management of patients admitted to the ED with suspected AMI (16), the demonstration of major clinical advantages over conventional CS techniques remain somewhat elusive (11,17,18).
According to the results of our investigation, the largest improvement obtained by replacing a CS cTnI immunoassay with a more recent HS cTnI technique commercialized by the same manufacturer was not actually seen in terms of overall improved diagnostic performance, since the AUC and the values of sensitivity and specificity were very similar between the CS and HS cTnI immunoassays (Figure 1). Nevertheless, when we calculated the imprecision corresponding to the assay-specific diagnostic thresholds, the new HS cTnI immunoassay displayed a CV that was approximately 6-fold lower than that of the CS technique (i.e., 3.4% vs. 22.0%) (Figure 2). Translating these figures into the risk of patient misclassification at ED admission, the use of the HS cTnI immunoassay was hence associated with a more than 6-fold lower risk of misclassifying patients (i.e., 0.5% vs. 3.2%) (Figure 3). Taken together, this data attests that the accuracy of diagnosing AMI may be certainly improved by replacing CS cTnI techniques with HS cTnI immunoassays, especially in the subset of patients displaying admission values close to the diagnostic cut-off calculated from ROC curve analysis. This is especially important in view of the meaningful number of “early presenters”, i.e., patients presenting to the ED within 2 hours from symptoms onset and with cTnI values still laying in the so-called “grey zone” (i.e., slightly lower or slightly higher cTnI concentrations than the diagnostic threshold), in whom AMI cannot be safely ruled out or diagnosed without serial measurement, with the second sample collected from 1 to 3 hours afterwards. In these patients, representing approximately 15% of all those admitted to the ED with suspected AMI (19), the risk of unsafe discharge or unjustified stay in the ED due to assay imprecision was found to be decreased by over 6-fold in our study, thus possibly leading to improved clinical outcomes and less inconvenience.
Conclusions
The major diagnostic accuracy, combined with recent data showing that the introduction of HS cTnI immunoassays may improve ED efficiency and decrease overall costs in the emergency room, represents an additional aspect in favor of introducing HS techniques for more timely and accurate management of patients admitted to the ED with suspected AMI, especially those displaying non-diagnostic cTnI values at presentation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Biomarkers in Cardiovascular Disease”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.12.03). The series “Biomarkers in Cardiovascular Disease” was commissioned by the editorial office without any funding or sponsorship. Fabian Sanchis-Gomar served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from December 2016 to November 2018. Giuseppe Lippi serves as the unpaid Editor-in-Chief of Journal of Laboratory and Precision Medicine from November 2016 to October 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). No ethical approval or patient’s consent were deemed necessary, since the study was based on pre-existing samples, no additional measurements were performed, and results did not affect the clinical management of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256. [Crossref] [PubMed]
- Lippi G, Cervellin G. Evidence and pitfalls in diagnosis and prognostication of acute coronary syndrome. Ann Transl Med 2016;4:250. [Crossref] [PubMed]
- Lackner KJ. High-sensitivity assays for cardiac troponins - continued. Clin Chem Lab Med 2017;55:1631-3. [Crossref] [PubMed]
- Lippi G, Cervellin G, Schena F. How much myocardium mass may be injured during endurance physical exercise? Clin Chim Acta 2017;470:29-30. [Crossref] [PubMed]
- Wallentin L, Kristensen SD, Anderson JL, et al. How can we optimize the processes of care for acute coronary syndromes to improve outcomes? Am Heart J 2014;168:622-31. [Crossref] [PubMed]
- Jaffe AS, Moeckel M, Giannitsis E, et al. In search for the Holy Grail: suggestions for studies to define delta changes to diagnose or exclude acute myocardial infarction: a position paper from the study group on biomarkers of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2014;3:313-6. [Crossref] [PubMed]
- Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. [Crossref] [PubMed]
- Ferraro S, Dolci A, Panteghini M. Fast track protocols using highly sensitive troponin assays for ruling out and ruling in non-ST elevation acute coronary syndrome. Clin Chem Lab Med 2017;55:1683-9. [Crossref] [PubMed]
- Lippi G, Bonfanti L, Dipalo M, et al. Clinical, organizational and economic analysis of high-sensitivity cardiac troponin testing in the emergency department. Ann Res Hosp 2017;1:44. [Crossref]
- Ferencik M, Mayrhofer T, Lu MT, et al. High-Sensitivity Cardiac Troponin I as a Gatekeeper for Coronary Computed Tomography Angiography and Stress Testing in Patients with Acute Chest Pain. Clin Chem 2017;63:1724-33. [Crossref] [PubMed]
- Sandoval Y, Smith SW, Thordsen SE, et al. Diagnostic Performance of High Sensitivity Compared with Contemporary Cardiac Troponin I for the Diagnosis of Acute Myocardial Infarction. Clin Chem 2017;63:1594-604. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Lippi G, Ferrari A, Gandini G, et al. Analytical evaluation of the new Beckman Coulter Access high sensitivity cardiac troponin I immunoassay. Clin Chem Lab Med 2017;56:157-61. [Crossref] [PubMed]
- Lippi G, Dipalo M, Avanzini P, et al. Analytical assessment of the Beckman Coulter Unicel DxI AccuTnI+3 immunoassay. Diagnosis 2014;1:195-7. [Crossref]
- Sheehan P, Blennerhassett J, Vasikaran SD. Decision limit for troponin I and assay performance. Ann Clin Biochem 2002;39:231-6. [Crossref] [PubMed]
- Body R. High-Sensitivity Troponin: Star Player but No Lone Hero. Clin Chem 2017;63:1555-6. [Crossref] [PubMed]
- Lippi G, Cervellin G, Robuschi E, et al. Comparison of high sensitivity and contemporary troponin I immunoassays for the early detection of acute myocardial infarction in the emergency department. Ann Clin Biochem 2012;49:205-6. [Crossref] [PubMed]
- Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011;306:2684-93. [Crossref] [PubMed]
- Boeddinghaus J, Nestelberger T, Twerenbold R, et al. Direct Comparison of 4 Very Early Rule-Out Strategies for Acute Myocardial Infarction Using High-Sensitivity Cardiac Troponin I. Circulation 2017;135:1597-611. [Crossref] [PubMed]
Cite this article as: Lippi G, Sanchis-Gomar F, Aloe R, Bonfanti L, Salvagno GL, Cervellin G. High-sensitivity cardiac troponin I immunoassay reduces the chance of patient misclassification in the emergency department. J Lab Precis Med 2017;2:93.


