Translational health economics—delivering the return on investment for laboratory medicine
Introduction
Healthcare systems throughout the world are known to be under increasing pressure to improve quality and value for money, against a background of increasing patient demand and a growing population. While advances in technology are seen as one solution to transforming care, it is questioned who will benefit, and at what cost (1). However, healthcare is also recognised as being a complex system with many challenges in both organisation and management (2). Allocating scarce resources to achieve these goals is in part the task of finance managers who are aiming for cost effectiveness and productivity of services (3,4). They are guided initially by health economic and health technology assessments which, through a variety of different analytical tools, seek to determine whether a new intervention, be it a drug, a procedure or a test, should be adopted based on these clinical and cost effectiveness analyses. (5,6). However, health economic analyses are primarily used to support policy and investment decisions based on value for money for the community at large. This type of analysis does not necessarily assist those responsible for applying policy at a local level. The problems of translating global evidence into local practice is one of the many commonly reported barriers to adoption of new technologies and practices in healthcare (7-9).
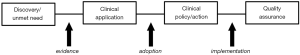
Cooksey in his review of health research funding in the United Kingdom identified two major gaps in translational research: (I) translating ideas from basic and clinical research into the development of new products and approaches to treatment of disease and illness; and (II) implementing those new products and approaches into clinical practice (10); similar observations have been made by others (11,12). These translational gaps are equally relevant to laboratory medicine (Figure 1).
Solutions for better translation of evidence into practice
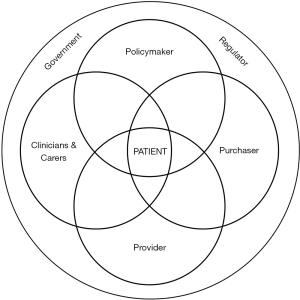
The discussion of translational gaps in healthcare, especially the second gap identified by Cooksey, has focussed particularly on improving the productivity and effectiveness of health research leading to an improved patient outcome. This is difficult due to the problems associated with health systems allocating resources to individual departments (or budgetary) silos, e.g. laboratory medicine, hence the call for bridging the gap between “service delivery silos” (13). Thus, during implementation there needs to be a recognition of the range of stakeholders involved in delivering a care pathway (Figure 2). The introduction of a new test will change that pathway and the potential benefits that will accrue to the various stakeholders as well as changes in resource allocation (14).
The laboratory medicine department is a classic example of a silo with its organisation and funding being based on its activities, namely the production of test results, with limited or no consideration of how they impact upon care pathways. This leads to several problems in the implementation of new technology (i.e., test). These include a long period of time until full implementation of new test is achieved (15,16), variability in the rates of test utilisation, including under and over utilisation (17-19) and the fragmented management of services is likely to be a factor in the observations that results may not be acted on after receipt (20,21). In addition, while evidence is generated on the clinical impact of a test result, little attention is paid to other changes in the care pathway, as reported in a narrative review of the use of point-of-care testing (POCT) for the measurement of HbA1c in the management of diabetes (22).
We suggest that these problems can be addressed through the use of a value proposition for laboratory medicine interventions (23), the core of which can be summarised in the series of questions posed in Table 1. These are built on the work of Donabedian, who proposed three approaches (or domains) to the assessment of quality in healthcare—outcomes, process and structure (24). In taking this approach he recognised the role played by all stakeholders involved in the delivery of care. Thus, in addition to a positive clinical outcome the introduction of a test will involve a process change as well as an impact on resource allocation (one of the elements of structure). Crucial to the implementation phase of a test intervention, articulated in the value proposition are (I) delineation of all of the changes in the pathway that result from the introduction of the test; (II) identification of all of the stakeholders contributing to the pathway; and (III) identification of the benefits expected by each of the stakeholders; see Table 2.
Table 1
| 1 What is the unmet clinical need? |
| 2. What is the patient population that will benefit? |
| 3. What is the nature of the test, e.g., biomarker and relation to pathology of condition? |
| 4. What is the utility of the intervention, e.g., screening, diagnosis, prognosis, risk stratification and/or monitoring? |
| 5. What are the expected outcomes: clinical, process and/or resource utilisation? |
| 6. Where will the test be performed: laboratory and/or point of care setting? |
| 7. What is the quality of evidence available? Trial and/or real world? |
| 8. In what part(s) of the care pathway will the test be used? |
| 9. Which stakeholders will be involved in the care pathway identified? |
| 10. What are the potential benefits for each stakeholder? |
| 11. What are the potential limitations and risks and any mitigation strategy? |
| 12. What is the [amount of] resource utilised for each service line contributing to the pathway? |
| 13. What is the resource allocation/reimbursement for delivering each service line contributing to the pathway? |
| 14. What are the metrics proposed for monitoring the implementation of the test? |
Table 2
| Stakeholder | Potential benefit | Specific examples of quality measures/outcomes |
|---|---|---|
| Patient | Quality of life | Mortality |
| Mobility | Care delivered at home | |
| Satisfaction with care | Reduced complication rate | |
| Family and carers | Quality of life | Improved health status |
| Access to care | Care delivered at home | |
| System efficiency | Reduced visits to hospital | |
| Clinician(s) | Aid to clinical decision making | Rapid diagnosis and treatment decisions |
| Aid to prognosis | Early detection of complications | |
| Efficiency of care | Reduced number of clinic visits | |
| Care provider unit | Patient outcome | Successful discharge |
| Efficiency of care | Reduced length of stay | |
| Structure/resource utilisation | Earlier discharge from Emergency Department | |
| Provider organisation | Patient outcome | Mortality statistics |
| Efficiency of care | Productivity of care units and service lines | |
| Structure/resource utilisation | Ability to invest in new technology | |
| Purchaser/insurer | Patient outcome | Reduction in prevalence of disease/complications |
| Organisational efficiency | Improved productivity of organisation | |
| Structure/resource utilisation | Reduction in care pathway costs | |
| Government/regulator | Population health statistics | Reduction in prevalence of disease/complications |
| System efficiency | More care delivered closer to home | |
| Structure/resource utilisation/costs | Cost per case |
This approach to bridging the translational gap in laboratory medicine described above using the framework of the value proposition can be supported with a range of translational tools, such as translational health economics which can be applied across laboratory medicine to determine the value of tests and how they can deliver a return on investment (ROI) (25).
Use of the value proposition concept
One way to overcome the translational gap described above is to apply the concept of a value proposition which, for a laboratory test, would be a complete description of the benefits of the test, to whom the benefits accrue, and how the benefits can be delivered (23). The information provided by the value proposition is summarised in Table 1. The value proposition for the application of a test is built on the evidence generated from clinical trials, which also helps to describe the new care pathway. The resource requirements can then be generated from knowledge of the care pathway developed, e.g., using simulation and resource management tools such as discrete event simulation (DES) (26) resource consumption analysis (RCA) (27), time driven activity-based costing (TDABC) (28), and patient level information costing systems (PLICS) (29). Such techniques are used in other areas of healthcare but rarely if ever in relation to the laboratory medicine contribution to a care pathway. The resource management tools are typically employed in service line management (30); an example has been in making the case for the use of POCT for intra-operative monitoring of haemostasis in cardiac surgical patients (31). The key points in using the value proposition are: (I) a test result, of itself has no value until it has been acted on; (II) benefits accrue to a range of stakeholders contributing to a care pathway; (III) these benefits (and any disbenefits) have to be measured and quantified in order the deliver the value of the test.
Translational health economics can be used to identify and guide the resource utilisation to support implementation of a new test. Furthermore, it can then be used in performance management and quality improvement, e.g., when monitoring productivity of services.
Linking ROI to value in laboratory medicine
Another approach to identifying the value of a test is to consider it as an investment. Resources will be required to establish and implement a new test on a routine basis and set against these costs will be the total benefits that the test brings to the pathway. All of these costs and benefits will be identified in the framework of the value proposition and measured using some of the translational economic tools mentioned above. The end result will be to calculate the return (benefits) on investment (cost of the test) which essentially is a measure of the efficiency of that investment
The ROI has been used to guide policymakers in relation to the adoption of a new clinical intervention and also as an evaluation tool for purchasers and providers of healthcare but is rarely described for tests. This is probably because the application of the value proposition and calculation of the ROI requires the collection of data outside of the laboratory, namely the impact on stakeholders including the patient, and conversion of the that data into economic metrics. In order to follow the approach advocated by Donabedian this will require data in the domains of clinical outcome, operational and resource management.
Thus, in the narrative review of the evidence on the clinical and cost effectiveness of POCT for HbA1c referred to earlier (22), the increased cost of the POCT would be expected to be covered by the saving made from the reduction in the number of clinic visits reported in a budget impact analysis (32). Similarly, in the case of adopting intra-operative POCT for haemostasis monitoring in cardiac surgical patients (31) the increased cost of POCT could be offset by the reduction in blood product utilisation and ward and clinic costs.
Challenges in the application of translational health economics
Some of the key barriers to the adoption of new laboratory medicine investigations are (I) a lack of data describing current practice; (II) lack of evidence on the clinical and cost effectiveness of new technology—including the impact of new technology on individual stakeholders; (III) reimbursement based on cost of technology rather than benefits; (IV) decommissioning of redundant resources; (V) a silo-based approach to budgeting; and (VI) lack of an innovation culture (9). These should be viewed in the context of the translational gaps for laboratory medicine illustrated in Figure 1.
Porter and Teisberg stated that in order to change healthcare it was necessary that (I) clinical practice be “organized around medical conditions and care cycles” (what we have referred to as pathways), and (II) “outcomes and costs—must be measured” (33,34). This has been encapsulated at a higher level with the promotion of integrated practice units (35). The framework of the value proposition provides a guide to the data required to enable most of the challenges above to be addressed: (I) by identifying what data needs to be collected in order to identify the contribution made by each of the stakeholders; (II) how that will alter the process of care and the clinical outcomes; and (III) changes in the data relating to resource utilisation, costs and other aspects of structure originally identified by Donabedian (24).
Improving cost effectiveness and system efficiency will create challenges for other stakeholders contributing to a given care pathway, as well as unintended consequences. Such unintended consequences may be negative for some stakeholders such as reduced income or increased costs, but these have to be taken into account and mitigated in order to demonstrate the full value of the test across the complete pathway. Another eventuality might be that use of a test leads to improvements in system efficiency by reducing for example, the number of clinic visits, but such clinic capacity needs to be reutilised in order to capture the value generated by the test. Service transformation in which the delivery of a care pathway is devolved from the hospital to the primary care setting, or to the home setting, with a change in provider can also create several transformational and translational challenges.
Conclusions
In any care pathway where a laboratory medicine investigation is employed the benefits will always accrue to the other stakeholders contributing to the pathway. The current approaches to budgeting in laboratory medicine, based on activity and costs, create a significant barrier to delivering the real value of laboratory medicine. The ROI of laboratory medicine is lost unless the benefits are realised in the other stakeholder contributions to the care pathway. The value proposition provides a means of leveraging the real value of laboratory medicine tests by highlighting the clinical, process and economic benefits that accrue across the whole stakeholder family. Translational health economics is a translational tool that can be used to guide the expected changes in resource utilisation as a result of introducing a new test into a care pathway. Such a tool can be employed with other change management tools to support a more integrated approach to care amongst a group of stakeholders (36). This approach applies in both the implementation and quality assurance phases of technology-supported healthcare delivery.
Acknowledgments
This paper is part of the work of the IFCC-WASPalM Committee for the Value Proposition in Laboratory Medicine.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mario Plebani and Giuseppe Lippi) for the series “2019 International Conference on Laboratory Medicine: From Bench to Diagnostic - Therapeutic Pathways” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.09.04). The series “2019 International Conference on Laboratory Medicine: From Bench to Diagnostic - Therapeutic Pathways” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Economic Forum. Shaping the Future of Health and Healthcare. Accessed 30 August 2019. Available online: https://www.weforum.org/system-initiatives/shaping-the-future-of-health-and-healthcare
- Burton C, Elliott A, Cochran A, et al. Do healthcare services behave as complex systems? Analysis of patterns of attendance and implications for service delivery. BMC Med 2018;16:138. [Crossref] [PubMed]
- Akachi Y, Kruk ME. Quality of care: measuring a neglected driver of improved health. Bulletin of the World Health Organization 2017;95:465-72. [Crossref] [PubMed]
- Porter ME. A strategy for health care reform — toward a value-based system. New Eng J Med 2009;361:109-12. [Crossref] [PubMed]
- Kernick DP. Introduction to health economics for the medical practitioner. Postgrad Med J 2003;79:147-50. [Crossref] [PubMed]
- Soares MO, Walker S, Palmer SJ, et al. Establishing the value of diagnostic and prognostic tests in health technology assessment. Med Decis Making 2018;38:495-508. [Crossref] [PubMed]
- Ivanov A. Barriers to the introduction of new medical diagnostic tests. Lab Medicine 2003;44:e132-6. [Crossref]
- Robert G, Greenhalgh T, MacFarlane F, et al. Adopting and assimilating new non-pharmaceutical technologies into health care: a systematic review. J Health Serv Res Policy 2010;15:243-50. [Crossref] [PubMed]
- NHS Institute for Innovation and Improvement. Organisational and Behavioural Barriers to Medical Technology Adoption. Accessed 30 August 2019. Available online: http://www.institute.nhs.uk/images/ResearchAndEvaluationReports/OBB_MTA.pdf
- Cooksey D. A review of UK health research funding. Accessed 30 August 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/228984/0118404881.pdf
- Waldman SA, Terzic A. Clinical and translational science: from bench-bedside to global village Clin Transl Sci 2010;3:254-7. [Crossref] [PubMed]
- Liebman MN, Franchini M, Molinaro S. Bridging the gap between translational medicine and unmet clinical needs. Technol Health Care 2015;23:109-18. [PubMed]
- Sherry M, Wolff JL, Ballreich J, et al. Bridging the silos of service delivery for high-need, high-cost individuals. Popul Health Manag 2016;19:421-8. [Crossref] [PubMed]
- Leviton LC, Melichar L. Balancing stakeholder needs in the evaluation of healthcare quality improvement. BMJ Qual Saf 2016;25:803-7. [Crossref] [PubMed]
- Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510-20. [Crossref] [PubMed]
- Rousseau F, Lindsay C, Labelle Y, et al. Measuring the chronology of the translational process of molecular genetic discoveries. Clin Chem Lab Med 2019;57:1136-41. [Crossref] [PubMed]
- Right Care. The NHS atlas of variation in diagnostic services. Accessed 30 August 2019. Available online: www.rightcare.nhs.uk/downloads/Right_Care_Diagnostics_Atlas_hi-res.pdf
- Zhi M, Ding EL, Theisen-Toupal J, et al. The landscape of inappropriate laboratory testing: A 15-year meta-analysis. PLoS One 2013;8:e78962 [Crossref] [PubMed]
- O'Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open 2018;8:e018557 [Crossref] [PubMed]
- Callen JL, Westbrook JI, Georgiou A, et al. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2012;27:1334-48. [Crossref] [PubMed]
- Callen J, Georgiou A, Li J, et al. The safety implications of missed test results for hospitalised patients: a systematic review. BMJ Qual Saf 2011;20:194-9. [Crossref] [PubMed]
- Price CP, St John A. The value proposition for point-of-care testing in healthcare: HbA1c for monitoring in diabetes management as an exemplar. Scand J Clin Lab Invest 2019;79:298-304. [Crossref] [PubMed]
- Price CP, St John A. Anatomy of a value proposition for laboratory medicine. Clin Chim Acta 2014;436:104-11. [Crossref] [PubMed]
- Donabedian A. The quality of care. How can it be assessed? JAMA 1988;260:1743-8. [Crossref] [PubMed]
- Price CP, Wolstenholme J, McGinley P, et al. Translational health economics: The key to accountable adoption of in vitro diagnostic technologies. Health Serv Manage Res 2018;31:43-50. [Crossref] [PubMed]
- Zhang X. Application of discrete event simulation in health care: a systematic review. BMC Health Serv Res 2018;18:687. [Crossref] [PubMed]
- Özyapıcı H, Tanış VN. Comparison of cost determination of both resource consumption accounting and time-driven activity-based costing systems in a healthcare setting. Aust Health Rev 2017;41:201-6. [Crossref] [PubMed]
- Keel G, Savage C, Rafiq M, et al. Time-driven activity-based costing in health care: A systematic review of the literature. Health Policy 2017;121:755-63. [Crossref] [PubMed]
- Llewellyn S, Chambers N, Ellwood S, et al. Patient-level information and costing systems (PLICSs): a mixed-methods study of current practice and future potential for the NHS health economy. Health Services and Delivery Research, No. 4.31. Southampton: NIHR Journals Library, 2016.
- NHS Improvement. Approved costing guidance 2019. Accessed 30 August 2019. Available online: https://improvement.nhs.uk/resources/approved-costing-guidance-2019/
- St John A, Price CP. Process change and outcomes: a patient centered approach. In Point-of-Care Testing. Making Innovation Work for Patient Centered Care. Available online: https://acutecaretesting.org/en/articles/poc-testing-making-innovation-work-for-patient-centered-care
- Chadee A, Blackhouse G, Goeree R. Point-of-care haemoglobin A1c testing: a budget impact analysis. Ont Health Technol Assess Ser 2014;14:1-23. [PubMed]
- Porter ME, Teisberg EO. How physicians can change the future of health care. JAMA 2007;297:1103-11. [Crossref] [PubMed]
- Porter ME, Teisberg EO. Redefining Health Care. Boston: Harvard Business School Publishing, 2006.
- van Harten WH. Turning teams and pathways into integrated practice units: appearance characteristics and added value. Int J Care Coord 2018;21:113-6. [Crossref] [PubMed]
- Singer SJ, Kerrissey M, Friedberg M, et al. A comprehensive theory of integration. Med Care Res Rev 2018; [Crossref] [PubMed]
Cite this article as: Price CP, St John A. Translational health economics—delivering the return on investment for laboratory medicine. J Lab Precis Med 2019;4:30.