
Pathophysiological mechanisms determining sex differences in circulating levels of cardiac natriuretic peptides and cardiac troponins
Background
It is well known that adult fertile women have higher circulating levels of cardiac natriuretic peptides (NPs) than men of the same age (1-5). On the contrary, men have on average higher circulating levels of cardiac troponin I (cTnI) and T (cTnT) than women (6). Furthermore, the circulating levels of both cardiac NPs and troponins progressively increase throughout the senescence in both sexes (1-6). These sex- dependent differences in circulating levels between NPs and cardiac troponins are closely related to complex physiological mechanisms, which are differently regulated in men and women (7-10). Some cardiac and extra-cardiac diseases with sex-related prevalence can also affect these physiological mechanisms, and in this way to allow more difficult the clinical interpretations of cardiac biomarker measurement with immunoassay methods (11-20).
The aim of this review article is to discuss the available evidence of the possible different mechanisms related to production, secretion and degradation of cardiac NPs and cardiac troponins in men and women.
NP system
Biochemical and physiological considerations
Human cardiomyocytes produce and secrete a family of related peptide hormones (i.e., cardiac natriuretic hormones) with similar biochemical structure and physiological activities (1,11). Cardiac NPs include atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and their related peptides, while other NPs, such as C-type natriuretic peptide (CNP), Dendroaspis (or D-type) natriuretic peptide (DNP) and urodilatin, structurally related to the ANP/BNP peptide family, are not produced and secreted by cardiomyocytes, but by other tissues (1,11). All NPs share a similar peptide structure characterized by a similar peptide ring (1,11).
All NPs share a direct diuretic, natriuretic and vasodilator effect and an inhibitory and protective action on inflammatory processes of myocardium, endothelium and smooth muscle cells, in this way modulating coagulation and fibrinolysis pathways, and inhibiting platelet activation (1,11). All the most important biological effects of NPs are mediated throughout two different guanylate cyclase-coupled receptors, NPR-A (more specific for ANP and BNP) and NPR-B (more specific for CNP) (12). A third specific receptor NPR-C, not coupled to a guanylate cyclase, has fundamentally a clearance function for all NPs (12).
Sex- and age-related physiological actions on cardiac NP system
Plasma BNP concentration is minimal in children with similar values in both sexes (Table 1), while sex-difference between hormone levels increase progressively throughout the adolescence, reaching values in normal cycling women about two-fold higher than men (Table 2 and Figure 1) (1,2,8,10,21-24). These data suggest that sex steroid hormones may play a relevant physiological role in the regulation of production/secretion of cardiac NPs (1,2,7,9,11,21). According to this hypothesis, some Authors suggested that female steroid hormones, in particular estrogens, have a stimulating effect on the cardiac natriuretic hormone system (1,25-28). In particular, the stimulatory action of female steroid hormones on production/secretion of cardiac natriuretic hormones was reported in post-menopausal women, where the administration of estrogens induced a greater increase in plasma BNP than ANP (26,27). On the other hand, the male steroid hormone may have an inhibitory action on the production/secretion of ANP and BNP by myocardiocytes (9,16,25). Indeed, transdermal testosterone administration to women with hypoandrogenemia due to hypopituitarism induced a significant fall in circulating NT-proBNP levels (29).
Table 1
| Groups (time periods from birth) | Number of individuals | Mean ± SD | Median | Range | 97.5th percentile | P value |
|---|---|---|---|---|---|---|
| 0–2 days | 68 | 280.3±167.5 | 243.5 | 41–866 | 758.7 | <0.0001 * |
| 3–30 days | 75 | 136.1±149.3 | 75 | 10–763 | 741.4 | <0.0001** |
| 1–12 months | 46 | 20.3±10.7 | 19 | 5–45 | 43.9 | – |
| 1–12 years | 64 | 15.7±8.9 | 13 | 4–46 | 39,8 | – |
| All groups (0-12 years) | 253 | 123.4±160.1 | 38 | 4–866 | 622.0 | – |
Range: minimum and maximum values; *, significantly higher than all the other next time period values; **, significantly higher than the values observed throughout the next time periods (i.e., 2–12 months and 2–12 years). The characteristics of the subjects studied are reported in detail elsewhere (8).
Table 2
| Age groups | Men | Women | P value* |
|---|---|---|---|
| AGE 20–50 years [N] | 5.9±6.0 [79] | 10.0±8.3 [91] | <0.0001 |
| AGE ≥50 years [N] | 10.1±7.8 [53] | 15.6±11.8 [68] | 0.0033 |
| P value* | 0.0009 | 0.0020 |
The subjects are the same reported in Figure 1A and B. The age cut-off of 50 years was chosen because corresponding to mean age of menopause in Western European countries. The number of subjects included in each subgroup is indicated within brackets. *, unpaired t-test using the logarithmic transformation of the original set of data. The characteristics of the studied population are reported in detail elsewhere (7). Plasma BNP was measured in the Authors’ laboratory with a commercial two-site immunoradiometric assay (Shionoria BNP, Shionogi & Co., Japan). BNP, brain natriuretic peptide.
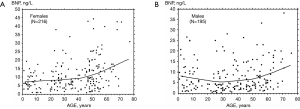
Unfortunately, there are few experimental studies in animal models specifically designed to investigate the influence of sex steroid hormones on gene expression of cardiac natriuretic hormones, and most of these are focused on ANP rather than BNP (1,2,7,9). Furthermore, these studies have some important limitations: rodents rather than primates, and castrated rather than fertile animals have been studied. As a consequence, the results of these studied should be applied with extreme caution to healthy fertile men and women. In rats, ovariectomy and orchiectomy decreased atrial ANP mRNA transcripts in vivo, while the pretreatment for 7 days of Wistar female rats with estradiol and progesterone increased atrial ANP gene expression (30). On the other hand, only scarce and contradictory data have been reported on the action of male steroid hormones on the ANP/BNP systems (7,9). In vivo pre-treatment with testosterone for 7 days of gonadectomized Wistar male rats increases atrial ANP gene expression (30). Another study observed increased plasma ANP concentrations and atrial stores in the castrated male rats, while testosterone replacement decreased plasma ANP concentration in castrated male rats, but not atrial stores (31). Other studies reported that testosterone stimulates synthesis and secretion of both ventricular and atrial ANP in newborn rat atrial cultured myocytes (32,33).
As far as studies in humans are concerned, androgen receptor blockade and, to a lesser extent, androgen suppression cause an increase in NT-proBNP in men with prostate cancer (34). These data, taken as a whole, indicate that female sex steroid hormones (especially estrogens) have a stimulating action on cardiac endocrine function, while androgens an inhibitory effect.
Two large population-based studies evaluated the role of male sex steroid hormones on the regulation of cardiac endocrine function in humans. The Dallas Heart Study (25) reported no association between estrogen status and NT-proBNP levels, whereas a strong inverse association was found between free testosterone and NT-proBNP in young women (age range 35–49 years). Saenger et al. (35) reported an inverse association of NT-proBNP with serum testosterone, a direct association with sex hormone binding globulin (SHBG), and any significant association with circulating levels of estradiol in a large population of girls and boys (age range from 2 months up to 18 years).
Taking as whole the above mentioned data (1,2,5,7-11,16,21-36), some authors assumed as working hypothesis that female and male sex steroid hormones together contribute to the regulation of production/secretion of ANP and BNP: estrogens may have a stimulatory, while androgens a inhibitory action (1,7,9,11,21). The inter-relationships between NPs and steroid hormones may be schematically represented by three distinct retroactive mechanisms (1,7-11,21,36). In normal cycling women NPs may share an inhibitory action on steroidogenesis, follicular development, granulosa cell maturation and ovulation, while estrogens have a stimulatory effect on production/secretion of ANP and BNP production/secretion by cardiomyocytes (1,7-11,21,36). Conversely, in adult men NPs may share a stimulatory effect on testis steroidogenesis, while androgens may have an inhibitory effect on ANP and BNP production/secretion by cardiomyocytes (1,7-11,21,36). Adrenal corticosteroids (including glucocorticosteroids and mineral corticosteroids) show both direct and indirect stimulating actions on cardiac endocrine function, while NPs inhibit the production of corticosteroids by adrenal gland both directly (i.e., throughout the specific NPs receptors on zona fasciculata and glomerulosa cells of adrenal gland) and indirectly (i.e., by inhibiting the action of renin-angiotensin system) (1,7-11,21,36). This working hypothesis, however, requires further evidences: in particular, it should be demonstrated that sex steroids are actually able to affect the production/secretion of BNP in mammalian cardiomyocytes both in cell cultures and in vivo.
Cardiac NP system: pathophysiological and clinical considerations
NP system shares complex interactions with the neuro-hormonal system: several studies aimed at clarifying the possible relationships among cardiac endocrine function, sex and cardiovascular risk. Indeed, NPs exert several cardio-protective actions, including: (I) vasodilation; (II) increase in natriuresis and diuresis; (III) anti-hypertrophic and anti-remodeling effects on myocardial tissue; (IV) improvement in endothelial function and anti-atherosclerotic effects; (V) counter-regulatory action on sympathetic nervous system and several hormones, including aldosterone, angiotensin II, endothelins, renin, and vasopressin (1,11,37).
Cardio-protective effects of female steroids may explain why cardiovascular risk is significantly lower in healthy premenopausal women compared to men (16,26,38-40), while the sex-related risk difference is progressively abolished after menopause (38-40). These data suggest an important beneficial role of female sex steroids on pathophysiological mechanisms related to cardiovascular diseases (7,9,16,21,26-28). On the other hand, the complex inter-relationship between NP system and sex-related gonadal function could play a relevant role in determining the sex and age-related differences in cardiovascular pathophysiological mechanisms, clinical presentation, and disease course. Indeed, it is well known that sex and age-specific differences are actually observed in cardiovascular function, electrocardiogram, heart chamber volumes, arterial vessels sizes, microcirculation function and circulating biomarkers between males and females (15).
Circulating levels of sex steroids and NPs show wide and differently related fluctuations throughout all the life span. Circulating levels of NPs are very high after birth and progressively fall throughout the first months of life (Table 1) (1,8,41-44), when, on the contrary, circulating sex steroids levels are lower than those of adult age. It is conceivable that high NP levels during neonatal period may help the adaptation of cardiovascular system of newborn to physiological conditions of extra-uterine life (8,43,44). In particular, NPs may alleviate the increased ventricular afterload of both ventricles after birth, and may also support heart function with a decreased preload in the first days of life (8,43-45).
During adolescence, NP levels of girls become progressively higher than boys, so that in the adult life fertile women (Figure 1A and Table 2) on average show BNP and NT-proBNP values about two-fold higher than men (Figure 1B) (1,8,16). It is conceivable that the NP action during the fertile period of women actually play a fundamental role in determining the sex-related differences in development, function, and disease susceptibility of cardiovascular system (16,46,47).
In particular during pregnancy, BNP and NT-proBNP levels show an early increase. NP levels are increased throughout all gestation until about 72 h after delivery with concentration peaks twice higher than in non-pregnant women (16,48-50). It is conceivable that NP system can significantly contribute to the regulation of fluids hemodynamics and cardiac function during pregnancy. Indeed, normal pregnant women show an increase in heart rate (from 10 to 20 bpm), cardiac output (up to 30–50%), but a fall in systemic vascular resistance (on average 20–30%) (50). In particular, the fall in systemic vascular resistance is observed only in the first period of pregnancy (up to 20 to 24 weeks of gestation), while in the last period of pregnancy there is a slow rise of systemic vascular resistance, however without reaching pre-pregnancy values (50). A similar trend is also found for mean arterial pressure with a fall (up to about 10%) in the first 20–24 weeks, and then a slow increase toward pre-pregnancy values approaching delivery (50). Adverse maternal cardiac events have been associated with high BNP concentrations (>100 ng/L), and its use as a negative predictive indicator appears to be of most value (50). In particular, NP assay was suggested to be useful in the diagnosis of pregnancy complications, such as acute HF, preeclampsia or eclampsia (50-52). In preeclampsia, it was also suggested that NT-proBNP may reflect ventricular stress and subclinical cardiac dysfunction worsening, especially if fetal growth restriction is present (53).
After menopause, NP levels progressively increase in women, and sex-related differences in BNP and NT-proBNP concentrations progressively tend to reduce (1,8,16). High NP levels in some individuals over 65 years are likely related to the parallel increase in the incidence of heart failure (HF), especially women with asymptomatic or paucisymptomatic HF with preserved ejection fraction (HFpEF) (16,46,54-56). As a result, BNP and NT-proBNP assay is recommended by all the most recent international guidelines for early diagnosis and risk assessment in HF patients (47,57,58).
Cardiac troponin I and T
Analytical and pathophysiological considerations
All international guidelines and expert documents recommend that cTnI and cTnT should be the preferred biomarkers for differential diagnosis of acute coronary syndrome (ACS), and also that the decision cut-off value (i.e., 99th URL) should be measured with an imprecision of ≤10 CV% (59-62). However, measurement of the 99th URL of cTnI and cTnT levels is a challenging task, due to low biomarker concentrations in healthy subjects, especially women and children (6,21,36,59,60,63,64). For this reason, only after the year 2006, some manufacturers set-up the first new generation of cTnI and cTnT immunoassays with improved analytical sensitivity in accordance with the quality specifications indicated by international guidelines and consensus documents (6,21,36,64-67). According to 2018 IFCC Task Force on Clinical Applications of Cardiac Bio-Markers (Academy of AACC and Task Force of IFCC) (61), immunoassay methods should be able to measure cTnI and cTnT circulating levels even in the majority of healthy adult subjects of both sexes. In particular, high-sensitivity methods should also be able to measure troponin levels in the majority of healthy adult subjects (>50%) enrolled in large populations (more than 300 individuals) including both men and women.
From a clinical point of view, it is important to stress that high-sensitivity cTnI and cTnT immunoassay methods should be able to detect even minimal (microscopic) amount of myocardial damage (36,59,60). In particular, some recent immunoassay methods should measure 99th URL values, corresponding to about 10–40 ng/L for cTnI (Table 3) and to 14 ng/L for cTnT, with an error ≤10% CV (Table 4) (64,68-72). Using an experimental protocol, including rat, ovine and human myocardial tissues, Marjot et al. (59) recently demonstrated that circulating cTnI and cTnT levels, corresponding to 99th URL value, are related to necrosis of about 40 mg of myocardium. In Table 4, the column named “Ratio” reports the calculation between the 99th URL values (suggested by the manufacturer) (Table 3) and the cTnI value measured with a CV% equal to 10% (i.e., LoQ 10%). These results indicate that the cTnI methods with ratio values ≥4 measure the 99th URL values with an imprecision (expressed as CV) of about 4–6% (63,68,69), which is significantly lower than the CV value required by international guidelines (61,62).
Table 3
| Populations | Architect method | Access hs cTnI method | ADVIA Centaur hs method |
|---|---|---|---|
| General population | 26.2 ng/L | 17.5 (12.6–20.7) ng/L | 47.34 (36.39–64.27) ng/L |
| Women | 15.6 ng/L | 11.6 (8.4–18.3) ng/L | 36.99 (30.22–72.63) ng/L |
| Men | 34.2 ng/L | 19.8 (14.0–42.9) ng/L | 57.27 (38.58–90.15) ng/L |
| Number of subjects | 1,531 | 1,098 | 2,010 |
The 99th URL values, reported in the table, are those suggested by the manufacturers. The 95% confidence limits are reported in brackets.
Table 4
| Methods | LoB (ng/L) | LoD (ng/L) | LoQ 20% CV (ng/L) | LoQ 10% CV (ng/L) | Ratio | References |
|---|---|---|---|---|---|---|
| cTnI | ||||||
| Architect | 0.7 | 1.3 | 1.8 | 4.7 | 5 | (64) |
| Access DxI | 0.6 | 1.3 | 2.1 | 5.3 | 4 | (68) |
| ADVIA | 1.0 | 2.2 | 3.5 | 8.4 | 5.6 | (69) |
| AIA | 1.1 | 2.1 | 15.0 | 30.9 | 1 | (70) |
| cTnT | ||||||
| ECLIA | 3 | 3-5 | 6 | 13 | 1.3 | (71) |
Data reported in this Table were obtained in the laboratory of Fondazione CNR Regione Toscana G. Monasterio (Pisa, Italy) according to the references (64,68-71). The ratio value was calculated by dividing the 99th URL value, suggested by the manufacturer, and the LoQ 10% CV evaluated in the reference laboratory. Architect, STAT Architect highly Sensitive TnI for Architect i1000SR platform (Abbott Diagnostics, Ref. B3P250); Access DxI, Access hsTnI (IUO) for DxI platform (REF B52699, Beckman Coulter, Inc. Brea, CA 92821 USA); ADVIA, ADVIA Centaur High-Sensitivity Troponin I (TNIH) (Ref. 10994774-5) for Centaur XPT platform (Siemens Healthineers Diagnostics, Erlangen, Germany); AIA, CLEIA method (CL AIA-PACK cTnI TEST) for automated AIA-CL2400 platform (TOSOH BIOSCIENCE, Tessenderlo, Belgium); ECLIA, ECLIA hs-cTnT method (Ref. 05092744) for Cobas e411 platform (Roche Diagnostics, Mannheim, Germany).
Therefore, according to the data reported in Tables 3 and 4, only the cTnI and cTnT immunoassay methods with a LoD value of about 1–2 ng/L and a ratio values ≥4 should be considered high-sensitivity methods. Of course, the high-sensitivity cTnI methods should be also able to measure cTnI levels in the majority of healthy adult men and women (61). However, at the moment of preparation of this review article, only for the Architect method several independent studies were available in literature demonstrating that this method is able to measure cTnI levels in the majority of healthy adult women and men (63).
It is important to note that the analytical sensitivity of the last generation of cTn immunoassays (Table 4) is much better than the spatial resolution of the most recent NMR cardiac imaging techniques, which are not able to detect necrosis of 40 mg of myocardial tissue (36,59,60). Experimental data indicate that the adult mammalian heart (including human) is capable of limited renewal of cardiomyocytes by means of mitosis and/or cellular replacement originating from stem cells (73-75). Consequently, circulating cTnI and cTnT levels, measured with high-sensitivity methods in healthy subjects, could be envisaged as a reliable index of the physiological renewal of human cardiomyocytes (36).
However, two important clinical points should be taken into-account. First, it is important to underline that increased levels of cardiac biomarker demonstrate the presence of myocardial damage, but cTnI or cTnT assay is not able to indicate the specific mechanism of myocardial injury (36,59,60,62). Thus, increased cTn values, in the absence of clinical evidence of ischemia, should prompt a search for other causes of cardiac damage (Table 5). Moreover, currently available high-sensitivity methods demonstrate no threshold below which cTn variations are harmless and without negative implications for prognosis (36,59,60,63,76-87). Therefore, the high-sensitivity cTnI and cTnT immunoassay methods are essential for risk assessment, clinical stratification and prognosis of patients with cardiovascular diseases even in general population (36,59,60,63,76-87).
Table 5
| Injury related to primary myocardial ischemia |
| Plaque rupture |
| Intraluminal coronary artery thrombus formation |
| Injury related to supply/demand imbalance of myocardial ischemia |
| Tachy-/brady-arrhythmias |
| Aortic dissection or severe aortic valve disease |
| Hypertrophic cardiomyopathy |
| Cardiogenic, hypovolemic, or septic shock |
| Severe respiratory failure |
| Severe anemia |
| Hypertension with or without LVH |
| Coronary spasm |
| Coronary embolism or vasculitis |
| Coronary endothelial dysfunction without significant cardiac disease |
| Injury not related to myocardial ischemia |
| Cardiac contusion, surgery, ablation, pacing, or defibrillator shocks |
| Rhabdomyolysis with cardiac involvement |
| Myocarditis |
| Cardiotoxic agents (such as anthracyclines), Herceptin, drugs of abuse (such as cocaine) |
| Multifactorial or indeterminate myocardial injury |
| Heart failure (chronic and acute) |
| Stress cardiomyopathy (such as Takotsubo disease) |
| Severe pulmonary embolism or pulmonary hypertension |
| Sepsis, critically ill patients |
| Renal failure (chronic and acute) |
| Severe acute neurological diseases (such as stroke and subarachnoid hemorrhage) |
| Infiltrative diseases (such as amyloidosis and sarcoidosis) |
| Strenuous exercise |
Sex and age-related difference in circulating levels of cTnI and cTnT
There are actually some fundamental issues to discuss about the estimation of 99th URL values: (I) there are sex-related differences in circulating cTnI and cTnT levels due to lower 99th URL values in women than men; (II) there are also age-related differences in circulating cTn levels; (III) there are still large variations among the 99th URL values suggested by manufacturers even among the most recent cTnI assays (Table 3). These issues can lead to misclassification in the differential diagnosis of ACS, and these may be particularly problematic for women, resulting in underdiagnosis of acute myocardial infarction (AMI) in women (88).
Using high-sensitivity methods, sex-related difference in circulating levels of both cTnI and cTnT has been confirmed in healthy adult subjects (16,36,60,63,65-67,71,72). In particular, considering the most recent studies including large populations of different ethnic origins, a meta-analysis reported that there is a significant mean difference of 10.97 ng/L (95% CI: 7.10–14.85 ng/L) between the cTnI concentrations (measured by Architect method) of adult men and women, while the sex-related difference for cTnT (measured by ECLIA method) is on average 4.59 ng/L (95% CI: 1.60–7.57 ng/L) (63). It is usually assumed that this sex difference in cTn circulating levels is due to different cardiac mass (15) (and so also cardiomyocyte renewal) between adult healthy men and women (36,59,60).
From a clinical point of view, a fundamental question is whether the use of sex-specific cutoff values for high-sensitivity cTnI and/or cTnT assays actually allows a more accurate detection of patients with non-ST-segment elevation acute coronary syndromes (NSTEMI-ACS), who are at higher risk of presenting major cardiovascular events in the short term. Considering high-sensitivity cTnI assays, several studies confirmed that the use of sex-specific decision values significantly improve both diagnostic accuracy of ACS and risk stratification in women (83,88-91). However, a very recent study (92) reported some conflicting results regarding the cost-benefit ratio of hs-cTnI assay concerning sex-related cut-off values in patients with suspected ACS. Indeed, Shah et al. (92) confirmed that the hs-cTnI method prompted reclassification of 1,771 (17%) of 10,360 patients with myocardial injury or infarction, but these reclassified patients did not show a lower subsequent incidence of myocardial infarction or cardiovascular death after a follow-up of 12 months. These results question whether the diagnostic threshold for AMI should be based on the 99th centile derived from a normal reference population. A very recent study demonstrated that intra-individual (within-subject) biological variation of cTnI in healthy adult subjects and patients with chronic kidney disease is about 8–10% (expressed as CV), while the between-subject variation is about 3-fold higher (on average about 61–62%), if evaluated by means of a high-sensitivity immunoassay method (93). These data open the question how to clinically interpreter the cTnI variations significantly greater than the LoD value, measured with high-sensitivity immunoassays, which still are within the 99th URL value in patients in the setting of ACS (60,67,76).
Considering the cTnT assay, several evidences indicate that sex-specific clinical decision values are not particularly useful for cTnT assay (94-96). Sex-related differences between the results of cTnI and cTnT in ACS patients are somewhat expected considering the lower sex-dependence of cTnT compared to cTnI (63).
As far as the age-related differences are concerned, several studies published after the year 2005 demonstrated that cTn levels progressively increase in apparently healthy adult subjects aged over 65 years (60,63,71,72). Higher circulating levels of cTnI and cTnT in elderly healthy subjects may be caused by an increased release of these proteins from cardiomyocytes as well as by a decreased turnover, or by the combined action of these two mechanisms (36,59,60). Indeed, several age-associated disorders can cause the death of cardiomyocytes, with the consequent release of sarcolemma proteins, including cTnI and cTnT (63,97,98).
It is conceivable that the improvement in analytical performance of immunoassay methods will continue in the next future until reaching the exceptional objective to measure the circulating levels of biomarker in more than 99% of individuals with cTnI and cTnT values above the LoD, including women and pediatric subjects. Indeed, comparing the results of cTnI immunoassay methods reported in 2004 (99) to those found in more recent studies (68-70,100), the bias among methods is greatly reduced (from about 20 folds to about two folds) and analytical performances are also significantly better. These results suggest that may be also possible to reach in a short period of time the ambitious objective to measure cTnI and cTnT concentrations at LoD level with an imprecision ≤20%.
Incremental diagnostic and prognostic risk using different cardiac specific biomarkers
In clinical routine, assessment of cardiovascular risk is usually based on the assay of several biomarkers, including NPs, cTn and, often, other biomarkers (in particular: inflammatory and fibrosis biomarkers, growth factors, neuro-hormones, cytokines, biomarkerts of oxidative stress, and biomarkers of renal function and injury) (Figure 2) (101-103,105,106). This approach is defined as Multi-Markers (MM) or global risk model (101-108). It is conceivable that only some of biomarkers included in MM models show sex-related variations. Novel risk biomarkers should be evaluated in several phases, including initial proof of concept, prospective validation in independent populations, documentation of incremental information in MM-models, assessment of effects on patient management and outcomes, and ultimately, cost-effectiveness (104,107-110).
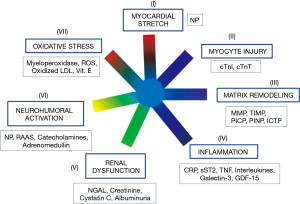
Biomarkers that do not change the management of a disease unlikely will significantly affect patient outcome and therefore will not be cost-effective (101,103-107,109,110). According to Braunwald (101), seven major classes of pathophysiological mechanisms are strictly related to pathogenesis of HF (Figure 2). Therefore, the biomarkers related to these seven classes of pathophysiological mechanisms are likely linked to progression and clinical severity of HF (Figure 2). As a result, a good strategy could be to include in MM models only one biomarker for each of these seven classes.
All the most recent guidelines (47,57,58,103) state that NPs and cTn assay should be considered as the first line biomarkers for risk evaluation in general population and HF patients. These recommendations are based on a huge number of experimental evidences, which have demonstrated that measurement of NPs and cTn significantly and independently improve both risk stratification and clinical outcome of HF patients (47,57,58,103). According to Figure 2, NPs and cTn independently contribute to cardiovascular risk assessment because are involved in different pathophysiological mechanisms related to cardiac dysfunction and progression of heart failure (i.e., detection of myocardial tissue injury and cardiac stress, respectively). Several studies (111-116) reported that these two biomarkers are increased even in the first two asymptomatic stages (A and B) of natural history of HF (47). However, the 2010 ACCF/AHA guidelines reported that NP measurement is not recommended for cardiovascular risk assessment in asymptomatic adults (Class III: no benefit; level of evidence: B) (117). Indeed, at present time, we need some specific clinical trials, based on cohorts of asymptomatic individuals, which are designed for early assessment of individuals at high risk of HF by means of NPs and cTn using high-sensitivity method. In particular, these clinical studies should demonstrate that early treatment is able to improve clinical outcomes, such as: reversion of adverse remodeling, slowing down in HF progression and/or reduction in mortality rate and major adverse cardiovascular events in individuals at high risk for HF with increased NP and cTn levels.
From a statistical viewpoint, when using decision values (such as 99th URL) it may be inadvisable to dichotomize continuous variables for multiple regression analyses, because this approach can significantly reduce information (118). Several recent studies, using high-sensitivity methods, demonstrated that a progressive increment in biomarker levels, even within the reference interval values, can significantly increase cardiovascular risk scores (76-79). Consequently, sex-related decision values have no clinical relevance in risk assessment.
Conflicting results have been reported when several biomarkers were added to regression models, already including NPs and cTn for risk stratification in general population or HF patients (47,57,58,109). In particular, although some inflammatory biomarkers (such as sST2, galectin-3 and GDF-15) are significantly related to clinical outcome in univariate analyses, correlations often vanished when these variables are tested in MM models including NPs and/or cTn measured with high-sensitivity methods (103). This effect is in part expected because these biomarkers (such as sST2, galectin-3 and GDF-15) have similar pathophysiological mechanisms, related to activation/regulation of cytokines system. As a result, these variables can introduce some collinearity when tested together in MM models (103). However, some recent results indicate that in MM models, also including NPs and cTn, some inflammatory biomarkers are also able to independently contribute to regression in patients with acute HF even when tested together in the same MM model (119).
Future perspectives
Due to the progressive improvement in analytical performance of immunoassays (6,36,60,63,66), LoQ values at 20% CV have progressively approximated those at LoD level of cTnI methods (Table 4). These results suggest that may be also possible to reach in a short time the objective to measure cTnI and cTnT concentrations at the LoD level with an imprecision ≤20%: a goal inconceivable only 5 years ago.
As far as the BNP immunoassay methods are concerned, several studies demonstrated that the IRMA method (by Shionogi Diagnostic Division, Japan), the ADVIA method for the Centaur platform (by Siemens Health Care Diagnostics) and ST AIA-PACK method for the AIA platform (by TOSOH Corporation, Tokyo, Japan) measured greatly lower (up to the half) BNP values in comparison with other immunoassays, such as the POCT Triage method (by Alere Diagnostics), the BNP Triage Biosite for Access and UniCell DxI platforms (by Beckman Coulter Diagnostics), the MEIA method for the AxSYM platform and the chemiluminescent microparticle immunoassay for ARCHITECT platform (both by Abbotts Diagnostics) (3). However, more recent results (120) indicate that the bias between BNP ADVIA and Access methods is significantly decreased (from approximately 50% to roughly 20%) compared to that reported with the previous generation of BNP ADVIA method (121-123). The results of this recent study also confirm that differences between NT-proBNP methods (i.e., <20%) are lower than that observed between BNP methods (120).
Further studies, including some based on results of external quality control programs, are needed to confirm that there is a trend to a progressive harmonization among the results of the most popular immunoassay methods for NPs and cTn. Some recent results (3,63,97,120,122,123), indicating both a progressive harmonization of results and better analytical performances, are good news for clinicians. If this trend to better harmonization for NP and cTn immunoassays will be confirmed also in the next future, it will be possible to adopt similar clinical decision values for the diagnosis of heart failure independently of the immunoassay method used. According to the Precision Medicine principles, this improvement in harmonization of clinical results and in analytical performance of NP and cTn methods will allow an early and more accurate diagnosis of cardiac disease, as well as a better stratification of cardiovascular risk within the reference interval values of biomarkers. These improvements will also allow to better distinguish features of specific groups of individuals/patients, and also to better individualize and personalize therapies (124).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fabian Sanchis-Gomar) for the series “The Effects of Demographic Factors on Cardiovascular Biomarkers” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.01.03). The series “The Effects of Demographic Factors on Cardiovascular Biomarkers” was commissioned by the editorial office without any funding or sponsorship. Aldo Clerico serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2017 to June 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clerico A, Giannoni A, Vittorini S, et al. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol 2011;301:H12-20. [Crossref] [PubMed]
- Lam CS, Cheng S, Choong K, et al. Influence of sex hormone status on circulating natriuretic peptides. J Am Coll Cardiol 2011;58:618-26. [Crossref] [PubMed]
- Clerico A, Passino C, Franzini M, et al. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin Chim Acta 2015;443:17-24. [Crossref] [PubMed]
- Goetze JP. Biosynthesis of cardiac natriuretic peptides. Results Probl Cell Differ 2010;50:97-120. [PubMed]
- Hamada M, Shigematsu Y, Takezaki M, et al. Plasma levels of atrial and brain natriuretic peptides in apparently healthy subjects: Effects of sex, age, and hemoglobin concentration. Int J Cardiol 2017;228:599-604. [Crossref] [PubMed]
- Clerico A, Fortunato A, Ripoli A, et al. Distribution of plasma cardiac troponin I values in healthy subjects: pathophysiological considerations. Clin Chem Lab Med 2008;46:804-8. [Crossref] [PubMed]
- Clerico A, Fontana M, Vittorini S, et al. The search for a pathophysiological link between sex, cardiac endocrine function, body mass regulation and cardiac mortality: Proposal for a working hypothesis. Clin Chim Acta 2009;405:1-7. [Crossref] [PubMed]
- Cantinotti M, Storti S, Parri MS, et al. Reference intervals for brain natriuretic peptide in healthy newborns and infants measured with an automated immunoassay platform. Clin Chem Lab Med 2010;48:697-700. [Crossref] [PubMed]
- Clerico A, Passino C, Emdin M. When gonads talk to the heart sex hormones and cardiac endocrine function. J Am Coll Cardiol 2011;58:627-8. [Crossref] [PubMed]
- Clerico A, Giannoni A, Vittorini S, et al. The paradox of low BNP levels in obesity. Heart Fail Rev 2012;17:81-96. [Crossref] [PubMed]
- Del Ry S, Cabiati M, Clerico A. Recent advances on natriuretic peptide system: New promising therapeutic targets for the treatment of heart failure. Pharmacol Res 2013;76:190-8. [Crossref] [PubMed]
- Potter LR. Guanylyl cylcase structure, function and regulation. Cell Signaling 2011;23:19216. [Crossref]
- Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev 2012;17:545-54. [Crossref] [PubMed]
- Wei YC, George NI, Chang CW, et al. Assessing sex differences in the risk of cardiovascular disease and mortality per Increment in systolic blood pressure: A systematic review and meta-analysis of follow-up studies in the United States. PLoS One 2017;12:e0170218 [Crossref] [PubMed]
- Kerkhof PLM, Peace RA, Macfarlane PW. Sex- and age-related reference values in cardiology, with annotations and guidelines for interpretation. Adv Exp Med Biol 2018;1065:677-706. [Crossref] [PubMed]
- Mingels AMA, Kimenai DM. Sex-related aspects of biomarkers in cardiac disease. Adv Exp Med Biol 2018;1065:545-64. [Crossref] [PubMed]
- Kim HL, Kim MA, Choi DJ, et al. Sex difference in the prognostic value of N-terminal pro-B type natriuretic peptide in patients with heart failure. A report from the Korean Heart Failure Registry (KorHF). Circ J 2017;81:1329-36. [Crossref] [PubMed]
- Sebastiani P, Thyagarajan B, Sun F, et al. Age and sex distributions of age-related biomarker values in healthy older adults from the Long Life Family Study. 2016;64:e189-94.
- Suthahar N, Meijers WC, Ho JE, et al. Sex specific associations of obesity and N-terminal pro-B-type natriuretic peptide levels in the general population. Eur J Heart Fail 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Taki M, Ishiyama Y, Mizuno H, et al. Sex difference in the prognostic power of Brain Natriuretic Peptide and N-terminal pro-Brain Natriuretic Peptide for cardiovascular events. The Japan Morning Surge-Home Blood Pressure Study. Circ J 2018;82:2096-102. [Crossref] [PubMed]
- Clerico A, Del Ry S, Maffei S, et al. The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin Chem Lab Med 2002;40:371-7. [Crossref] [PubMed]
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254-8. [Crossref] [PubMed]
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and sex. J Am Coll Cardiol 2002;40:976-82. [Crossref] [PubMed]
- Das SR, Drazner MH, Dries DL. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163-8. [Crossref] [PubMed]
- Chang AY, Abdullah SM, Jain T. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol 2007;49:109-16. [Crossref] [PubMed]
- Kuroski de Bold ML. Estrogen, natriuretic peptides and the renin-angiotensin system. Cardiovasc Res 1999;41:524-31. [Crossref] [PubMed]
- Maffei S, Del Ry S, Prontera C, et al. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci 2001;101:447-53. [Crossref] [PubMed]
- Kawano H, Nagayoshi Y, Soejima H, et al. B-type natriuretic peptide after hormone therapy in postmenopausal women with chest pain and normal coronary angiogram. Menopause 2008;15:352-6. [Crossref] [PubMed]
- Lin E, McCabe E, Newton-Cheh C, et al. Effects of transdermal testosterone on natriuretic peptide levels in women: a randomized placebo-controlled pilot study. Fertil Steril 2012;97:489-93. [Crossref] [PubMed]
- Hong M, Yan Q, Tao B, et al. Estradiol, progesterone and testosterone exposures affect the atrial natriuretic peptide gene expression in vivo in rats. Biol Chem Hoppe Seyler 1992;373:213-8. [Crossref] [PubMed]
- Hwu CM, Tsai SC, Lau CP, et al. Increased concentrations of atrial and plasma atrial natriuretic peptide in castrated male rats. Life Sci 1993;52:205-12. [Crossref] [PubMed]
- Matsubara H, Hirata Y, Yoshimi H. Effects of steroid and thyroid hormones on synthesis of atrial natriuretic peptide by cultured atrial myocytes of rat. Biochem Biophys Res Commun 1987;145:336-43. [Crossref] [PubMed]
- Matsubara H, Hirata Y, Yoshimi H. Ventricular myocytes from neonatal rats are more responsive to dexamethasone than atrial myocytes in synthesis of atrial natriuretic peptide. Biochem Biophys Res Commun 1987;148:1030-8. [Crossref] [PubMed]
- Dockery F, Bulpitt CJ, Agarwal S. Anti-androgens increase N-terminal pro-BNP levels in men with prostate cancer. Clin Endocrinol (Oxf) 2008;68:59-65. [Crossref] [PubMed]
- Saenger AK, Dalemberg DA, Bryant SC, et al. Pediatric Brain Natriuretic Peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem 2009;55:1869-75. [Crossref] [PubMed]
- Giannoni A, Giovannini S, Clerico A. Measurement of circulating concentrations of cardiac troponin I and T in healthy subjects: a tool for monitoring myocardial tissue renewal? Clin Chem Lab Med 2009;47:1167-77. [Crossref] [PubMed]
- Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 2014;35:419-25. [Crossref] [PubMed]
- Stramba-Badiale M, Fox KM, Priori SG, et al. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J 2006;27:994-1005. [Crossref] [PubMed]
- Maas AH, Appelman YE. Sex differences in coronary artery heart disease. Neth Heart J 2010;18:598-602. [Crossref] [PubMed]
- Shufelt CL, Pacheco C, Tweet MS, Miller VM. Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol 2018;1065:433-54. [Crossref] [PubMed]
- Cantinotti M, Law Y, Vittorini S, et al. The potential and limitations of plasma BNP measurement in the diagnosis, prognosis, and management of children with heart failure due to congenital cardiac disease: an update. Heart Fail Rev 2014;19:727-42. [Crossref] [PubMed]
- Nir A, Lindinger A, Rauh M, et al. NT-Pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol 2009;30:3-8. [Crossref] [PubMed]
- Mir TS, Flato M, Falkenberg J, et al. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and sex. Pediatr Cardiol 2006;27:73-7. [Crossref] [PubMed]
- Yoshibayashi M, Kamiya T, Saito Y, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: marked and rapid increase after birth. Eur J Endocrinol 1995;133:207-9. [Crossref] [PubMed]
- Kunii Y, Kamada M, Ohtsuki S, Araki T, Kataoka K, Kageyama M. Plasma brain natriuretic peptide and the evaluation of volume overload in infants and children with congenital heart disease. Acta Med Okayama 2003;57:191-7. [PubMed]
- van Riet EES, Hoes AW, Limburg A, et al. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 2014;16:772-77. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-327. [PubMed]
- Yoshimura T, Yoshimura M, Yasue H, et al. Plasma concentration of atrial natriuretic peptide and brain natriuretic peptide during normal human pregnancy and the postpartum period. J Endocrinol 1994;140:393-7. [Crossref] [PubMed]
- Hameed AB, Chan K, Ghamsary M, et al. Longitudinal changes in the B-type natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol 2009;32:E60-2. [Crossref] [PubMed]
- Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2017;135:e50-87. [Crossref] [PubMed]
- Resnik JL, Hong C, Resnik R, et al. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am J Obstet Gynecol 2005;193:450-4. [Crossref] [PubMed]
- Tanous D, Siu SC, Mason J, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol 2010;56:1247-53. [Crossref] [PubMed]
- Giannubilo SR, Pasculli A, Tidu E, et al. Relationship between maternal hemodynamics and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia and fetal growth restriction. J Perinatol 2017;37:484-7. [Crossref] [PubMed]
- Reddy YN, Borlaug BA. Heart failure with preserved ejection fraction. Curr Probl Cardiol 2016;41:145-88. [Crossref] [PubMed]
- Ferrari R, Böhm M, Cleland JG, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17:665-71. [Crossref] [PubMed]
- Clerico A, Zaninotto M, Passino C, et al. Obese phenotype and natriuretic peptides in patients with heart failure with preserved ejection fraction. Clin Chem Lab Med 2018;56:1015-25. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the American Heart Association. Circulation 2017;135:e1054-e1091. [Crossref] [PubMed]
- Marjot J, Kaier TE, Martin ED, et al. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin Chem 2017;63:990-6. [Crossref] [PubMed]
- Mair J, Lindahl B, Hammarsten O, et al. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care 2018;7:553-60. [Crossref] [PubMed]
- Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: Expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem 2018;64:645-55. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J 2019;40:237-69. [Crossref] [PubMed]
- Clerico A, Zaninotto M, Ripoli A, et al. The 99th percentile of reference population for cTnI and cTnT assay: methodology, pathophysiology and clinical implications. Clin Chem Lab Med 2017;55:1634-51. [Crossref] [PubMed]
- Caselli C, Cangemi G, Masotti S, et al. Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a highly sensitive immunoassay method: Highly sensitive troponin I in pediatric age. Clin Chim Acta 2016;458:68-71. [Crossref] [PubMed]
- Sandoval Y, Apple FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem 2014;60:455-62. [Crossref] [PubMed]
- Apple FS, Jaffe AS, Collinson PInternational Federation of Clinical Chemistry (IFCC) Task Force on Clinical Applications of Cardiac Bio-Markers, et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem 2015;48:201-3. [Crossref] [PubMed]
- Apple FS, Sandoval Y, Jaffe AS, et al. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 2017;63:73-81. [Crossref] [PubMed]
- Masotti S, Prontera C, Musetti V, et al. Evaluation of analytical performance of a new high-sensitivity immunoassay for cardiac troponin I. Clin Chem Lab Med 2018;56:492-501. [Crossref] [PubMed]
- Musetti V, Masotti S, Prontera C, et al. Evaluation of the analytical performance of a new ADVIA immunoassay using the Centaur XPT platform system for the measurement of cardiac troponin I. Clin Chem Lab Med 2018;56:e229-e231. [Crossref] [PubMed]
- Masotti S, Musetti V, Prontera C, et al. Evaluation of analytical performance of a chemiluminescence enzyme immunoassay (CLEIA) for cTnI using the automated AIA-CL2400 platform. Clin Chem Lab Med 2018;56:e174-e176. [Crossref] [PubMed]
- Franzini M, Lorenzoni V, Masotti S, et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, sex, and population selection: A multicenter study in Italy. Clin Chim Acta 2015;438:376-81. [Crossref] [PubMed]
- Krintus M, Kozinski M, Boudry P, et al. European multicenter analytical evaluation of the Abbott ARCHITECT STAT highly sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657-65. [PubMed]
- Bergmann O, Zdunek S, Felker A, et al. Dynamics of cell generation and turnover in the human heart. Cell 2015;161:1566-75. [Crossref] [PubMed]
- Haubner BJ, Schneider J, Schweigmann U, et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ Res 2016;118:216-21. [Crossref] [PubMed]
- Eschenhagen T, Bolli R, Braun T, et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017;136:680-6. [Crossref] [PubMed]
- Eggers KM, Jaffe AS, Lind L, et al. Value of cardiac troponin I cutoff concentrations below the 99th percentile for clinical decision-making. Clin Chem 2009;55:85-92. [Crossref] [PubMed]
- Sinning C, Keller T, Zeller T, et al. Association of high-sensitivity assayed troponin I with cardiovascular phenotypes in the general population: the population-based Gutenberg health study. Clin Res Cardiol 2014;103:211-22. [Crossref] [PubMed]
- de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503-12. [Crossref] [PubMed]
- Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the ARIC Study. Circulation 2011;123:1367-76. [Crossref] [PubMed]
- Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation 2012;126:1596-604. [Crossref] [PubMed]
- Hussein AA, Gottdiener JS, Bartz TM, et al. Cardiomyocyte Injury Assessed by a highly sensitive troponin assay and sudden cardiac death in the community: The Cardiovascular Health Study. J Am Coll Cardiol 2013;62:2112-20. [Crossref] [PubMed]
- Blankenberg S, Salomaa V, Makarova N, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J 2016;37:2428-37. [Crossref] [PubMed]
- Everett BM, Zeller T, Glynn RJ, et al. High-sensitivity cardiac troponin I and B-type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation 2015;131:1851-60. [Crossref] [PubMed]
- van der Linden N, Klinkenberg LJ, Bekers O, et al. Prognostic value of basal high-sensitive cardiac troponin levels on mortality in the general population. Medicine 2016;95:e5703 [Crossref] [PubMed]
- Parikh RH, Seliger SL, de Lemos J, et al. Prognostic significance of high-sensitivity cardiac troponin T concentrations between the limit of blank and limit of detection in community-dwelling adults: a meta-analysis. Clin Chem 2015;61:1524-31. [Crossref] [PubMed]
- Willeit P, Welsh P, Evans JDW, et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol 2017;70:558-68. [Crossref] [PubMed]
- Sigurdardottir FD, Lyngbakken MN, Holmen OL, et al. Relative prognostic value of cardiac troponin I and C-Reactive Protein in the general population (from the Nord-Trøndelag Health [HUNT] Study). Am J Cardiol 2018;121:949-55. [Crossref] [PubMed]
- Shah AS, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873. [Crossref] [PubMed]
- Kavsak PA, Shortt C, Pond G, Worster A. High-sensitivity cardiac troponin I for predicting death in a female emergency department population. Clin Chem 2014;60:271-324.e1. [Crossref] [PubMed]
- Bohula May EA, Bonaca MP, Jarolim P, et al. Prognostic performance of a high-sensitivity cardiac troponin I assay in patients with non-ST-elevation acute coronary syndrome. Clin Chem 2014;60:158-64. [Crossref] [PubMed]
- Eggers KM, Johnston N, James S, et al. Cardiac troponin I levels in patients with non-ST-elevation acute coronary syndrome-the importance of sex. Am Heart J 2014;168:317-24. [Crossref] [PubMed]
- Shah ASV, Anand A, Strachan FE, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919-28. [Crossref] [PubMed]
- van der Linden N, Hilderink JH, Cornelis T, et al. twenty-four-hour biological variation profiles of cardiac troponin I in individuals with or without chronic kidney disease. Clin Chem 2017;63:1655-6. [Crossref] [PubMed]
- Giannitsis E. Sex-specific troponin measures for diagnosis of acute coronary syndrome. Heart 2016;102:91-2. [Crossref] [PubMed]
- Mueller-Hennessen M, Lindahl B, Giannitsis E, et al. Diagnostic and prognostic implications using age- and sex-specific cut-offs for high-sensitivity cardiac troponin T - Sub-analysis from the TRAPID-AMI study. Int J Cardiol 2016;209:26-33. [Crossref] [PubMed]
- Rubini Giménez M, Twerenbold R, Boeddinghaus J, et al. Clinical effect of sex-specific cutoff values of high-sensitivity cardiac troponin T in suspected myocardial infarction. JAMA Cardiol 2016;1:912-20. [Crossref] [PubMed]
- Dhalla NS, Rangi S, Babick AP, et al. Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev 2012;17:671-81. [Crossref] [PubMed]
- Lakatta EG. So! What’s aging? Is cardiovascular aging a disease? J Mol Cell Cardiol 2015;83:1-13. [Crossref] [PubMed]
- Panteghini M, Pagani F, Yeo KT, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem 2004;50:327-32. [Crossref] [PubMed]
- Clerico A, Ripoli A, Masotti S, et al. Pilot study on harmonization of cardiac troponin I immunoassays using patients and quality control plasma samples. On behalf of the Italian Section of the European Ligand Assay Society (ELAS) and of the Study Group on Cardiovascular Biomarkers of the Società Italiana di Biochimica Clinica (SIBioC). Clin Chim Acta 2016;456:42-8. [Crossref] [PubMed]
- Braunwald E. Heart Failure. JACC Heart Fail 2013;1:1-20. [Crossref] [PubMed]
- Vittorini S, Clerico A. Cardiovascular biomarkers: increasing impact of laboratory medicine in cardiology practice. Clin Chem Lab Med 2008;46:748-63. [Crossref] [PubMed]
- Aspromonte N, Gulizia MM, Clerico A, et al. ANMCO/ELAS/SIBioC Consensus Document: biomarkers in heart failure. Eur Heart J Suppl 2017;19:D102-D112. [Crossref] [PubMed]
- Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a Scientific Statement from the American Heart Association. Circulation 2009;119:2408-16. [Crossref] [PubMed]
- Emdin M, Vittorini S, Passino C, et al. Old and new biomarkers of heart failure. Eur J Heart Fail 2009;11:331-5. [Crossref] [PubMed]
- Passino C, Barison A, Vergaro G, et al. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin Chim Acta 2015;443:29-38. [Crossref] [PubMed]
- Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 2011;123:551-65. [Crossref] [PubMed]
- Muthén B. A general structural equation model with dichotomous, ordered categorical, and continuous latent variable indicators. Psycometrika 1984;49:115-32. [Crossref]
- Brotman DJ, Walker E, Lauer MS, et al. In search of fewer independent risk factors. Arch Intern Med 2005;165:138-45. [Crossref] [PubMed]
- Price CP, Christenson RH. The clinical question: a system for formulating answerable questions in laboratory medicine. In Evidence Based Laboratory Medicine - Principles, Practice, and Outcome. Second edition 2007, AACCPress, Washington DC, 2007:25-52.
- Emdin M, Passino C, Prontera C, et al. Comparison of Brain Natriuretic Peptide (BNP) and amino-terminal proBNP for early diagnosis of heart failure. Clin Chem 2007;53:1289-97. [Crossref] [PubMed]
- Swoboda PP, McDiarmid AK, Erhayiem B, et al. Diabetes Mellitus, microalbuminuria, and subclinical cardiac disease: identification and monitoring of individuals at risk of heart failure. J Am Heart Assoc 2017;6:e005539 [Crossref] [PubMed]
- Álvarez I, Hernández L, García H, et al. High-sensitivity troponin T assay in asymptomatic high cardiovascular risk patients. The TUSARC Registry. Rev Esp Cardiol (Engl Ed) 2017;70:261-6. [Crossref] [PubMed]
- Hasler S, Manka R, Greutmann M, et al. Elevated high-sensitivity troponin T levels are associated with adverse cardiac remodelling and myocardial fibrosis in hypertrophic cardiomyopathy. Swiss Med Wkly 2016;146:w14285 [PubMed]
- Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013;61:187-95. [Crossref] [PubMed]
- Sundström J, Ingelsson E, Berglund L, et al. Cardiac troponin-I and risk of heart failure: a community-based cohort study. Eur Heart J 2009;30:773-81. [Crossref] [PubMed]
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adult: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50-103. [Crossref] [PubMed]
- Altman DG, Royston P. The cost of dichotomizing continuous variables. BMJ 2006;332:1080. [Crossref] [PubMed]
- Demissei BG, Cotter G, Prescott MF, et al. A multi-marker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail 2017;19:1001-10. [Crossref] [PubMed]
- Masotti S, Musetti V, Pronetra C, et al. Evaluation of analytical performances using standardized analytical protocols and comparison of clinical results of the new ADVIA BNP and NT-proBNP immunoassays for Centaur XPT platform. Clin Chem Lab Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Franzini M, Masotti S, Prontera C, et al. Systematic differences between BNP immunoassays: Comparison of methods using standard protocols and quality control materials. Clin Chim Acta 2013;424:287-91. [Crossref] [PubMed]
- Prontera C, Zaninotto M, Giovannini S, et al. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NT-proBNP) immunoassays: the CardioOrmoCheck study. Clin Chem Lab Med 2009;47:762-8. [Crossref] [PubMed]
- Clerico A, Zaninotto M, Prontera C, et al. Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry. State of the art of BNP and NT-proBNP immunoassays: The CardioOrmoCheck study. Clin Chim Acta 2012;414:112-9. [Crossref] [PubMed]
- Kohane IS. Health Care Policy. Ten things we have to do to achieve precision medicine. Science 2015;349:37-8. [Crossref] [PubMed]
Cite this article as: Clerico A, Masotti S, Musetti V, Passino C. Pathophysiological mechanisms determining sex differences in circulating levels of cardiac natriuretic peptides and cardiac troponins. J Lab Precis Med 2019;4:8.


