Circulating blood biomarkers in essential hypertension: a literature review
Introduction
Hypertension (HTN) is the leading cause of preventable death and disability worldwide and is a major global risk factor for cardiovascular diseases (1). It has an estimated prevalence of 30–45% in the general population, resulting in an increased burden of heart diseases, vasculopathy, nephropathy and cerebrovascular damage, often with devastating consequences (1). HTN is responsible for at least 45% of deaths due to heart diseases, 51% of deaths due to stroke, and an estimated 16.5% (9.4 million) of all deaths annually (2). The diagnosis of HTN is dependent on the most recent classification of a consistent systolic blood pressure (SBP) greater than or equal to 130 mmHg and diastolic blood pressure (DBP) greater than or equal to 80 mmHg (3). Blood pressure (BP) values vary vastly and tend to increase with age in the population. The risk of vascular complications linearly increases with higher BP values (4). There are many hemodynamic factors involved in BP regulation, which include cardiac output, total peripheral resistance, stroke volume, and heart rate. At least four systems are responsible for direct BP regulation, which include the cardiac system, renal system, blood vessel tone and hormones (3).
Approximately 90% of patients with HTN have elevated BPs with no known reason and are termed having essential hypertension (EH) (5). EH defined as an idiopathic chronic elevation of systemic BP (5). This diagnosis is often one of exclusion typically after ruling out other causes of HTN (secondary hypertension). Some causes of secondary HTN include white coat HTN, pulmonary HTN, pregnancy-related HTN, Cushing syndrome, renovascular causes, renal parenchymal disease, primary hyperaldosteronism, and pheochromocytoma (6). The methods of measuring BP have evolved from the traditional office measurements with sphygmomanometers to ambulatory BP monitoring, and home BP monitoring (7). Non-invasive central BP devices have been developed, which can detect differences between the central and the peripheral BP but their use in clinical practice is controversial because large studies have not revealed a clinically significant difference in estimation of these approaches (8,9). Regardless of the device used to assess BP, the conventional practice only provides us with values and patterns. They do not answer other pertinent questions required to effectively manage and individualize management of HTN such as the precise underlying pathogenic mechanism (3). They also do not effectively relay and predict pre-existing, imminent or incident end-organ damage caused by HTN.
Biomarkers are objective, quantifiable characteristics of biological processes, which can be measured accurately and reproducibly (10). They may or may not necessarily correlate with patient’s clinical symptoms. Clinical endpoints, on the other hand, represent a study subject’s health and wellbeing from the subject’s perspective. Some specific biomarkers, however, have been well characterized and repeatedly shown to correctly predict relevant clinical outcomes across a variety of disease treatments and affected populations. As such, their presence as primary endpoints in clinical trials is widely accepted (3). Multiple blood biomarkers for HTN have been identified over the years and may shed light on the underlying processes involved in the development and progression of HTN.
Therefore, in this review, we will examine the applicability of numerous blood biomarkers studied to better understand the pathophysiology, diagnosis, progression, and therapeutic efficacy of EH. A schematic representation of sections covered in this review is shown in Figure 1.
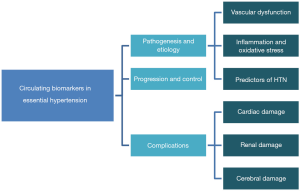
Biomarkers in the pathogenesis and diagnosis of HTN
In this section, we will review biomarkers in relation to the HTN and the following:
- Vascular dysfunction;
- Inflammation and oxidative stress;
- Predictors of hypertension.
Vascular dysfunction
Vascular dysfunction is a well-established mechanism involved in the development of EH (7). While vascular dysfunction plays a key role in the development of HTN, it can also occur as a result of HTN. Thus, evaluation of markers of vascular dysfunction, not only enable us to understand the pathogenesis of HTN, but also help us in recognizing disease progression, monitoring the efficacy of management, and the development of associated complications (7).
The renin-angiotensin-aldosterone system plays an important and central role in BP regulation (11). Some biomarkers can be quantified to help us better understand mechanisms of HTN development and can serve as potential treatment targets. Angiotensin A (Ang A), and vasoconstriction inhibiting factor (VIF) are novel vasoactive substances, readily detectable by mass spectrometry, which are involved in creating equilibrium between vasoconstriction and vasodilation (11,12). Ang A is a biologically active octapeptide, a decarboxylated derivative of angiotensin II (Ang II). It has been found that in normal human plasma, Ang A may modulate the harmful effects of Ang II. Ang A also has dose-dependent pressor and renal vasoconstrictive effects. It has been demonstrated to cause an increase in mean arterial pressure (MAP) in mice, which is blocked by AT 1 receptor antagonists such as losartan. Knocking out the AT 1 receptors nearly eliminates the vascular effects of Ang A (13). While Angiotensin A has some evidence of effecting MAPs in animal studies, clinical studies are needed. Clinical data would determine whether Angiotensin A could, in fact, be used as a target in therapeutics for BP management.
VIF is a chromogranin A peptide with vasoregulatory properties which are mediated by the AT2 receptor (12) VIF modulates the vasoconstrictive effects of Ang II. Levels of VIF are significantly increased in patients with renal and heart failure, which suggest that VIF may be a protective factor counteracting the harmful effects of Ang II. Targeting this biomarker could potentially assist in developing new treatment strategies against HTN and ultimately cardiovascular disease (12).
Endothelial microparticles (EMPs) are submicron membrane fragments, which are released into the extravascular space by cells that are either under stress or damaged (14). Though EMPs are present in the plasma and other body fluids of healthy people, their levels are also altered in many conditions such as HTN, diabetes, dyslipidemia, and chronic kidney disease among others (14). Studies have demonstrated that there is an increase in CD144+ EMP and possibly CD 31+/CD41− EMPs in HTN (15). It is also known that anti-hypertensive medications including beta-blockers, angiotensin receptor blockers, and calcium channel blockers have an impact on a certain subset of circulating EMPs (15). Though EMPs could further explain the pathophysiology of vascular dysfunction in HTN, the measurement is cumbersome, and there are limitations that make these novel biomarkers not yet clinically applicable. EMPs have been assessed in very few large samples because they require specialized specimen handling and thus are difficult to measure (15,16). Measurement of circulating EMPs relies on different centrifugation techniques in platelet-free plasma and on the identification cell-surface antigens. Moreover, the concentrations are typically measured by flow cytometry to identify cellular and molecular markers in plasma cells. Future prospective cohort studies could utilize suitable samples for EMP and other cell-based phenotyping (16). Therefore, additional research needs to be done to assess the role of EMPs in the management of HTN.
Inflammation and oxidative stress
Growing body of evidence has shown that inflammation plays a crucial role in the pathogenesis and development of HTN (17-19). Essential HTN, in particular, is characterized by increased peripheral vascular resistance to blood flow in arteries. These resistant arteries eventually undergo vascular remodeling (such as increased media width and reduced lumen size) which leads to structural and functional changes in the endothelium (18). These changes are often apparent in the early stages of HTN in part due to systemic inflammation. In addition, extracellular matrix deposition and inflammation are involved in vascular remodeling (18). In both animal and human studies, pro-inflammatory components of the renin-angiotensin-aldosterone system have been demonstrated to play a role in vascular changes (17). Vascular changes in HTN are often associated with mechanical factors that modulate downstream signaling effects, resulting in abnormal function, extracellular matrix deposition, and inflammation. HTN affects endothelium through hemodynamic changes, leading to its dysfunction, and increased levels of inflammatory markers such as interleukin-6 (IL-6), intracellular adhesion molecule 1 (ICAM1), P-selectin, and tumor necrosis factor-α (TNF-α) (18,19).
Cross-sectional studies have consistently linked higher levels of IL-6 and C-reactive protein (CRP) to cardiovascular disease and HTN (16). CRP is a well-known acute phase reactant that has been studied extensively in relation to EH (20-22). Sesso et al. studied the association of CRP, and the development of HTN in a prospective cohort consisted of 525 females aged ≥45 years with normal BP (21). The participants were followed for a median period of 7.8 years. Baseline plasma CRP and coronary risk factors were collected. The findings of the study demonstrated that higher CRP levels were significantly associated with an increased risk of developing HTN, including those with low levels of baseline BP and with no coronary risk factors (21).
Another study by Sesso et al. assessed CRP along with other plasma inflammatory markers and the risk of developing HTN in men in a prospective, nested, case-control study (20). The diagnosis of HTN was self-reported through follow-up questionnaires given to participants every 6 months. Baseline blood samples were drawn, and inflammatory markers were analyzed. The study had results including higher levels of Plasma IL-6 and D-dimer in HTN cases versus control, which was non-significant. They found that men in the highest quartile of CRP had higher baseline BP, were older, heavier, and were more likely to have a history of hyperlipidemia, smoking, and inactivity. The highest versus lowest quartile of plasma IL-6 found no differences in SBP. However, this study demonstrated that CRP and IL-6 were not significantly associated with higher risk of developing HTN in middle-aged and older men, particularly after adjusting for BMI (20).
A Study by Wang et al. evaluated about 3,500 participants to identify biomarkers with the strongest association of HTN risk and characteristics of a multimarker approach to predict the incidence of HTN (23). The studied inflammatory biomarkers included CRP, fibrinogen, and plasminogen activator-1 (PAI-1) along with brain natriuretic peptide (BNP), homocysteine, serum aldosterone, plasma renin, and urinary albumin/creatinine ratio (UACR). Results were significant for a set of three biomarkers: CRP, PAI-I, and UACR, which were associated with higher risk of developing HTN. Interestingly, the association between serum aldosterone and incidental HTN was not significant after adjusting for CRP, PAI-I, and UACR. This may suggest that interactions between aldosterone and these inflammatory markers may exist. The magnitude of the association of each biomarker was similar and also had similar regression coefficients. The study demonstrated that the association of CRP persists after accounting for other biomarkers (23). The study was limited in that it used multimarker analyses rather than individual biomarkers. The authors state that this approach is limited by practical constraints, as it is not feasible to examine all possible biomarkers as biomarkers for inclusion may vary over time and advancements in medical knowledge. Another limitation was that the cohort was predominantly a white population, the findings of which may not be generalizable to individuals of other races.
In experimental models, HTN promotes endothelial dysfunction by suppressing the endogenous production of nitric oxide (24,25). It also up-regulates angiotensin-I receptors in vascular smooth muscle, and subsequently the vasoconstrictive and proatherogenic effects of angiotensin-II (22,26). Angiotensin-II perpetuates vascular inflammation by stimulating inflammatory cytokines, recruiting monocytes, and inducing oxidative stress (22,26).
Serum uric acid levels have also found to be a useful marker of inflammation and oxidative stress in HTN. Studies have shown a significant association between uric acid levels, HTN and its cardiovascular complications (27,28). A study by Feig et al. found that by administering drugs that reduce uric acid level such as allopurinol in obese adolescents with pre-hypertension, resulted in marked BP control and reduction in systemic vascular resistance (29). The researchers recruited 30 adolescents with newly diagnosed stage 1 EH and serum uric acid levels of 6.0 mg/dL or higher. Allopurinol treatment was found to be associated with a significant decrease in SBP and DBP. The mean decrease in 24-hour ambulatory BP during allopurinol treatment was −6.3 mmHg SBP and −4.6 mmHg DBP. The decrease in ambulatory BP directly correlated with the treatment with allopurinol. The limitations of this study were its small size and population, which consisted of 30 adolescents with mild, newly, diagnosed HTN.
Moreover, several studies have shown a positive association between HTN and the neutrophil to lymphocyte ratio (NLR), which is also a reliable biomarker of systemic inflammatory status. NLR is an emerging biomarker for both cardiac and non-cardiac disorders. From a large Chinese cohort of 28,850 HTN-free individuals who were followed for about 6 years, Liu et al. demonstrated that elevated NLR significantly correlates with an increased risk of development of HTN after multivariate analysis (30). Another study conducted by Sun et al. on 341 patients ≥80 years of age, found that hypertensive patients with higher quartile NLR had higher all-cause mortality in 90 days (31). The receiver operating curve (ROC) of the NLR for all-cause mortality showed an area under the curve (AUC) =0.714 (95% CI: 0.629–0.798, P=0.000), with a critical value of 2.97, with a sensitivity and specificity of 92.6% and 52.5% respectively (31). Similar results have been obtained about the NLR with resistant HTN (32), BP variability (33) and diastolic dysfunction in hypertensive patients (34).
Recently, red blood cell distribution width (RDW) has gained immense popularity as a marker of inflammation, and prognostic factor in various disorders (35). RDW is a part of routine complete blood count (CBC) measurement and reflects the index of heterogeneity of erythrocytes in circulation. Studies have shown that it can predict prognosis in many disease states such as coronary artery disease (CAD), stroke, heart failure, peripheral artery disease, and pulmonary arterial HTN (36). A study by Bilal et al. showed that the mean RDW levels are higher in hypertensive patients giving support to the hypothesis of the role of inflammation in HTN (36). This supports the hypothesis that RDW and inflammation are closely linked, and that chronic inflammation leads to increased RDW levels. As RDW is a relatively simple and readily available test, RDW may be helpful for early diagnosis and identifying patients at a greater risk for adverse outcomes from cardiovascular disease.
Oxidative stress results in redox imbalance following the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Several signaling pathways are triggered, which ultimately results in arterial wall remodeling and arterial stiffness (5). Furthermore, ROSs are involved in renal homeostatic regulation of BP, with recent studies revealing the contribution of site-specific expression of sources of ROS such as nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) in the kidneys (37). Increased oxidative stress results in decreased availability of Nitric oxide, which is essential for maintaining vascular tone and stability.
In subjects with EH, oxidized low-density lipoprotein (ox-LDL), and plasma 8-Isoprostanglandin F2a (8-ISO PGF2a) are significantly increased in circulation. Ox-LDL has a pro-inflammatory, prothrombotic, and pro-apoptotic properties in patients with heightened oxidative stress as seen in HTN. In subjects with HTN and those with decreased arterial elasticity, there is an increase in the level of circulating ox-LDL, suggesting its role in decreasing arterial distensibility in HTN. It leads to stimulation of monocytic infiltrates; migration and proliferation of smooth muscle cell, ultimately contributing to endothelial dysfunction and damage (38). Patients with EH also have increased lipid peroxidation. There is a significant increase in the plasma levels of 8-ISO PGF2a in such patients. This is an isomer of prostaglandins generated through a free radical catalyzed attack of esterified arachidonic acid in cell membranes and lipoproteins. 8-ISO PGF2a is a highly sensitive and specific biomarker of oxidative stress in vivo (38,39). It is a very stable isoprostane, which acts as a potent renal vasoconstrictor and also stimulates endothelin-1 release in the aortic endothelial cells. Studies have shown that administering an endothelin antagonist to Pigs with hypertrophic cardiomyopathy depletes the 8-ISO PGF2a, thus reducing the oxidative stress (38). Other biomarkers increased in oxidative stress include asymmetric dimethylarginin (ADMA), symmetric dimethylarginin (SDMA) that are found to be markedly elevated in uncontrolled HTN (37).
Biomarkers that predict development of hypertension
Overt HTN is preceded by abnormalities in multiple biological pathways (38). It is thus plausible that biomarkers involved in these multi-pathway abnormalities in normotensive or pre-hypertensive patients could foretell the increased risk of HTN. Studies have shown that pharmacological treatment of patients with prehypertension can delay the onset of HTN (40,41). It is, therefore, important to isolate biomarkers that can predict the high risk of developing HTN. As discussed above, because the obese adolescents with increased uric acid are young, have no established vascular disease, and are not hypertensive, it is proposed that increased uric acid could serve as a biomarker that adds to risk stratification, and could be a potential target for therapy.
In multiple cohorts, high levels of CRP in normotensive patients have predicted the development of HTN on follow up (41-43). Also, increased levels of plasminogen activator inhibitor-1 (PAI-1), a serine protease inhibitor, which is an inhibitor of tissue plasminogen activator, a marker of reduced fibrinolytic potential and a well-established predictor of EH. While this review is focused on blood biomarkers, we should note that data from multiple cohorts have revealed that urinary albumin excretion (UAE) predicted the incidence of HTN better than CRP and PAI-1 (23). Furthermore, high UAE, which indicates mild renal disease, is also involved in the development of HTN. This was further tested in a study by Brantsma and colleagues, which found that the risk of developing HTN was highest in groups with elevated UAE and low glomerular filtration rates (GFRs) (42). It is well established that the renal system plays a central role in the pathogenesis of HTN in patients with severe renal damage. This study shows that even individuals with mild renal damage may be at risk of developing HTN.
There is also data suggesting that biomarkers like fibrinogen, renin, aldosterone, B-type natriuretic peptide, N-terminal pro-atrial natriuretic peptide, vitamin D3, and homocysteine are associated with the development of HTN. Homocysteine is a sulfur-containing amino acid that is derived from methionine via methyl group metabolism. It is widely known that hyperhomocysteinemia plays a critical role in the development of cardiovascular disease. There is a positive correlation between the levels of homocysteine and the incidence of myocardial infarction. Homocysteine has been shown to negatively impact endothelial cell function, affecting cardiovascular health (44). The impact on endothelial cell death may also contribute to the development of hypertension. In a study of 500 patients, those with a high plasma concentration of homocysteine had an odds ratio of 1.66 for the development of HTN within ten years. Homocysteine levels, intima-media thickness, and CRP were significantly higher in hypertensive patients. Moreover, in pre-hypertensive patients, circulating homocysteine levels are related to increased arterial stiffness, and therefore, may be a valuable biomarker in the development of HTN (44).
While a number of these biomarkers have significantly altered levels in circulation, the change noticed is often modest. Also, some markers correlate with each other. An example is aldosterone level, which increases in HTN, but correlates closely with CRP and PAI-1, such that the change in aldosterone levels becomes insignificant once corrected for CRP and PAI-1. As such, it has been proposed that rather than looking at the biomarkers individually, having a ‘multimarker score’ may predict HTN with better sensitivity and specificity (23).
Biomarkers in hypertension progression and control
BP remains the best method for HTN control, and it is the primary target of all therapeutic approaches in HTN. Regardless of the pathogenesis and forms of HTN, lowering BP has been found to reduce cardiovascular risk. Monitoring biomarkers and targeting the mechanisms by which they are expressed, offers the opportunity to develop ‘disease-modifying therapy, possibly resulting in reduced or delayed incidence of HTN (7).
Biomarkers in disease progression
In the event of disease progression, there is further endothelial dysfunction, oxidative stress, and inflammation. As such, there is an increase in biomarkers such as CRP, PAI-1, ox-LDL, leptin, TNF alpha, ADMA, and SDMA (5). Some biomarkers are expressed with imminent or incident target organ dysfunction, and detecting such biomarkers could enable us to target and manage such complications. A study by Morillas et al. found that serum levels of matrix metalloproteinase-1 (MMP-1) and tissue inhibitor metalloproteinase-1 (TIMP-1) were higher in EH patients with target organ dysfunction. Also increasing levels of these biomarkers were progressively associated with an increase in the number of organs damaged (45).
The myocardial injury may also be involved in the progression of EH towards heart failure. Sato et al. demonstrated that by using high sensitivity cardiac troponin-T as a biomarker, many patients with treated EH had similar levels compared to that of patients with heart failure. This study also showed a significant correlation between left ventricular hypertrophy and troponin T. High sensitivity troponin T levels >0.003 ng/mL were independently correlated with age, renal function, and ECG voltage suggesting left ventricular hypertrophy (46).
Biomarkers in control of HTN
It is typically difficult to predict individual response to anti-hypertensive drugs. It has however been proposed that the presence of some biomarkers could help predict good treatment response to specific types of antihypertensive drug. This could eliminate the practice of switching the patient’s medications in case patient’s BP is not controlled (7).
The National Institute for Health and Care Excellence (NICE) guidelines in the United Kingdom consider age and ethnicity as a surrogate for Renin, and thus Renin-Angiotensin-Aldosterone System (RAAS) activity (7). Therefore, in patients with increased RAAS activity, administering RAAS blocking antihypertensive medications will more than likely than not control their BP (47,48). Animal experimental studies in Mice by Wang et al., revealed the angiotensin II unregulated the expression of the CXCR2 protein, leading to an increase in CD45+, and CXCR2+ cells in the aorta (49). The CXCR2 chemokine receptor mediates chemotaxis of inflammatory cells, but its exact mechanism in the pathogenesis of HTN is largely unknown and requires more research. However, in this study, CXCR2 inhibition led to the reduction of angiotensin-II-induced endothelial dysfunction, vascular superoxide generation, accumulation of inflammatory cells in the vascular walls, cytokines expression, collagen deposition, reduction in BP, and reversal of HTN induced by increased angiotensin-II levels (45). In the future, studies on how this relates to human subjects may be beneficial in managing EH.
Biomarkers of target organ damage
Given the already established causal relationship between HTN and resulting target organ damage, the degree of correlation between the level of BP and level of end-organ damage is not as much as expected. As such, biomarkers are potential tools that can be used to reclassify individuals, particularly with intermediate risk into the high or low risk of end-organ damage (50,51). To serve as a restratification tool, a biomarker should be able to provide information independent of BP and other risk factors. BP variability, for example, is an independent risk factor for stroke regardless of the level of BP (52).
Cardiac damage
In the early stages of cardiac damage, left ventricular hypertrophy and impaired diastolic filling are subclinical changes in cardiac structure and function (7,53). There is evidence that left ventricular hypertrophy is an independent risk factor for cardiovascular events, and responds differently to various antihypertensives (1,54). It can also be a target for pharmacotherapy and is currently screened for by electrocardiography and echocardiography (54).
A subclinical elevation in microalbuminuria is frequently seen in EH (55). Microalbuminuria is an important predictor of cardiovascular events and renal failure in diabetic patients. There has been an association of microalbuminuria and the development of cardiac damage in essential hypertensive patients (56). A study consisting of 112 subjects who had EH, found a significant correlation between 24-hour SBP, DBP, and UAE. UAE was found to be associated with a subclinical decrease of left ventricular function (55). It was found that microalbuminuria is a marker for the presence of higher values of BP throughout a 24-hour period. The pathogenic mechanism of microproteinuria in EH is not well understood. It is suggested that atrial natriuretic factor may play a role as a mediator of hyperfiltration. Increased intraglomerular pressures and other hemodynamic changes in the renal system could be the cause of hyperfiltration and worsening proteinuria. Nonetheless, microalbuminuria may be an early marker of cardiac involvement in patients with EH (55). A novel biomarker; Galectin 3 (gal-3) a carbohydrate-binding protein that has an important regulatory role in inflammation, immunity, and cancer is increased in HTN, and its levels strongly correlate with left ventricular hypertrophy (53). It could, therefore, be a biomarker of subclinical cardiac damage in newly diagnosed hypertensive patients (51). Studies also suggest that another biomarker; soluble angiotensin-converting enzyme 2 (sACE 2) activity correlates with heart failure and HTN with imminent heart failure (57).
Levels of other markers such as BNP, cardiac troponins, and markers of collagen turnover, are altered in the event of cardiac damage, which is independent of HTN (58,59). However, measurement of all these markers has little value at present to justify their use in routine clinical practice over the use of the traditional screening methods, BP measurement, assessment of other risk factors, EKGs and ECHOs (7).
Vascular damage
The changes in vasculature due to HTN range from endothelial dysfunction in the early stages to vascular stiffness and atherosclerotic burden (7,60). No single biomarker at present can give a full picture of pathology in the vasculature structure and function (5). However, markers of endothelial cell function and activation, such as E-selectin and fibrinogen, as well as markers of inflammation like CRP have found to correlate with some of these processes in the vasculature (5,61). Another factor that may be involved in vascular damage is plasma vascular endothelial growth factor (plasma VEGF) (62).
Plasma VEGF is a multifunctional peptide capable of inducing receptor-mediated cell proliferation and angiogenesis of the endothelium (62,63). A link has been found between VEGF and development of cardiovascular disease. It has also been shown that VEGF and its soluble receptor, Flt- is often increased in HTN and can decrease after treatment (62). A study by Tsai et al. found that VEGF levels were significantly higher in hypertensive patients with retinopathy compared to normotensive patients. The study concluded that plasma VEGF levels could be a useful marker for the detection of early microvascular damage in HTN (62). Serum cystatin-C concentration is positively and independently correlated with increased carotid artery thickness in hypertensive patients who are non-responsive to treatment or have subclinical cardiovascular dysfunction (55). The traditional methods of screening such as carotid doppler ultrasound and retinal imaging/fundoscopy remain the methods of choice in clinical use (64).
Renal damage
Serum creatinine, GFR, and UAE are considered as early biomarkers of renal dysfunction, which assess renal excretory function (7). Serum cystatin-C, which correlates with GFR, could also be very relevant regarding risk prediction of elevated BP (64). Adrenomedullin (ADM) is a marker that has a variety of biological functions such as vasodilation, natriuresis, inhibition of secretion of renin, aldosterone, vascular smooth muscle cell proliferation and migration (65). In a prospective study carried out by Hu et al., the plasma concentration of ADM, ANP, and BNP w initially measured in patients with Stage I, II, and III with or without renal dysfunction. They were then re-measured four weeks after onset of treatment with antihypertensives. The circulating level of ADM correlated positively with atrial natriuretic peptide (ANP) and BNP (P<0.05). It also correlated positively with MAP, and serum creatinine, but negatively with GFR (P<0.05). It increases with disease severity along with ANP and BNP in patients with EH, and its function may be to initiate or perpetuate mechanisms that counteract further increase in BP just like ANP and BNP. It is excreted via the kidneys. Therefore, in renal damage occurring as a complication of HTN, the levels of ADM is significantly increased compared to the control. The circulating levels of ADM, ANP, and BNP improve with an improvement in MAP and renal function on antihypertensive treatment (65).
In the future, a possible therapeutic system may be the administration of adrenomedullin to tissues or stimulation of endogenous production of ADM, ANP, and BNP to manage EH (65).
Cerebral damage
With regards to the brain, many of the studies are regarding acute cerebrovascular events as there is already an established strong correlation between uncontrolled HTN and stroke (50). Studies are being carried out to relate HTN with cognition (52). However, there is evidence of a correlation between cognition and aortic stiffness (66). Recent findings have shown a correlation between ubiquitin C-terminal hydrolase-L1 (UCH-L1) levels and cerebral white matter lesions (67). In HTN, vascular cell adhesion molecule-1 (VCAM-1) can provide information on cerebral blood flow and may identify those at highest risk of falls (68).
Several biomarkers have been explored as means of predicting cerebrovascular disease secondary to HTN, but none has been introduced into clinical practice because of the exorbitant cost, the requirement of a specific technology (68). Brain-specific proteins such as neuron-specific enolase (NSE) are promising future biomarkers that will be more readily accessible and less costly to help curb the burden of stroke and cognitive decline (50,68).
Conclusions
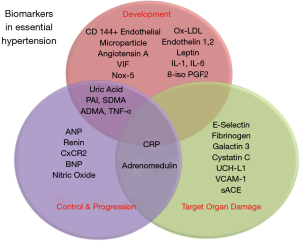
EH is a major risk factor for CAD, heart failure, stroke, renal disease, and peripheral vascular disease. As the diagnosis and treatment of HTN are mainly guided by conventional BP readings, circulating biomarkers may prove to be useful in understanding the pathogenesis, diagnosis, progression, and therapeutic efficacy. The image shown in Figure 2 depicts the biomarkers discussed in this review and their respective roles in EH. While various biomarkers already have established roles with regards to EH, most of them may not be applicable for routine clinical use for various reasons. These range from the need for further studies, exorbitant costs, absent technology, and lack of expertise. Circulating biomarkers could, however, be the future of primordial prevention, better control, and decrease in morbidity and mortality related to HTN.
Acknowledgments
We would like to thank Dr. Abhilash Perisetti, MD, FACP in systematic arrangement and Endnote styles for the manuscript references.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fabian Sanchis-Gomar) for the series “Biomarkers in cardiovascular disease” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2017.12.06). The series “Biomarkers in Cardiovascular Disease” was commissioned by the editorial office without any funding or sponsorship. Hemant Goyal serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from May 2017 to April 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schmieder RE. End organ damage in hypertension. Deutsches Arzteblatt international 2010;107:866-73. [PubMed]
- WHO. A global brief on hypertension. World Health Organization, 2013.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;2017. [Epub ahead of print].
- Malyszko J, Muntner P, Rysz J, et al. Blood pressure levels and stroke: J-curve phenomenon? Curr Hypertens Rep 2013;15:575-81. [Crossref] [PubMed]
- Kossaify A, Garcia A, Succar S, et al. Perspectives on the value of biomarkers in acute cardiac care and implications for strategic management. Biomark Insights 2013;8:115-26. [PubMed]
- Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens 2011;2011:236239.
- Currie G, Delles C. Use of Biomarkers in the Evaluation and Treatment of Hypertensive Patients. Curr Hypertens Rep 2016;18:54. [Crossref] [PubMed]
- Herbert A, Cruickshank JK, Laurent S, et al. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J 2014;35:3122-33. [Crossref] [PubMed]
- Mitchell GF. Central pressure should not be used in clinical practice. Artery Res 2015;9:8-13. [Crossref] [PubMed]
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS 2010;5:463-6. [Crossref] [PubMed]
- Jankowski V, Vanholder R, van der Giet M, et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol 2007;27:297-302. [Crossref] [PubMed]
- Salem S, Jankowski V, Asare Y, et al. Identification of the Vasoconstriction-Inhibiting Factor (VIF), a Potent Endogenous Cofactor of Angiotensin II Acting on the Angiotensin II Type 2 Receptor. Circulation 2015;131:1426-34. [Crossref] [PubMed]
- Yang R, Smolders I, Vanderheyden P, et al. Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent. Hypertension 2011;57:956-64. [Crossref] [PubMed]
- Burger D, Schock S, Thompson CS, et al. Microparticles: biomarkers and beyond. Clin Sci (Lond) 2013;124:423-41. [Crossref] [PubMed]
- Amabile N, Cheng S, Renard JM, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J 2014;35:2972-9. [Crossref] [PubMed]
- Leopold JA. Chapter 2 - The Endothelium. In: Creager MA, Beckman JA, Loscalzo J, editors. Vascular Medicine: A Companion to Braunwald's Heart Disease (Second Edition). Philadelphia: W.B. Saunders, 2013:14-24.
- Rodríguez-Iturbe B, Pons H, Quiroz Y, et al. The immunological basis of hypertension. Am J Hypertens 2014;27:1327-37. [Crossref] [PubMed]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens 2006;15:152-8. [PubMed]
- Huang Z, Chen C, Li S, et al. Serum Markers of Endothelial Dysfunction and Inflammation Increase in Hypertension with Prediabetes Mellitus. Genetic testing and molecular biomarkers 2016;20:322-7. [Crossref] [PubMed]
- Sesso HD, Jimenez MC, Wang L, et al. Plasma Inflammatory Markers and the Risk of Developing Hypertension in Men. J Am Heart Assoc 2015;4:e001802 [Crossref] [PubMed]
- Sesso HD, Wang L, Buring JE, et al. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 2007;49:304-10. [Crossref] [PubMed]
- Wang CH, Li SH, Weisel RD, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 2003;107:1783-90. [Crossref] [PubMed]
- Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension 2007;49:432-8. [Crossref] [PubMed]
- Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002;106:913-9. [Crossref] [PubMed]
- Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 2002;106:1439-41. [Crossref] [PubMed]
- Brasier AR, Recinos A 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 2002;22:1257-66. [Crossref] [PubMed]
- Mazzali M, Kanbay M, Segal MS, et al. Uric acid and hypertension: cause or effect? Curr Rheumatol Rep 2010;12:108-17. [Crossref] [PubMed]
- Sundström J, Sullivan L, D'Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005;45:28-33. [Crossref] [PubMed]
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008;300:924-32. [Crossref] [PubMed]
- Liu X, Zhang Q, Wu H, et al. Blood Neutrophil to Lymphocyte Ratio as a Predictor of Hypertension. Am J Hypertens 2015;28:1339-46. [Crossref] [PubMed]
- Sun X, Luo L, Zhao X, et al. The neutrophil-to-lymphocyte ratio on admission is a good predictor for all-cause mortality in hypertensive patients over 80 years of age. BMC Cardiovasc Disord 2017;17:167. [Crossref] [PubMed]
- Belen E, Sungur A, Sungur MA, et al. Increased Neutrophil to Lymphocyte Ratio in Patients With Resistant Hypertension. Journal of clinical hypertension 2015;17:532-7. [Crossref] [PubMed]
- Kılıçaslan B, Dursun H, Kaymak S, et al. The relationship between neutrophil to lymphocyte ratio and blood pressure variability in hypertensive and normotensive subjecs. Turk Kardiyol Dern Ars 2015;43:18-24. [Crossref] [PubMed]
- Karagöz A, Vural A, Gunaydin ZY, et al. The role of neutrophil to lymphocyte ratio as a predictor of diastolic dysfunction in hypertensive patients. Eur Rev Med Pharmacol Sci 2015;19:433-40. [PubMed]
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017;23:4879-91. [Crossref] [PubMed]
- Bilal A, Farooq JH, Kiani I, et al. Importance of Mean Red Cell Distribution Width in Hypertensive Patients. Cureus 2016;8:e902 [PubMed]
- Holterman CE, Thibodeau JF, Towaij C, et al. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 2014;25:784-97. [Crossref] [PubMed]
- Dhawan V, Sharma I, Mahajan N, et al. Implication of Endothelin-2 and Oxidative Stress Biomarkers in Essential Hypertension. J Hypertension 2014;3:1095-2167.
- Praticò D, Iuliano L, Mauriello A, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest 1997;100:2028-34. [Crossref] [PubMed]
- Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006;354:1685-97. [Crossref] [PubMed]
- Lüders S, Schrader J, Berger J, et al. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens 2008;26:1487-96. [Crossref] [PubMed]
- Brantsma AH, Bakker SJ, de Zeeuw D, et al. Extended prognostic value of urinary albumin excretion for cardiovascular events. J Am Soc Nephrol 2008;19:1785-91. [Crossref] [PubMed]
- Hage FG. C-reactive protein and hypertension. J Hum Hypertens 2014;28:410-5. [Crossref] [PubMed]
- Schalinske KL, Smazal AL. Homocysteine imbalance: a pathological metabolic marker. Adv Nutr 2012;3:755-62. [Crossref] [PubMed]
- Morillas P, Quiles J, de Andrade H, et al. Circulating biomarkers of collagen metabolism in arterial hypertension: relevance of target organ damage. J Hypertens 2013;31:1611-7. [Crossref] [PubMed]
- Sato Y, Yamamoto E, Sawa T, et al. High-sensitivity cardiac troponin T in essential hypertension. J Cardiol 2011;58:226-31. [Crossref] [PubMed]
- Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059-68. [Crossref] [PubMed]
- Bühler FR, Bolli P, Kiowski W, et al. Renin profiling to select antihypertensive baseline drugs. Renin inhibitors for high-renin and calcium entry blockers for low-renin patients. Am J Med 1984;77:36-42. [PubMed]
- Wang L, Zhao XC, Cui W, et al. Genetic and Pharmacologic Inhibition of the Chemokine Receptor CXCR2 Prevents Experimental Hypertension and Vascular Dysfunction. Circulation 2016;134:1353-68. [Crossref] [PubMed]
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. [Crossref] [PubMed]
- Devereux RB, Pickering TG, Alderman MH, et al. Left ventricular hypertrophy in hypertension. Prevalence and relationship to pathophysiologic variables. Hypertension 1987;9:II53-60. [Crossref] [PubMed]
- Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895-905. [Crossref] [PubMed]
- Nar G, Aksan G, Gorgulu O, et al. Galectin-3 as a novel biomarker for the diagnosis of essential hypertension with left ventricular hypertrophy. J Exp Clin Med 2016;33:123-8.
- Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561-6. [Crossref] [PubMed]
- Mettimano M, Specchia ML, Migneco A, et al. Microalbuminuria as a marker of cardiac damage in essential hypertension. Eur Rev Med Pharmacol Sci 2001;5:31-6. [PubMed]
- Palatini P. Microalbuminuria in hypertension. Curr Hypertens Rep 2003;5:208-14. [Crossref] [PubMed]
- Úri K, Fagyas M, Manyine Siket I, et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PloS One 2014;9:e87845 [Crossref] [PubMed]
- Takeda T, Kohno M. Brain natriuretic peptide in hypertension. Hypertens Res 1995;18:259-66. [Crossref] [PubMed]
- Tanindi A, Cemri M. Troponin elevation in conditions other than acute coronary syndromes. Vasc Health Risk Manag 2011;7:597-603. [Crossref] [PubMed]
- Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens 2014;27:114-21. [Crossref] [PubMed]
- de la Sierra A, Larrousse M. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens 2010;24:373-9. [Crossref] [PubMed]
- Tsai WC, Li YH, Huang YY, et al. Plasma vascular endothelial growth factor as a marker for early vascular damage in hypertension. Clin Sci (Lond) 2005;109:39-43. [Crossref] [PubMed]
- Nadar SK, Blann AD, Lip GY. Plasma and platelet-derived vascular endothelial growth factor and angiopoietin-1 in hypertension: effects of antihypertensive therapy. J Intern Med 2004;256:331-7. [Crossref] [PubMed]
- Čabarkapa V, Ilinčić B, Đerić M, et al. Cystatin C, vascular biomarkers and measured glomerular filtration rate in patients with unresponsive hypertensive phenotype: a pilot study. Ren Fail 2017;39:203-10. [Crossref] [PubMed]
- Hu W, Zhou PH, Zhang XB, et al. Plasma concentrations of adrenomedullin and natriuretic peptides in patients with essential hypertension. Exp Ther Med 2015;9:1901-8. [Crossref] [PubMed]
- Pase MP, Himali JJ, Mitchell GF, et al. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension 2016;67:513-9. [PubMed]
- Li Y, Sun Y, Li J, et al. Changes of ubiquitin C-terminal hydrolase-L1 levels in serum and urine of patients with white matter lesions. J Neurol Sci 2015;357:215-21. [Crossref] [PubMed]
- González-Quevedo A, González-García S, Peña-Sánchez M, et al. Blood-Based Biomarkers Could Help Identify Subclinical Brain Damage Caused by Arterial Hypertension. MEDICC Review 2017;18:46-53.
Cite this article as: Shere A, Eletta O, Goyal H. Circulating blood biomarkers in essential hypertension: a literature review. J Lab Precis Med 2017;2:99.