Laboratory evaluation of deranged liver chemistries in pregnancy
Introduction
The liver plays a pivotal role in normal physiologic function, aiding in metabolism, detoxification, immune function, bile production and secretion, synthesis of albumin and clotting factors, storage of glycogen and minerals and more (1). Given the complex role of the liver, assessing and quantifying its function is an important but an arduous task. Several tests have been assembled to help monitoring liver synthetic function including albumin, prothrombin time (PT)/international normalized ratio (INR), platelet count, and evaluation of hepatocellular injury and cholestasis by measurement of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, gamma-glutamyl transpeptidase (GGT) and lactate dehydrogenase (LDH) (2). All of these tests together have been arbitrarily defined as “liver chemistries” (LCs) (3). Abnormal levels of these tests may indicate an intrinsic hepatic or a systemic disease affecting the liver. In special populations such as pregnant women, liver diseases can have significant short- and long-term consequences on mother and fetus. Risks to the fetus are varied depending on the specific type of liver disease present. Short term consequences may include preterm delivery, placental insufficiency, nutritional deficits, fetal growth restriction, distress, bradycardia and meconium stained amniotic fluid, while long term risks include congenital malformations, stillbirth and fatality (4,5). Short term maternal consequences include infection, nutritional deficits, hemorrhage, placental abruption, acute kidney failure and subcapsular hematomas (5,6). Long term, maternal risks may be severe as neuroglial impairment, psychosocial stress, disease recurrence in subsequent pregnancies and mortality (7). Therefore, careful and considerate investigation of abnormal LCs in this patient population is paramount (8).
Anticipated changes in LCs in pregnant women
Despite physiologic changes, the majority of liver tests remain within normal limits throughout the course of pregnancy (Table 1). Generally, levels of maternal ALP are expected to increase secondary to excess secretion of ALP by the placenta as well as development of fetal bones. Similarly, levels of alpha fetoprotein (AFP) will also rise as a result of added production by the fetal liver (9).
Table 1
| Parameter | Change observed in pregnancy |
|---|---|
| Alkaline phosphatase | Increase |
| Alpha-fetoprotein | Increase |
| Aspartate aminotransferase | Remain within normal limits |
| Total bilirubin | Remain within normal limits |
| Alanine aminotransferase | Remain within normal limits |
| Gamma-glutamyl transpeptidase | Remain within normal limits |
| Prothrombin time | Remain within normal limits |
| Activated partial thromboplastin time | Remain within normal limits |
| Thrombin time | Remain within normal limits |
| Serum albumin | Decrease |
| Total protein | Decrease |
| Serum bile acids | Remain within normal limits |
In anticipation of hemorrhage after delivery, the maternal coagulation system undergoes changes to promote hemostasis. Therefore, there is an increase in clotting factors VII, VIII, IX, X, and fibrinogen (10). On the contrary, there is a decrease in serum albumin, total protein, and biological anticoagulants like protein S and antithrombin (11). However, coagulation related liver tests such as PT, activated partial thromboplastin time (aPTT), and thrombin time (TT), still tend to remain within normal limits in patients who do not have an underlying bleeding disorder. Similarly, AST, ALT, bilirubin, and GGT remain normal throughout pregnancy (11). As such, any abnormal presentation of these labs warrants additional investigation.
Abnormal liver test
While there are expected laboratory changes in pregnant women, some may also present with unanticipated changes in LCs, which may indicate a disease process involving the liver (Table 2). Abnormal LCs occur at a rate of 3–5% in pregnant women (12). Detection of abnormal levels of AST, ALT, GGT, and/or bilirubin require further evaluation.
Table 2
| ALT | AST | ALP | Platelets | Albumin | GGT | PT | Bilirubin | |
|---|---|---|---|---|---|---|---|---|
| Liver disorders induced by pregnancy | ||||||||
| Hyperemesis gravidarum | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Pre-eclampsia | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Eclampsia | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Acute fatty liver of pregnancy | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↑ | ↑ |
| Hemolysis, elevated liver test and low platelets (HELLP) | ↑ | ↑ | ↔ | ↓ | ↔ | ↔ | ↔ | ↑ |
| Liver rupture | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Intrahepatic cholestasis of pregnancy | ↑ | ↑ | ↑ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Liver disease emerging coincidentally during pregnancy | ||||||||
| Viral hepatitis | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Herpes simplex | ↑ | ↑ | ↔ | ↓ | ↔ | ↔ | ↔ | ↔ |
| Budd-Chiari syndrome | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Cholelithiasis | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Pregnancy with pre-existing liver disease | ||||||||
| Cirrhosis of liver/portal hypertension | ↑ | ↑ | ↑ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Primary sclerosing cholangitis | ↑ | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Chronic active hepatitis | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Primary biliary cholangitis | ↔ | ↔ | ↑ | ↔ | ↔ | ↑ | ↔ | ↔ |
| Autoimmune hepatitis | ↑ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Wilson’s disease | ↑ | ↑ | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; PT, prothrombin time. ↑ increase, ↓ decrease, ↔ remains the same.
Liver diseases in pregnancy
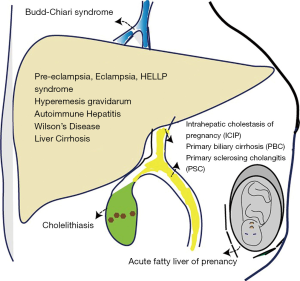
Liver diseases in pregnancy can be divided into three main categories: (I) liver diseases that are caused by physiologic changes of pregnancy, (II) liver diseases that have developed coincidentally during the course of pregnancy and (III) liver diseases that are chronic or pre-existing prior to pregnancy (Table 3, Figure 1).
Table 3
| Induced by pregnancy | Preexisting worsened by pregnancy | Emerging coincidental with pregnancy |
|---|---|---|
| Hyperemesis gravidarum | Chronic hepatitis B virus | Acute viral hepatitis |
| Pre-eclampsia | Chronic hepatitis C virus | Cholelithiasis |
| Eclampsia | Wilson’s disease | Budd-Chiari syndrome |
| HELLP syndrome | Liver cirrhosis/portal hypertension | Drug induced liver injury |
| Acute fatty liver of pregnancy | Autoimmune hepatitis | Hepatic tumor |
| Intrahepatic cholestasis of pregnancy | Primary sclerosing cholangitis | |
| Liver rupture/infarction | Primary biliary cholangitis |
Liver disorders induced by pregnancy
A number of diseases that affect the liver during pregnancy occur as a result of the physiologic changes in pregnancy. When severe, the fetus and mother can experience significant morbidity and mortality (13).
Hyperemesis gravidarum (HG)
During the first trimester, approximately 0.3–3% of pregnant women can experience HG, characterized by severe, intractable nausea and vomiting, and subsequent dehydration, volume depletion and weight loss. Laboratory values that aid in the diagnosis include serum electrolytes, complete blood count, LCs, amylase, lipase, urinalysis, creatinine, blood urea nitrogen, thyroid function tests and beta-human chorionic gonadotropin (hCG) (14). Commonly, lab abnormalities present in HG include decreases serum electrolytes (primarily potassium, sodium, and chloride). An elevated hematocrit can also be seen due to a fluid volume contraction. Laboratory examination can also demonstrate an increase in the urinary ketones, specific gravity, and blood urea nitrogen. Additionally, hCG and thyroxine, have been shown to increase with the severity of HG, while thyroid stimulating hormone decreases (15,16). Pertaining to LCs, an increase of ALT of up to two to five times the upper limit of normal can occur, while bilirubin levels tend to remain unchanged (17).
Pre-eclampsia, eclampsia, HELLP syndrome
Previously normotensive pregnant patients can develop hypertension (HTN) and experience hypertension-related liver disorders, which include pre-eclampsia, eclampsia, and hemolysis, elevated liver test and low platelets (HELLP) syndrome. Pre-eclampsia, which occurs in nearly 3–5% of pregnant patients, is a maternal multisystemic organ dysfunction disorder associated with new-onset HTN occurring beyond the 20th gestational week in a patient with no prior history of HTN, and greater than 300 mg of proteinuria within 24 hours (18). Eclampsia presents with the same criteria, with the only additional distinguishing feature of seizures. Associated LC abnormalities in patients with pre-eclampsia include elevated AST and ALT (in about 30% of patients) and mild increase in bilirubin. HELLP syndrome occurs in up to 0.8% of pregnant patients and represents a more severe form of pre-eclampsia. HELLP syndrome constitutes an increase in bilirubin, AST and ALT (200–700 IU/L range), and LDH (>600 IU/L) including thrombocytopenia (<100 K/μL platelets) (19).
Liver rupture and infarction
A rare, but potentially fatal complication on the spectrum of eclampsia includes liver hemorrhage or rupture which is associated with up to 50% mortality. Patients usually present with severe abdominal pain, fever, and other symptoms related to HELLP. LCs are notable for significantly elevated AST and ALT (typically greater than 1,000 U/L), anemia, and leukocytosis (20).
Acute fatty liver of pregnancy (AFLP)
AFLP is development of sudden microvascular steatosis hepatic cells that’s leads to liver failure and usually occur in the late stages of pregnancy. AFLP can lead to fatality of both mother and fetus if left untreated (5). Several LC derangements are recognized in AFLP including significantly elevated levels of AST and ALT (up to 1,000 IU) and bilirubin (usually does not exceed 0.0855 mmol/L), in addition to prolonged PT and decreased fibrinogen. Other laboratory abnormalities include elevations in levels of lactic acid, ammonia, and amino acids, which indicates hepatic mitochondrial failure. Patients may also present with thrombocytopenia, leukocytosis, and severe renal dysfunction (13).
Intrahepatic cholestasis of pregnancy (ICP)
ICP is the most common pregnancy-related liver disorder. It is a reversible cholestatic disorder presenting with pruritus on the soles of feet and palms of the hand with spontaneous relief within 6 weeks of delivery (21). Serum bilirubin levels are rarely elevated and measuring serum bile acids is the most effective biochemical test to diagnose ICP. There is a correlation between the level of maternal bile acids and the risk of fetal harm. In ICP, levels of total serum bile acids are typically elevated by 1.5–15 times. Levels exceeding 40 µmol/L have been associated with unfavorable effects on the fetus (22). Serum AST and ALT can also be elevated (typically increased by 1.5 to 10 times) with variable levels of ALP and GGT (22,23). Similarly, PT typically remains normal, but can be prolonged in cases of vitamin K malabsorption (22).
Liver disease emerging coincidentally during pregnancy
Acute hepatitis
Acute viral hepatitis can be acquired during the course of pregnancy. Acute hepatitis as a result of hepatitis A, B, C, D, and E, cytomegalovirus (CMV), Epstein-Barr virus (EBV) and herpes simplex virus (HSV) are collectively responsible for about 40% of cases of jaundice in pregnant women. Infection with hepatitis A, B, C, or D during pregnancy has little to no risk of fetal or maternal mortality or teratogenicity (24). On the other hand, pregnant women who acquire hepatitis E virus are at increased risks of developing fulminant hepatitis failure. Laboratory analysis most notably reveals an acute increase in ALT, AST, and bilirubin (25).
HSV
HSV is a rare but serious cause of acute hepatitis in pregnant women, with an estimated mortality rate of 40%. The clinical presentation is often vague and nonspecific. LC values that may aid in diagnosis include coagulopathy, thrombocytopenia and increased levels of aminotransferases, while bilirubin tends to remain normal (26).
Budd-Chiari syndrome (BCS)
The physiologic changes in pregnancy increase levels of certain coagulation factors inducing a hypercoagulable state. As a result, thrombophilic vascular diseases can occur at increased frequency. BCS is a rare disorder that results from outflow obstruction of the hepatic venous system secondary to hepatic vein thrombosis. This leads to sinusoidal congestion, ischemic liver injury, and portal hypertension. One out of five cases of BCS occur in women who are pregnant, have a recent history of pregnancy, or have a history of contraceptive use (27). The clinical presentation typically includes right upper quadrant pain, jaundice, hepatomegaly, and ascites. LCs do not typically aid in the diagnosis, as doppler ultrasound is needed to demonstrate venous outflow obstruction (28). However, these patients can have increased levels of clotting factors (I, II, V, VII, X and XII) along with fibrinogen levels and physiologic reduction of anticoagulant protein C concentration.
Cholelithiasis
Pregnant patients are at higher risk of development of gall stones due to deceased gallbladder motility and increased cholesterol secretion due to increase in estrogen and progesterone. Symptomatic gallstone disease during pregnancy has been associated with increased risk of maternal and fetal morbidity, as well as preterm birth (29). Therefore, making a diagnosis is essential. Any elevations in the LCs warrant additional investigation for other causes of acute abdominal pain including choledocholithiasis, acute cholecystitis, gallstone pancreatitis, acute cholangitis, gallstone ileus and more (30).
Drug induced liver injury (DILI)
Obtaining a thorough history of medication use is essential to determining causes of DILI, in addition to review of laboratory values. Patients with hepatocellular injury will have elevated aminotransferases with emphasis on ALT sometimes as high as 25 times the normal limit. Contrarily, patients with cholestatic injury will primarily have a significant rise in ALP that is at least twice the normal limit. Increased levels of bilirubin can be seen in cholestatic and hepatocellular injury and should be considered significant when total bilirubin values are higher than twice the normal limit in conjunction with an increased ALP or ALT (31,32).
Pregnancy with pre-existing liver disease
Pregnancy can also occur in women with pre-existing liver pathology. The most common chronic liver disorder in pregnant women is chronic viral hepatitis which requires diligent monitoring throughout the course of pregnancy and delivery.
Chronic viral hepatitis
Pregnant patients with pre-existing chronic hepatitis B (HBV) or hepatitis C (HCV) infection typically have an uneventful course of pregnancy, with minimal effect on existing liver disease in noncirrhotic patients. The primary concerns in these patients are prevention of vertical transmission and flare of chronic hepatitis, especially in endemic areas. As described earlier, the shift in immunological factors during pregnancy seemingly downregulate the maternal immune system to tolerate the genetically variable tissues between mother and fetus better. This altered immune status can potentially predispose patients to hepatitis flares by HBV or HCV viremia (33). Laboratory analysis in HBV typically show a rise in ALT that is at least three times the upper limit of normal limits, and hepatitis B surface antigen is present for at least six months duration (34). In chronically infected HCV patients, levels of ALT decrease in the later stages of pregnancy, with a return to baseline following delivery (35).
Autoimmune hepatitis (AIH)
AIH is a chronic inflammatory liver disorder with a predilection towards women. In pregnant patients with AIH, there is an increased risk of premature birth, low birth weight, and fetal demise (36). Because of immunological nature of AIH, the diagnosis is typically based on the clinical, serological, and histological data. Most studies describe elevated levels of ALT and AST, in addition to low complement C4 levels. Additionally, hypergammaglobulinemia (IgG) as well as increase in the autoantibodies aid in diagnosis. Testing for autoantibodies such as serum anti-nuclear, anti-mitochondrial, anti-smooth muscle, and anti-liver/kidney microsomal-1 antibodies is recommended in patients with suggestive clinical features. Lastly, liver biopsy can also aid in confirming the diagnosis although it is not always required (37).
Primary biliary cholangitis (PBC)
PBC is a T-cell immune-mediated disease of the small intralobular bile ducts with a predilection for women. Though post-menopausal women are typically affected, up to 25% of women of childbearing age can also be affected (38). Little data exists regarding changes in disease course and maternal and fetal outcomes in patients with PBC. However, existing studies have reported significantly elevated ALP and GGT values with presence of positive antimitochondrial and antinuclear antibodies. Additionally, liver transaminases may be mildly abnormal, but are typically normal. Lastly, bilirubin levels can increase significantly which are indicative of poor prognosis (39).
Primary sclerosing cholangitis (PSC)
PSC is an idiopathic disorder characterized by chronic inflammation and fibrosis of intrahepatic and extrahepatic bile ducts resulting in alternating ductal strictures and dilation. The clinical presentation typically includes right upper quadrant abdominal tenderness, weight loss, fatigue, and pruritus (40). Up to 70% of diagnosed patients are male. However, women can also be affected and the development of PSC may be influenced by pregnancy. Data is scarce, but there are some reported cases of PSC in pregnancy (41). Laboratory values show an increase in the levels of ALP, AST, ALT, serum IgG as well as magnetic resonance cholangiographic evidence of multifocal biliary tree strictures. Bilirubin levels tend to remain within the normal limits at diagnosis (42,43).
Wilson’s disease (WD)
WD disorder is characterized by the accumulation of copper primarily in the liver and brain as a result of defective biliary copper excretion related to mutations in the ATP7B gene. The data on pregnancy outcomes in patients with WD is scant, with only a few cases reporting a mix of successful and complicated outcomes (44). Clinical presentation varies, but commonly includes acute or chronic liver failure with or without neuropsychiatric decline. Laboratory abnormalities typically include elevated bilirubin, increased AST and ALT, Coombs-negative hemolytic anemia, and low serum ALP. When severe enough to cause liver failure, laboratory abnormalities in WD reveal an AST/ALT ratio often greater than two. Other labs noted include decreased levels of serum ceruloplasmin and platelets, as well as vitamin k refractory coagulopathy (45).
Cirrhosis of liver/portal hypertension
In women with pre-existing liver cirrhosis, pregnancy is rare due to irregular estrogen metabolism in combination with disturbances in the hypothalamic-pituitary axis that lead to anovulation and infertility (46). When pregnancy does occur, fetal risks include prematurity, spontaneous abortion, and stillbirth. The patient can present with laboratory abnormalities such as increased AST and ALT, with variable elevations in GGT and ALP (47). Bilirubin tends to remain unchanged. As synthetic function declines, the patient can also present with low levels albumin. In advanced fibrosis, laboratory changes include thrombocytopenia, anemia, and leukopenia (48).
Conclusions
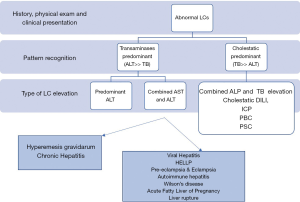
In conclusion, liver diseases in pregnancy can be potentially harmful to both mother and fetus. Pregnant patients who present with jaundice or abnormal LCs require laboratory investigations to prevent potential complications. As in any patient, a comprehensive history and physical examination should be performed, in addition to collection of appropriate labs (Figure 2). Deranged LCs in pregnant patients should be interpreted in the appropriate clinical context and further work should be performed for any deviation from laboratory changes that occur as a natural response to the state of pregnancy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Lippi, Martina Montagnana and Zhi-De Hu) for the series “Laboratory Medicine in Pregnancy” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.10.05). The series “Laboratory Medicine in Pregnancy” was commissioned by the editorial office without any funding or sponsorship. Hemant Goyal serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from May 2019 to April 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoekstra LT, de Graaf W, Nibourg GA, et al. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg 2013;257:27-36. [Crossref] [PubMed]
- Lala V, Minter DA. Liver Function Tests. StatPearls. Treasure Island (FL), 2019.
- Goyal H, May E. Roadmap for evaluation of abnormal liver chemistries. Journal of Laboratory and Precision Medicine 2017;2.
- Pusl T, Beuers U. Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis 2007;2:26. [Crossref] [PubMed]
- Hay JE. Liver disease in pregnancy. Hepatology 2008;47:1067-76. [Crossref] [PubMed]
- Cappell MS. Hepatic disorders severely affected by pregnancy: medical and obstetric management. Med Clin North Am 2008;92:739-60. vii-viii. [Crossref] [PubMed]
- Terrabuio DR, Abrantes-Lemos CP, Carrilho FJ, et al. Follow-up of pregnant women with autoimmune hepatitis: the disease behavior along with maternal and fetal outcomes. J Clin Gastroenterol 2009;43:350-6. [Crossref] [PubMed]
- Sharma AV, John S. Liver Disease In Pregnancy. StatPearls. Treasure Island (FL), 2019.
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, et al. Physiological changes in pregnancy. Cardiovasc J Afr 2016;27:89-94. [Crossref] [PubMed]
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409-14. [Crossref] [PubMed]
- Hill CC, Pickinpaugh J. Physiologic changes in pregnancy. Surg Clin North Am 2008;88:391-401. vii. [Crossref] [PubMed]
- Panther E, Blum HE. Liver diseases in pregnancy. Dtsch Med Wochenschr 2008;133:2283-7. [Crossref] [PubMed]
- Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant 2010;10:2520-6. [Crossref] [PubMed]
- Broussard CN, Richter JE. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am 1998;27:123-51. [Crossref] [PubMed]
- Gill BK, Jindal P, Kumar R, et al. A study of thyroid status in hyperemesis gravidarum. Indian J Clin Biochem 2007;22:148-51. [Crossref] [PubMed]
- Lockwood CM, Grenache DG, Gronowski AM. Serum human chorionic gonadotropin concentrations greater than 400,000 IU/L are invariably associated with suppressed serum thyrotropin concentrations. Thyroid 2009;19:863-8. [Crossref] [PubMed]
- Erick M, Cox JT, Mogensen KM. ACOG Practice Bulletin 189: Nausea and Vomiting of Pregnancy. Obstet Gynecol 2018;131:935. [Crossref] [PubMed]
- Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104. [Crossref] [PubMed]
- Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol 2011;35:182-93. [Crossref] [PubMed]
- Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol 1993;169:1000-6. [Crossref] [PubMed]
- Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol 2016;64:933-45. [Crossref] [PubMed]
- Ozkan S, Ceylan Y, Ozkan OV, et al. Review of a challenging clinical issue: Intrahepatic cholestasis of pregnancy. World J Gastroenterol 2015;21:7134-41. [Crossref] [PubMed]
- Floreani A, Carderi I, Paternoster D, et al. Intrahepatic cholestasis of pregnancy: three novel MDR3 gene mutations. Aliment Pharmacol Ther 2006;23:1649-53. [Crossref] [PubMed]
- Hieber JP, Dalton D, Shorey J, et al. Hepatitis and pregnancy. J Pediatr 1977;91:545-9. [Crossref] [PubMed]
- Licata A, Ingrassia D, Serruto A, et al. Clinical course and management of acute and chronic viral hepatitis during pregnancy. J Viral Hepat 2015;22:515-23. [Crossref] [PubMed]
- McCormack AL, Rabie N, Whittemore B, et al. HSV Hepatitis in Pregnancy: A Review of the Literature. Obstet Gynecol Surv 2019;74:93-8. [Crossref] [PubMed]
- Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med 1980;68:113-21. [Crossref] [PubMed]
- Darwish Murad S, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med 2009;151:167-75. [Crossref] [PubMed]
- Ibiebele I, Schnitzler M, Nippita T, et al. Outcomes of Gallstone Disease during Pregnancy: a Population-based Data Linkage Study. Paediatr Perinat Epidemiol 2017;31:522-30. [Crossref] [PubMed]
- Date RS, Kaushal M, Ramesh A. A review of the management of gallstone disease and its complications in pregnancy. Am J Surg 2008;196:599-608. [Crossref] [PubMed]
- Yu YC, Mao YM, Chen CW, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int 2017;11:221-41. [Crossref] [PubMed]
- Firoz T, Webber D, Rowe H. Drug-induced fulminant hepatic failure in pregnancy. Obstet Med 2015;8:190-2. [Crossref] [PubMed]
- Soderstrom A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis 2003;35:814-9. [Crossref] [PubMed]
- ter Borg MJ, Leemans WF, de Man RA, et al. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat 2008;15:37-41. [PubMed]
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology 2000;31:751-5. [Crossref] [PubMed]
- Lee MG, Hanchard B, Donaldson EK, et al. Pregnancy in chronic active hepatitis with cirrhosis. J Trop Med Hyg 1987;90:245-8. [PubMed]
- Sebode M, Hartl J, Vergani D, et al. Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver Int 2018;38:15-22. [Crossref] [PubMed]
- Trivedi PJ, Kumagi T, Al-Harthy N, et al. Good maternal and fetal outcomes for pregnant women with primary biliary cirrhosis. Clin Gastroenterol Hepatol 2014;12:1179-85.e1. [Crossref] [PubMed]
- Reshetnyak VI. Primary biliary cirrhosis: Clinical and laboratory criteria for its diagnosis. World J Gastroenterol 2015;21:7683-708. [Crossref] [PubMed]
- Broome U, Olsson R, Loof L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610-5. [Crossref] [PubMed]
- Wellge BE, Sterneck M, Teufel A, et al. Pregnancy in primary sclerosing cholangitis. Gut 2011;60:1117-21. [Crossref] [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [Crossref] [PubMed]
- Boberg KM, Fausa O, Haaland T, et al. Features of autoimmune hepatitis in primary sclerosing cholangitis: an evaluation of 114 primary sclerosing cholangitis patients according to a scoring system for the diagnosis of autoimmune hepatitis. Hepatology 1996;23:1369-76. [Crossref] [PubMed]
- Sinha S, Taly AB, Prashanth LK, et al. Successful pregnancies and abortions in symptomatic and asymptomatic Wilson's disease. J Neurol Sci 2004;217:37-40. [Crossref] [PubMed]
- Garcia-Romero CS, Guzman C, Cervantes A, et al. Liver disease in pregnancy: Medical aspects and their implications for mother and child. Ann Hepatol 2019;18:553-62. [Crossref] [PubMed]
- Cundy TF, O'Grady JG, Williams R. Recovery of menstruation and pregnancy after liver transplantation. Gut 1990;31:337-8. [Crossref] [PubMed]
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988;95:734-9. [Crossref] [PubMed]
- Qamar AA, Grace ND, Groszmann RJ, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol 2009;7:689-95. [Crossref] [PubMed]
Cite this article as: Johnson KD, Perisetti A, Thandassery R, Inamdar S, Cheryala M, Jecmenica M, Tharian B, Goyal H. Laboratory evaluation of deranged liver chemistries in pregnancy. J Lab Precis Med 2020;5:4.