
Detection of membrane antigens of extracellular vesicles by surface plasmon resonance
Extracellular vesicles (EVs) are membrane-bound vesicles released from cells to the extracellular milieu, playing an important role in the biological processes, including intercellular communications (1). EVs can be generally divided into exosomes, microvesicles (MVs), and apoptotic bodies largely by their size, despite debate about their definition (2). Recently, the application of EVs as a tool for non-invasive liquid biopsy in tumor specimens has been investigated in prostate cancer (3), glioblastoma (4), and pancreatic cancer (5). Therefore, it is important to develop a better detection technique for the surface antigen of EVs, leading to the discovery of a precise biomarker for the diagnosis and prognosis of tumor. Currently, there are several techniques being used for the detection and characterization of EVs, including nanoparticle tracking analysis, dynamic light scattering analysis, zeta potential analysis, tunable resistive pulse sensing, Raman spectroscopy, electron microscopy, enzyme-linked immunosorbent assay, western blot, and “omics” such as proteomics and RNA-seq (6). In addition, surface plasmon resonance (SPR) sensing has emerged as a widespread biophysical sensing tool for the analysis of membrane molecules, including surface proteins and carbohydrates. Recently, a plethora of research on the detection of EVs by SPR technique has been carried out for various purposes (Table 1).
Table 1
| Technique | Type of EV detected | Peculiarity | Reference |
|---|---|---|---|
| nPLEX (nano-plasmonic exosome) or nanohole-based SPR sensor | Exosomes | Characterization of exosomes | (7) |
| SPR | EVs derived from inflammation-triggered endothelial cells | Characterization of EV biomarkers in primary cells derived from patients with acute CHD | (8) |
| LSPR with self-assembly gold nanoislands (SAM-AuNIs) | Exosomes and MVs | Direct detection without functionalization of the LSPR chip | (2) |
| Dual-Wavelength SPR | Exosomes | Determining the size and concentration of sub-populations of EVs | (9) |
| SPR | Clinically relevant exosomes | Detection of human epidermal growth factor receptor 2 (Her2)-positive exosomes | (10) |
| Biacore 3000 instrument (GE Healthcare) | Exosomes | Molecular screening of exosomes derived from cancer | (11) |
| Aptamer-based SPR | Exosomes | CD63-specific aptamer-based SPR for biosensing exosomes | (12) |
| SPRi | Exosomes | Quantitative detection of exosomes | (13) |
| SPR | Exosomes | Determination of concentration of exosome in solution | (14) |
| SPR | Exosomes | Detection of exosome internalization by prostate cancer cells | (15) |
EVs, extracellular vesicles; SPR, surface plasmon resonance; SPRi, surface plasmon resonance imaging; MVs, microvesicles; CHD, coronary heart disease.
In the paper entitled “Surface plasmon resonance is an analytically sensitive method for antigen profiling of extracellular vesicles”, Gool EL et al. established a sensitive method to detect membrane proteins, such as Her2 and EGFR, in the EVs derived from human breast cancer cell lines, including HS578T, MCF7, and SKBR3. Importantly, the density of surface antigens in these cells and their exosomes was accurately measured via employing surface plasmon resonance imaging (SPRi) and the technique was much sensitive to detect small amount of surface antigens above the limit of detection (LOD) (16). The common Kretschmann configuration setup of the SPR for the detection of EVs has been represented in Figure 1.
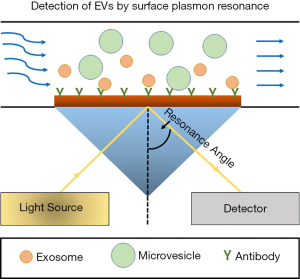
Gool EL et al. established the SPRi device in which the sensor surface was precoated with a gold- and 3D hydrogel- layer, leading to the reduction of nonspecific background. An array of 48 spots functionalized with various antibodies was used to capture EVs, making the conduction of high-throughput experiments much easier. Once the chip is illuminated, light goes through the coupling crystal at a constant incident angle and the alteration of reflected light from the SPR gold surface are transformed into the altered refractive index resulting from the binding of EVs. Furthermore, this whole set of event is recorded by a charge-coupled device (CCD) camera which eventually yields plot as a component of time. The stage is quick and exceptionally subtle and requires a very low volume of samples. For high throughput multiplex estimations, a multichannel fluidic cell framework is necessary, alongside the identification framework incorporated with optics comprising of a laser diode and a CCD camera (16).
In recent studies, various exosomal proteins, including exosomal membrane antigens, have been proposed as diagnostic biomarkers for cancer, such as Glypican-1 (GPC1), Apbblip, Daf2, Foxp1, Incenp, BCO31781, Aspn, and Gng2 in pancreatic cancer, CD34 in acute myeloid leukemia (AML), phosphatase and tensin homolog (PTEN) in prostate cancer, and EDIL-3/Del1 in bladder cancer (6). However, the membrane of EVs generally contains small level of antigens associated with tumors, resulting in the challenge of the phenotyping of EVs by various biosensor tools, including flow cytometry (FCM). Gool EL et al. in their paper demonstrated that SPRi had high sensitivity to detect 31 out of 33 antibody-EV combinations above the LOD by the reduction of the background signals via using hydrogel on the sensor as well as the increase of the “back-and-forth” flow. However, only 5 among 33 antibody-EV combinations exceeded the LOD by FCM, suggesting its low sensitivity for phenotyping of EVs. Particularly, in comparison with FCM, SPRi can be efficiently used as a high-throughput screening platform for the discovery of novel exosomal biomarkers in various diseases, including tumors. However, FCM can only analyze a number of biomarkers ranging from 6 to 11 at a time on a routine basis, nonetheless various types of liquid biopsies still can be conducted by FCM via using the samples from plasma, cell culture supernatant, urine, and cerebrospinal fluid (18).
Importantly, Gool EL et al. demonstrated that the SPRi response was proportional to the antigen (Her2) density of SKBR3 cells and their EVs, which was correlated with the specific fluorescence intensity (SFI) of SKBR3 cells by FCM, confirming the precise detection of exosomal Her2 by SPRi. In addition, the sensitivity of the detection by the SPRi also was determined; for example, 2×105 cells/mL were necessary on Her2 spots to surpass the LOD within 10 min of binding, whereas 2×108 SKBR3 EVs/mL were necessary to surpass the LOD.
Concluding remarks
Gool EL et al. (16) clearly demonstrated the strength of SPRi in the accurate detection of exosomal biomarkers, such as Her2, through the experimental tests of the specificity and sensitivity for various exosomal proteins. Qualitative determination of exosomal antigen could be achieved by the SPRi, however, quantitative phenotyping of EVs was not clearly demonstrated because the contribution of the concentrations and diameters of the EV particles on the SPRi response could not be determined. Finding both optimal detection methods and targets of membrane antigens of EVs might be critical for conducting better quantitative analysis of EVs by SPRi. Nonetheless, in future, quantitative analysis of exosomal biomarkers in disease progression, including tumors, can be a promising biosensing method as a liquid biopsy in the laboratories as well as the clinics.
One of the major concerns in the research of EVs is the dearth of appropriate terminology and techniques for the description of experimental results. Although the authors have referred EVs to all the vesicles in the aforementioned article, accumulated evidence has demonstrated a clear distinction between three major types of EVs, exosomes, MVs, and apoptotic bodies. Each type of EVs are synthesized and released by different biological pathways. Importantly, different type of EVs has different membrane property (2) and it contains different components, prompting us to investigate their specific role in physiological- and pathological- conditions. Therefore, the detection of specific type of EVs by SPRi might be a good diagnostic method and it also can provide a great insight into the underlying mechanism of each type of EVs in the progression of disease, such as tumor metastasis.
Acknowledgments
Funding: This work was supported by the start-up grants of City University of Hong Kong awarded to Dr. Y. Lee (Grant No. 9610340 and 7200472).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhi-Yang Li (Department of Laboratory Medicine, the Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.12.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983;33:967-78. [Crossref] [PubMed]
- Thakur A, Qiu G, Ng SP, et al. Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens Bioelectron 2017;94:400-7. [Crossref] [PubMed]
- Minciacchi VR, Zijlstra A, Rubin MA, et al. Extracellular vesicles for liquid biopsy in prostate cancer: where are we and where are we headed? Prostate Cancer Prostatic Dis 2017;20:251-8. [Crossref] [PubMed]
- Santiago-Dieppa DR, Steinberg J, Gonda D, et al. Extracellular vesicles as a platform for ‘liquid biopsy’ in glioblastoma patients. Expert Rev Mol Diagn 2014;14:819-25. [Crossref] [PubMed]
- Chronopoulos A, Lieberthal TJ, del Río Hernández AE. Exosomes as a platform for ‘liquid biopsy’in pancreatic cancer. Converg Sci Phys Oncol 2017;3:013005 [Crossref]
- Sunkara V, Woo HK, Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst 2016;141:371-81. [Crossref] [PubMed]
- Im H, Yang K, Lee H, et al. Characterization of Extracellular Vesicles by Surface Plasmon Resonance. Methods Mol Biol 2017;1660:133-41. [Crossref] [PubMed]
- Hosseinkhani B, van den Akker N, D’Haen J, et al. Direct detection of nano-scale extracellular vesicles derived from inflammation-triggered endothelial cells using surface plasmon resonance. Nanomedicine 2017;13:1663-71. [Crossref] [PubMed]
- Rupert DL, Shelke GV, Emilsson G, et al. Dual-Wavelength Surface Plasmon Resonance for Determining the Size and Concentration of Sub-Populations of Extracellular Vesicles. Anal Chem 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Sina AA, Vaidyanathan R, Dey S, et al. Real time and label free profiling of clinically relevant exosomes. Sci Rep 2016;6:30460. [Crossref] [PubMed]
- Grasso L, Wyss R, Weidenauer L, et al. Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy. Anal Bioanal Chem 2015;407:5425-32. [Crossref] [PubMed]
- Zhou Q, Rahimian A, Son K, et al. Development of an aptasensor for electrochemical detection of exosomes. Methods 2016;97:88-93. [Crossref] [PubMed]
- Zhu L, Wang K, Cui J, et al. Label-free quantitative detection of tumor-derived exosomes through surface plasmon resonance imaging. Anal Chem 2014;86:8857-64. [Crossref] [PubMed]
- Rupert DL, Lässer C, Eldh M, et al. Determination of exosome concentration in solution using surface plasmon resonance spectroscopy. Anal Chem 2014;86:5929-36. [Crossref] [PubMed]
- Suutari T. Surface Plasmon Resonance in Living Cell Sensing-SPR Responses in Cell-Exosome Interactions. Available online: http://hdl.handle.net/10138/135725
- Gool EL, Stojanovic I, Schasfoort RBM, et al. Surface Plasmon Resonance is an Analytically Sensitive Method for Antigen Profiling of Extracellular Vesicles. Clin Chem 2017;63:1633-41. [Crossref] [PubMed]
- Sabban S. Development of an in vitro model system for studying the interaction of Equus caballus IgE with its high-affinity FcεRI receptor. Available online: http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.555643
- Pugholm LH, Revenfeld AL, Søndergaard EK, et al. Antibody-Based Assays for Phenotyping of Extracellular Vesicles. Biomed Res Int 2015;2015:524817.
Cite this article as: Thakur A, Qiu G, NG SP, Wu CML, Lee Y. Detection of membrane antigens of extracellular vesicles by surface plasmon resonance. J Lab Precis Med 2017;2:98.


