
Cholylglycine determination by an automated chemiluminescence immunoassay: preliminary results in the intrahepatic cholestasis of pregnancy
Introduction
Bile acids are C24 steroids synthesised by liver from cholesterol. Through hydroxylation steps, cholic acid and chenodeoxycholic acid, namely primary bile acids, are formed. The primary bile acids are then dehydroxylated to secondary bile acids, principally deoxycholic acid and lithocholic acid through the action of intestinal bacteria. An important step in the creation of the pool of bile acids is the conjugation of primary and secondary bile acids with glycine and taurine, leading to the synthesis of the so-called conjugated bile acids (1). In the serum of healthy subjects, conjugated bile acids are present, with more than 70% constituted by conjugated primary bile acids, with cholylglycine (CG) and chenodeoxycholylglycine the most present forms (2).
Total bile acids (TBA) are frequently requested in liver diseases, such us liver cirrhosis and hepatitis. One of the most frequent reasons to determine TBA in a routine laboratory is for the diagnosis and management of intrahepatic cholestasis of pregnancy (ICP), also called obstetric cholestasis. ICP is a reversible cholestasis characterized by intense pruritus in pregnancy (most frequently in the third trimester of pregnancy) with spontaneous signs and symptoms disappearing after delivery (3). ICP is associated with perinatal mortality, meconium staining, intrapartum fetal distress, preterm labor and preterm (4). The most frequent laboratory abnormality noted in ICP is the elevation in the serum TBA. In particular, the most increases bile acids in ICP are CG and chenodeoxycholylglycine (5). The first-line management of ICP is the administration of ursodeoxycholic acid (UDCA), that acts through different effects: protection of cholangiocytes against cytotoxicity of hydrophobic bile acids, stimulation of hepatobiliary secretion, and protection of hepatocytes against bile acid–induced apoptosis (4).
In routine medical laboratory, the analytical methods that can be implemented for TBA determination are enzymatic and immunometric methods (6,7). Radioimmunoassays were introduced 25 years ago for the determination of TBA: the main drawback is the specificity of the antibody, particularly at low concentration of bile acids, and consequently the difference in cross-reactivities between the various bile acids. Moreover, these methods are manuals, with long analytical runtimes. The enzymatic methods are the most common laboratory assays: they use 3-α hydroxysteroid dehydrogenase to convert bile acids to 3-ketosteroids and NADH, follows a spectrophotometric detection. These methods are simple to implement, with no sample preparation, no use of radioactive materials and they can be carried out in fully-automated clinical chemistry analyzers. However, a study conducted by a UK External Quality Assessment provider demonstrates that enzymatic methods suffers of significant cross-reactivity with UDCA, the principal drug administered in the ICP management (8).
Bile acids profile can be obtained by mass spectrometric based methods, with the simultaneous separation and quantification of the serum bile acids. These methods represent the reference method for bile acids determination, but they are not easily implementable in a routine laboratory (9)
Recently, a new fully-automated chemiluminescence immunoassay (CLIA) has been developed to determine specifically CG (10). The aim of this paper is to evaluate the clinical utility of this CLIA in the ICP.
Methods
Subjects and samples
Two cohorts of patients with a singleton pregnancy were retrospectively recruited between 2015 and 2017 in a tertiary center. The first cohort (namely controls, (age range, 21–48 years) was represented by 56 patient hospitalized in the third trimester, for a different reason respect to ICP, in particular: 30% itching (not ICP), 19% gestational hypertension, 15% preterm delivery risk, 6% fetal problems (biometrical or morphological), 33% maternal diseases (renal, rheumatological, hematological, trauma). The second cohort (namely ICP women) was composed of 32 women at the third trimester of pregnancy (age range, 22–45 years) with a final diagnosis of ICP, before the treatment with UDCA. There were no other maternal pregnancy or maternal pathologies. The diagnosis of ICP was based on current clinical practice guidelines (3).
Fasting venous blood samples were collected for all participants to measure serum CG. Blood samples were kept at room temperature for at least 30 min and then centrifuged at 4,000 g for 5 min. Serum was separated and stored at 4 °C until assay. All the analyses were completed in at maximum 3 days.
The study was designed in accordance with the Helsinki Declaration. All participants were fully informed about the nature, purpose, procedures and risks of the study, and gave their informed consent.
CG determination by CLIA
Serum CG concentrations were measured using a single step competitive CLIA (MAGLUMI Cholylglycine, REF 130209005M, Snibe, Shenzhen, China) with nano-magnetic microbeads coated with a polyclonal antibody anti CG and the purified antigen conjugated to the luminescence substrate N-(aminobutil)- N-(ethyl)-isoluminol ABEI. Sample or Calibrator, with CG labelled with ABEI and nano magnetic microbeads coated with AbαCG are mixed thoroughly and incubated for 15 min at 37 °C; the supernatant is decanted after the sedimentation in a magnetic field. After a single cycle washing, the starter reagents are added to start the chemiluminescent reaction, with a total turnaround time of 30 min. This assay was carried out on the MAGLUMI 2000 plus platform (Snibe) following the manufacturer’s instructions. The required sample volume is 10 µL for the reaction, with a dead volume of 200 µL. The analytical measurement range was 0.13–86 µmol/L, being 0.13 µmol/L the analytical sensitivity declared by the manufacturer. Samples with concentrations higher than 86 µmol/L can be diluted re-tested and the final concentration calculated multiplying by the dilution factor. The declared imprecisions were less than 6% and 10% for repeatability (n=20) and reproducibility (n=21), respectively. The mean recovery was 102%.
Imprecision study
Three serum samples were repeated 12 times in a single batch to calculate the intra-assay imprecision. To evaluate the intra-laboratory imprecision, two serum pools, stored in aliquots at –20 °C, were repeated over one year, in each analytical batch.
Comparison with a TBA radioimmunometric assay
In routine, TBA were analysed with a RIA method (Conjugated Bile Acids 125I RIA Kit, REF 06B242918, MP BIOMEDICALS, LLC). This assay is manual, with an incubation time of 1 h at 37 °C. The analytical measurement range was 0.2–50 µmol/L, being 0.2 µmol/L the analytical sensitivity declared by the manufacturer. Samples with concentrations higher than 50 µmol/L shall be report as >50 µmol/L. The intra-laboratory imprecision obtained over one year with two serum pools was compared with that obtained with CLIA method. Serum samples (n=67) with TBA request were analysed in parallel for TBA with RIA and for CG with CLIA.
Statistical analysis
Analyse-it version 2.07 (Analyse-it® Software Ltd, Leeds, UK) was used for comparability study. The comparability between RIA and CLIA was assessed through Passing-Bablok regression analysis. Samples with analyte concentrations higher than 50 µmol/L with TBA assay were excluded from the analysis. MedCalc version 19.0.7 (MedCalc® Software, Ostend, Belgium) was used for reference intervals estimation and ROC curve analysis.
Results
Imprecision study
Intra-assay imprecisions were 5%, 4% and 3% at 3.2, 5.0 and 8.5 µmol/L, respectively. Intra-laboratory imprecision calculated over 1 year (n=65) was 6% for both serum pool levels (3.2 and 7.3 µmol/L).
Comparison with a TBA radioimmunometric assay
For TBA RIA method, intra-laboratory imprecisions calculated over 1 year (n=64) were 26% and 12% at 0.5 and 10.4 µmol/L, respectively. Passing-Bablok equation obtained with 67 serum samples (concentration range, 0.6–50 µmol/L) was CG = 0.94 TBA +0.44 with 95% CI from 0.81 to 1.07 for slope from –0.30 to 0.78 for intercept.
Serum CG levels in pregnant women
The levels of CG determined in the control cohort ranged from 0.5 to 9.3 µmol/L (median 2.8 µmol/L). The 95% CI were 0.9–9.1 µmol/L.
The levels of CG determined in the ICP women cohort ranged from 2.3 to 57.4 µmol/L (median 12.1 µmol/L). In Figure 1, the Box and whisker plot of the serum CG is represented.
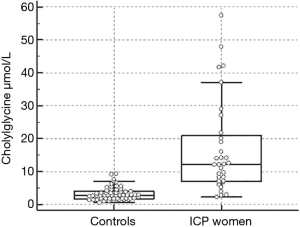
The optimal cut-off of CG for ICP was 5.5 µmol/L which corresponded to a sensitivity of 84% and a specificity of 92% with a Youden index of 0.74.
Discussion
TBA determination is a useful tool to evaluate hepatobiliary disease and bile acid malabsorption. Moreover, TBA are the most frequently requested biochemical markers in the ICP, both for diagnosis and management of the disease (3). Traditionally, TBA are measured by radioimmunoassay, with long analytical runtime, manual testing and the main drawback related to the radioactive waste management (6). The tendency in the clinical laboratories is to remove radioactive material and so there is an important drive to substitute RIA methods with no-radioactive assays (11). The most feasible methods for TBA determination in a routine medical laboratory are the enzymatic methods, which allow a rapid turnaround time, with no sample preparation, easily to automatize and without technical skills (6). Despite these advantages, Thomas et al. demonstrated that enzymatic methods suffered by significant interference by UDCA (8), that is the principal drug administered to the pregnant women with intrahepatic cholestasis to control the disease postponing the delivery as much as possible to guarantee the safety of the foetus (3,4). A manufacturer, in fact, declares that samples of patients in treatment with UDCA are not appropriate for the method (instructions for use not available online). Another possibility is to adopt mass spectrometric-based methods: liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) is the reference method for bile acids profiling (9), but this technology is difficult to integrate in a routine laboratory if the numerosity of the requests is large with time to report less than 3 days. More importantly, specific technical skills are necessary to carried out analyses with this technology. Therefore, it’s not feasible to shift all methods with analytical limitations to LC-MS/MS (12,13).
Recently a new chemiluminescent immunoassay to measure CG, the prevalent bile acid present in serum, was developed. So, when the manufacturer of our routine method released a declaration of no more availability of the kit, we decided to evaluate this new CLIA assay, considering that our laboratory is the reference for the Ginecology and Obstetrics Unit of the Hospital of Padova. The method demonstrated an imprecision less or equal than 6% for both repeatability and intra-laboratory imprecision over one year. The calculated imprecisions were less than the coefficients of variation declared by manufacturer (intra assay imprecision ranged from 5,3% to 5,9%; inter assay imprecision ranged from 8.7% to 9.1%). Moreover, the obtained imprecisions were better than the routine RIA method. Indeed, using the linear interpolation equation, the imprecisions at the same levels tested for the CLIA method (3.2 and 7.3 µmol/L) were 22.2% and 16.4%, respectively. Taking into account the impossibility to the supply of the RIA kit declared by manufacturer, a comparison study was made, and the results demonstrated a no significant difference between the methods (Passing Bablok equation with 95% CI for slope 0.81 to 1.07 and for intercept from –0.30 to 0.78.). Considering these results, we transfer the reference intervals for healthy subjects adopted for the RIA method (<6 µmol/L). In literature, to the best of our knowledge, there are no data about CG levels obtained with this CLIA method and, in particular, for ICP. The levels measured in pregnant women at the third trimester without ICP were higher than healthy subjects, suggesting the need for a specific reference interval for this population. Moreover, with evaluation of pregnant women at the third trimester with ICP, a cut-off for CG of 5.5 µmol/L was obtained with a sensitivity of 84% and a specificity of 92%. The obtained diagnostic accuracy was in line with the others present in literature, considering the methods heterogeneity and the diversity in the patients’ recruitment (14). Recently Manzotti et al. published a review to assess and compare the diagnostic accuracy of total serum bile acids for the diagnosis of ICP in pregnant women, presenting with pruritus: when considering all studies with a cut-off of 10 µmol/L, TSBA overall sensitivity ranged from 0.72 to 0.98 and specificity ranged from 0.81 to 0.97 (14). Our results fall within this range but with a lower cut-off. This can be explained by two main reasons. The first is certainly due to an analytical aspect. Our results were obtained by a CLIA method released to measured CG and in the systematic review no study used this kit. The second point is related to the patients’ recruitment, considering that our controls include pregnant women with and without pruritus.
This study has at least two limitations. The first is related to the number of patients. The cohort of controls should be increased to establish specific reference intervals and the possibility to divide this cohort in two, pregnant women with and without pruritus, should be taking into account. The second is related to the gestational age. We consider only women at the third trimester, without any classification in gestational weeks and with no women at the second trimester of pregnancy. Therefore, the obtained cut-off could be influenced by this factor and it should be confirmed in the future with more expanded cohorts of pregnancy women.
In conclusion, CG determination by CLIA method has demonstrated first of all a reliability from the analytical point of view. Secondly, the results obtained in the pregnancy women with ICP have demonstrated a diagnostic accuracy similar to the traditional assays carried out for TBA determination. Moreover, the turnaround time of CG analysis (about 30 min) allows a rapidly answer to clinical suspects of cholestasis in pregnancies avoiding potential foetal and maternal risks connected to high bile acid levels.
Acknowledgments
The authors thank Medical Systems for kindly providing the reagents used to perform the study.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Lippi, Martina Montagnana and Zhi-De Hu) for the series “Laboratory Medicine in Pregnancy” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The series “Laboratory Medicine in Pregnancy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of University of Padova and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marin JJ, Macias RI, Briz O, et al. Bile Acids in Physiology, Pathology and Pharmacology. Curr Drug Metab 2015;17:4-29. [Crossref] [PubMed]
- Kuntz E, Kuntz HD. Hepatology, Principles and Practice. Springer, 2008.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237-67. [Crossref] [PubMed]
- Diken Z, Usta IM, Nassar AH. A clinical approach to intrahepatic cholestasis of pregnancy. Am J Perinatol 2014;31:1-8. [Crossref] [PubMed]
- Chen J, Deng W, Wang J, et al. Primary bile acids as potential biomarkers for the clinical grading of intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet 2013;122:5-8. [Crossref] [PubMed]
- Sharma KR. Review on bile acid analysis. Int J Pharm Biomed Sci 2012;3:28-34.
- Griffiths WJ, Sjövall J. Bile acids: analysis in biological fluids and tissues. J Lipid Res 2010;51:23-41. [Crossref] [PubMed]
- Thomas M, Morris J, Ducroq D, et al. Specificity of Cholic acid, Chenodeoxycholic acid, Deoxycholic acid and Ursodeoxycholic acid in routine. Ann Clin Biochem 2013;50:29.
- Krautbauer S, Liebisch G. LC-MS/MS Analysis of Bile Acids. Methods Mol Biol 2018;1730:103-10. [Crossref] [PubMed]
- Snibe. Product. Immunoassay Kit. Available online: http://www.snibe.com/zh_en/en_productArtical.aspx?id=39
- International Atomic Energy Agency. Management of radioactive waste from the use of radionuclides in medicine. IAEA-TECDOC-1183. Available online: https://www.iaea.org/publications/6063/management-of-radioactive-waste-from-the-use-of-radionuclides-in-medicine
- Vogeser M, Zhang VY. Understanding the strategic landscape surrounding the implementation of mass spectrometry in the clinical laboratory: A SWOT analysis. Clin Mass Spectrom 2018;9:1-6. [Crossref]
- Antonelli G, Marinova M, Artusi C, et al. Mass spectrometry or immunoassay: est modus in rebus. Clin Chem Lab Med 2017;55:1243-45. [Crossref] [PubMed]
- Manzotti C, Casazza G, Stimac T, et al. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst Rev 2019;7:CD012546 [PubMed]
Cite this article as: Antonelli G, Visentin S, Tasinato A, Rocco G, Zemin F, Volentiera E, Faggian D, Cosmi E, Plebani M. Cholylglycine determination by an automated chemiluminescence immunoassay: preliminary results in the intrahepatic cholestasis of pregnancy. J Lab Precis Med 2019;4:35.


