
In pursuit of novel biomarkers reflecting intestinal inflammation: temporal variability and phenotypic characterisation of serum calprotectin and lactoferrin
Introduction
Ageing is characterized by a state of chronic low-grade inflammation predisposing the development of multiple chronic diseases and all-cause mortality (1, 2). However, the specific triggers of the pathogenic pro-inflammatory environment with advanced age remain largely unclear. Ageing is further indicated by pronounced impairment of homeostasis of intestinal microbiota, characterized by increased intestinal permeability and reduced number of beneficial commensal microbes. This environment may increase exposure to pathogenic bacteria and viruses in the gastrointestinal tract and facilitate the flow of microbial species into the bloodstream (3). In animal models using old germ-free mice, colonization of germ-free mice with microbiota from old mice were shown to drive intestinal permeability and translocation of bacterial components, further fueling inflammation and impairing cellular antibacterial functions (4). Co-housing, but not young, conventionally raised mice were shown to increase pro-inflammatory cytokines in the blood. In contrast, in tumor necrosis factor-deficient mice no age-related microbiota changes have been observed (4). Microbial dysbiosis could therefore play an important role as a contributor to age-associated disease risk (3). However, to test this hypothesis there is a need of easy to measure biomarkers of gut inflammation in bio samples collected in large human cohorts.
So far in clinical practice, faecal calprotectin and lactoferrin have emerged as common biomarkers used to detect gastrointestinal inflammation. Calprotectin is a zinc-binding protein that is believed to play a role in the defence against bacteria and viruses. This molecule consists of a complex of two intracellular proteins, S100A8 and S100A9, that is translocated as a heterodimer from the cytosol to the neutrophil cell membrane following calcium mobilization (5). It serves as a danger-associated molecular pattern protein (DAMP) due to its response to infectious agents, tissue damage, and other cellular deviations (6). Next to measuring in stool, calprotectin can be quantified in blood serum and plasma. Elevated serum calprotectin concentrations have previously been described in patients with arthritis (7-9), cardiometabolic diseases (10), diabetes (11), and cancer (12) among others.
Lactoferrin is a multifunctional glycoprotein belonging to the transferrin family (13). By interacting with specific receptors on monocytes and macrophages, lactoferrin attenuates inflammation and contributes to tissue repair (13). Furthermore, lactoferrin has the ability to scavenge free iron, which provides protection against pathogens and controls the release of pro-inflammatory cytokines (14). Growing evidence suggests multiple roles of lactoferrin in metabolic disorders including obesity, type-2 diabetes, cardiovascular disease and cancer (15-18).
The link between concentrations of calprotectin and lactoferrin and physiological or pathological effects on body functions, however, is not yet well characterized. The aim of this study was to assess the temporal reliability of serum calprotectin and lactoferrin over a 4-month period in a population-based sample of 207 apparently healthy individuals within the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam cohort. Furthermore, we aimed to characterize cross-sectional associations with anthropometric indicators of adiposity and a range of inflammatory and metabolic biomarkers.
Methods
Study population
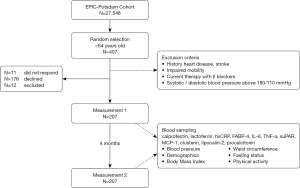
The study included 407 individuals taking part in a validation study conducted within the EPIC-Potsdam study (19) (see Figure 1). Individuals were randomly selected among all EPIC-Potsdam study participants younger than 64 years old. Exclusion criteria included history of heart disease (myocardial infarction, heart failure, cardiomyopathy, stroke, angina pectoris), impaired mobility, used β-blockers, and had systolic or diastolic blood pressure above 180 or 110 mmHg, respectively. Of the 407 invited participants, the total number of eligible participants with two blood samples was 207 (n=11 individuals did not respond; n=176 declined to participate; n=12 used β-blockers; n=1 provided one blood sample). Blood was drawn on two occasions, 4 months apart. The first blood samples were collected between October 2007 and March 2008 and the second between February and July 2008. The blood collection took place in the morning between 8–11 am. Written informed consent was obtained from all participants and the Ethics Committee of the Medical Association of Brandenburg approved the study procedures.
Biomarker measurement
After blood draw, blood fractions were separated and stored at −80 °C by qualified laboratory technicians. Calprotectin, lactoferrin, monocyte chemoattractant protein 1 (MCP-1), lipocalin-2, and high sensitivity C-reactive protein (hsCRP) were measured in serum with sandwich ELISA [BioVendor, limit of detection (LoD) 0.22 ng/mL, 1.1 ng/mL, 2.3 pg/mL, and 0.02 µg/mL, respectively]. Concentrations of fatty acid-binding protein 4 (FABP-4), procalcitonin, soluble urokinase-type plasminogen activator receptor (suPAR), and clusterin were measured in EDTA-plasma with sandwich ELISA [BioVendor, LoD 0.05 ng/mL, 15 pg/mL, 5.1 pg/mL, and 0.5 ng/mL, respectively]. Concentrations were measured at the Department of Clinical Nutrition, DIfE, Germany and according to the manufacturer’s instructions. The repeated samples from each study participant were assessed in the same batch. Cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α) were measured with a multiplex platform (MSD V-Plex Proinflammatory Panel 1 human Kit, LoD 0.06 pg/mL, 0.07 pg/mL, and 0.04 pg/mL, respectively) in plasma and with single samples.
Anthropometric measurement
Measurements of height, weight, waist circumference (WC), and systolic- and diastolic blood pressure were collected at the first and second visits. Height was measured with a rigid stadiometer; weight was measured using a standard scale or bio-impedance scale (20). Body mass index (BMI) was calculated from height and weight (kg/m2). Level of physical activity was assessed with a physical activity questionnaire (20).
Statistical analysis
All statistical analyses were performed using SAS software package, release 14.2 (SAS Institute, Cary, NC, USA). P value <0.05 was considered statistically significant, and statistical tests used were two-sided.
Variable distribution was assessed by visual inspection of histograms and evaluation of quantile-quantile plots. Non-normally distributed data was transformed using Box-Cox transformation, in order to allow for parametric testing. Serum calprotectin and lactoferrin concentrations were presented as medians and interquartile ranges (IQRs). Strata-specific median concentrations were calculated for sex and inflammatory status represented by hsCRP (below and above median concentration). For each biomarker, Wilcoxon signed rank test was used to compare concentrations between first and second measurements. Wilcoxon rank sum test (Kruskal Wallis) was used to compare concentrations between men and women for each measurement. As a measure of reliability between the two measurements, the intraclass correlation coefficient (ICC) was calculated for each biomarker, total and stratified by sex and hsCRP median cut-off. ICCs were calculated as ratios of between-person variance and total variance (between-person variance + within-person variance). Bland-Altman plots were further created as a complementing procedure to assess the agreement between two measurements for each individual (21). ICCs were calculated within BMI, WC, hsCRP and age strata (subdivided by respective medians as cut-off points).
Using Spearman partial correlation analysis, associations of serum calprotectin and lactoferrin were assessed with anthropometric measures of adiposity (BMI and WC) and a range of pre-selected inflammatory biomarkers including hsCRP, MCP-1, clusterin, lipocalin-2, procalcitonin, IL-6, IL-8, TNF-α, suPAR, and FABP-4. Correlations with BMI were adjusted for age and sex, and the remaining correlations were additionally adjusted for BMI. Fisher’s z transformation was used to produce 95% CIs for each correlation coefficient.
In linear regression analysis anthropometric measures and inflammatory biomarkers were modelled as predictors of calprotectin and lactoferrin concentrations to estimate the explained variance represented by the adjusted coefficient of determination (adjusted R2). In both correlation and regression analysis the baseline concentration measurements were used.
To facilitate potential application of biomarker measurements in future observational studies, we calculated the degree of attenuation of risk estimates that arises due to biological variability of the biomarker. The relative risk (RR) estimate is based on the following formula:
Results
Table 1 presents the baseline characteristics of the study population. The median age of the study participants was 56.7 years. Participants had a median BMI of 26.1 kg/m2, WC of 93.0 cm, and serum hsCRP level of 1.2 µg/mL.
Table 1
| Characteristics | All participants (n=207) | Men (n=83) | Women (n=124) |
|---|---|---|---|
| Age (years) | 56.7 (53.7, 59.5) | 57.6 (55.8, 60.4) | 55.4 (51.5, 58.9) |
| Range | 44.8–63.9 | 51.5–63.7 | 44.8–63.9 |
| BMI (kg/cm2) | 26.1 (23.3, 28.8) | 27.8 (25.3, 29.5) | 25.0 (22.6, 27.9) |
| Range | 19.1–41.7 | 19.8–37.0 | 19.1–41.7 |
| Overweight (BMI >25) | 61% | 78% | 50% |
| Waist circumference (cm) | 93.0 (83.8, 101.8) | 100.8 (96.1, 107.5) | 86.3 (77.6, 93.3) |
| Range | 68.3–126.3 | 79.3–126.3 | 68.3–115.8 |
| hsCRP (μg/mL) | 1.2 (0.7, 2.5) | 1.5 (0.7, 2.9) | 1.1 (0.6, 2.2) |
| Range | 0.1–13.4 | 0.1–12.9 | 0.2–13.4 |
| Systolic blood pressure (mm Hg) | 136.0 (128.0, 144.0) | 137.0 (130.0, 145.0) | 134.8 (124.0, 142.0) |
| Range | 100.0–206.0 | 100.0–206.0 | 100.0–163.0 |
| Diastolic blood pressure (mm Hg) | 88.0 (80.0, 94.0) | 90.0 (85.0, 96.0) | 86.0 (79.0, 92.0) |
| Range | 62.0–120.0 | 62.0–120.0 | 67.0–106.0 |
| Sports in winter (h per week) | 1.0 (0, 2.5) | 0.5 (0, 2.0) | 1.0 (0, 3.0) |
| Range | 0–14.0 | 0–12.0 | 0–14.0 |
| Sports in summer (h per week) | 1.0 (0, 3.0) | 0 (0, 2.0) | 1.0 (0, 3.0) |
| Range | 0–14.0 | 0–12.0 | 0–14.0 |
| Non-fasting | 10% | 13% | 8% |
Values are expressed as medians (25th, 75th percentile), or percentages. BMI, body mass index; hsCRP, high sensitivity C-reactive protein.
Table 2 presents the repeated measurements of calprotectin and lactoferrin, overall and stratified by sex, BMI (below or above 25 kg/m2), and hsCRP (below or above 1.2 µg/mL). Median serum calprotectin concentrations for first and second measurements were 1,494 ng/mL (IQR: 1,123–2,029) and 1,648 ng/mL (IQR: 1,139–2,486), respectively. Median serum lactoferrin concentrations for first and second measurements were 455.9 ng/mL (IQR: 304.8–620.4) and 517.6 ng/mL (IQR: 352.5–734.2), respectively.
Table 2
| Biomarkers | First measurement | Second measurement | P difference* | ICC (95% CI) | |||
|---|---|---|---|---|---|---|---|
| N | Median [IQR] | N | Median [IQR] | ||||
| Calprotectin (ng/mL) | |||||||
| All | 207 | 1,494 [1,123–2,029] | 207 | 1,648 [1,139–2,486] | 0.205 | 0.38 (0.26, 0.49) | |
| Gender | |||||||
| Men | 83 | 1,421 [1,115–1,861] | 83 | 1,723 [1,154–2,582] | 0.129 | 0.48 (0.30, 0.63) | |
| Women | 124 | 1,514 [1,123–2,208] | 124 | 1,604 [1,125–2,483] | 0.674 | 0.33 (0.16, 0.47) | |
| P difference** | 0.427 | 0.931 | |||||
| BMI | |||||||
| <25 kg/m2 | 80 | 1,465 [1,163–1,873] | 80 | 1,456 [1,072–2,163] | 0.629 | 0.41 (0.21, 0.58) | |
| ≥25 kg/m2 | 127 | 1,555 [1,120–2,446] | 127 | 1,731 [1,167–2,741] | 0.223 | 0.37 (0.21, 0.51) | |
| P difference** | 0.513 | 0.068 | |||||
| hsCRP | |||||||
| <1.2 μg/mL | 130 | 1,399 [1,082–1,859] | 130 | 1,500 [1,124–2,540] | 0.076 | 0.39 (0.24, 0.53) | |
| ≥1.2 μg/mL | 77 | 1,673 [1,202–2,510] | 77 | 1,813 [1,227–2,437] | 0.815 | 0.35 (0.14, 0.53) | |
| P difference** | 0.01 | 0.211 | |||||
| Lactoferrin (ng/mL) | |||||||
| All | 163 | 455.9 (304.8–620.4) | 171 | 517.6 (352.5–734.2) | <0.0001 | 0.62 (0.51, 0.71) | |
| Gender | |||||||
| Men | 66 | 415.3 (328.3–511.4) | 68 | 486.2 (355.3–666.1) | <0.0001 | 0.45 (0.24, 0.62) | |
| Women | 97 | 488.6 (297.1–694.1) | 103 | 526.7 (330.7–805.0) | 0.012 | 0.69 (0.57, 0.78) | |
| P difference** | 0.04 | 0.545 | |||||
| BMI | |||||||
| <25 kg/m2 | 67 | 434.6 (292.9–694.1) | 69 | 526.7 (350.9–758.2) | 0.003 | 0.65 (0.48, 0.76) | |
| ≥25 kg/m2 | 96 | 467.3 (320.8–610.6) | 102 | 497.3 (353.4–688.3) | 0.0008 | 0.59 (0.45, 0.71) | |
| P difference** | 0.726 | 0.595 | |||||
| hsCRP | |||||||
| <1.2 μg/mL | 88 | 418.3 (270.7–610.6) | 95 | 476.4 (331.6–672.0) | 0.0008 | 0.64 (0.51, 0.75) | |
| ≥1.2 μg/mL | 75 | 477.9 (391.9–621.8) | 76 | 576.6 (366.1–841.7) | 0.003 | 0.56 (0.39, 0.70) | |
| P difference** | 0.034 | 0.032 | |||||
Values are expressed as medians (25th, 75th percentile). *, P value for difference based on Wilcoxon signed rank test between first and second measurements. **, P value for difference based on Wilcoxon rank sum test between men and women. BMI, body mass index; hsCRP, high sensitivity C-reactive protein; ICC, intraclass correlation coefficient.
Serum calprotectin and lactoferrin concentrations were higher in participants with elevated hsCRP (above 1.2 µg/mL). The overall ICCs over a 4-month period were moderate (ICC: 0.38, 95% CI: 0.26, 0.49 for calprotectin; ICC: 0.62, 95% CI: 0.51, 0.71 for lactoferrin). Information from the Bland-Altman plots supported a good agreement for both biomarkers observed mostly at lower concentrations, as seen by the symmetrical distribution of the individual differences within the limits of agreement (Figure 2). There have been more participants with extreme calprotectin concentrations and respectively a higher number of individuals with low agreement between repeated measurements for this biomarker.

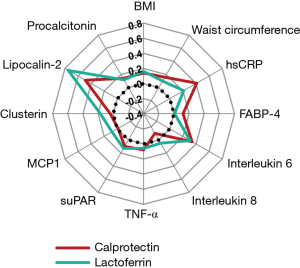
Figure 3 presents the results from the correlation analyses between lactoferrin, calprotectin, and biomarkers representing metabolic and immune response variables, adjusted for age, sex and BMI. Both calprotectin and lactoferrin showed positive correlations towards lipocalin-2 [Rho: 0.49 (95% CI: 0.38, 0.59); 0.75 (0.67, 0.81)], IL-6 [0.34 (0.21, 0.46); 0.31 (0.16, 0.44)], and hsCRP [0.41 (0.26, 0.53); 0.21 (0.05, 0.36) (see Table S1]. Another albeit weaker correlation calprotectin and lactoferrin showed was with BMI [Rho 0.14 (0.00, 0.27) and 0.16 (0.00, 0.30), respectively]. Mutual adjustments did not essentially change the correlations of calprotectin and lactoferrin with further biomarkers of inflammation. In multivariable-adjusted linear regression lipocalin-2 explained largest variation in circulating calprotectin and lactoferrin (23.4% and 54.6%, respectively) (Table S2).
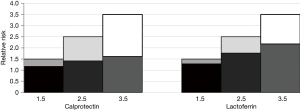
The attenuation of hypothetical true risk due to intra-individual variability was calculated based on the overall ICCs of calprotectin and lactoferrin (Figure 4). Considering true relative risks of 1.5, 2.5, and 3.5, the risk estimates of calprotectin would be attenuated by 22%, 43%, and 54%, respectively, and lactoferrin by 14%, 29%, and 38%, respectively.
Discussion
In this population-based study sample, serum calprotectin and lactoferrin showed moderately good reliability over a 4-month time period. Both biomarkers were positively associated with biomarkers of chronic inflammation (hsCRP), innate immune response (IL-6) and bacterial infection (lipocalin-2). These findings suggest calprotectin and lactoferrin as reliable biomarkers that could potentially reflect the activity and size of an inflammatory process in the gut.
Quantifying calprotectin and lactoferrin concentrations in blood may provide non-invasive estimations of systemic immunomodulatory activities, including regulation of microbial activity and intestinal homeostasis (22). Serum calprotectin represents a danger signal which is actively put into action by sentinel cells rather than being released once tissue damage has already occurred (23). Lactoferrin on the other hand acts as an anti-inflammatory factor when released by neutrophils. A potential mechanism for the influence of lactoferrin on metabolism may implicate its ability to change microbial composition (24,25). Since many systemic diseases are considered to depend on interactions with intestinal permeability and microbiota, calprotectin and lactoferrin could serve as important novel biomarkers in associated disease risk outperforming established non-specific inflammatory biomarkers such as traditionally used CRP measurements.
In line with our expectations, both calprotectin and lactoferrin were strongly correlated with lipocalin-2 as an established biomarker of bacterial infection and associated inflammatory response. Similar to lactoferrin, increased lipocalin-2 has been suggested to prevent intestinal inflammation and supress microbial growth (14).
Elevated calprotectin and lower lactoferrin serum levels have been reported in obesity-related chronic low grade inflammation (10,15,17,26-28), suggesting the potential utility of these biomarkers in the monitoring of metabolic complications. Indeed, research suggests perturbations in the gut microbial composition arise with obesity, increasing the number of Firmicutes and Bacteroides in obese patients (29). Studies of associations between microbiota profiles and different phenotypes and BMI have adventured positive and negative associations among different phyla of the intestines. Despite these findings, we could not find clear links between serum calprotectin and lactoferrin with obesity-related measures in our population, which is in line with reports suggesting that circulating concentrations of calprotectin and lactoferrin could be linked to chronic inflammation beyond obesity (30).
It could be questioned to what extent serum concentrations of calprotectin and lactoferrin are representative of intestinal inflammation. Previously reported correlations between serum and faecal concentrations ranged from low to moderate strength (see Table S3). It has been suggested that decreased serum levels of calprotectin may mirror local inflammation, because inflammatory cells expressing calprotectin are activated and transmigrate from peripheral circulation, through the endothelium, to the inflamed tissues (31). Whether serum calprotectin and lactoferrin may better reflect systemic inflammation rather than intestinal inflammation warrants further validation in large cohorts.
As calprotectin and lactoferrin may be of valuable interest to predict or monitor microbial activities implicated in health and disease, issues related to different methods of measurements should be considered. Lack of assay standardization may limit comparisons in different study outcomes. In our study, calprotectin and lactoferrin were measured using ELISA. ELISA kits from different manufacturers have varying detection limits, and some kits may not reach the clinical threshold of biomarkers especially in early stage of diseases or in healthy individuals with low concentrations (32). In the kits we used, the detection limit of lactoferrin and calprotectin were 1.1 and 0.22 ng/mL, respectively. When comparing detection limits of varying ELISA kits we observed notable differences. Indeed, a recent study from the UK National External Quality Assessment Service revealed there can be 3.8-fold differences between calprotectin quantification by ELISAs from different manufacturers (33). Although calprotectin and lactoferrin are often measured with ELISA, this type of quantification has its drawbacks because measurements can be time consuming and mostly suited for analysing samples in batch. Faster and more user-friendly techniques have been developed for quantifying calprotectin, including enzyme fluoroimmunoassay, quantitative immunochromatography, and semi-quantitative immunochromatography. A study comparing a range of different calprotectin assays for the assessment of IBD, including these, still found large quantitative differences between assays (34). As it is not possible to use different methods interchangeably, these findings again highlight the need for standardization of methods.
The large in-between assay variability may be a reason why optimal threshold parameters for serum calprotectin and lactoferrin concentrations are still not fully defined. These parameters should be specific for different populations, because their concentrations can differ according to individual characteristics such as age, phenotypic traits, or infection and disease state. In general, physiologic serum levels of calprotectin and lactoferrin are low in healthy populations. In prior research serum calprotectin has been measured in diabetic populations as surrogate biomarker endpoint of dietary interventions (35,36), and levels were found to be elevated above the reference range of <1 mg/L (23). Due to the growing evidence that circulating calprotectin and lactoferrin levels are elevated in chronic inflammatory conditions, the use of these biomarkers for the early identification of age-related diseases before they develop any clinical manifestations holds promise for the development of appropriate primary prevention strategies.
Our study has several strengths. We are the first, to our knowledge, to assess the variability of serum calprotectin and lactoferrin concentrations. Our findings may provide methodological guidance for future studies interested in quantifying the degree of intestinal inflammatory activity and gut homeostasis in the circulation. Rather than relying on a limited number of generic non-specific markers common to both acute and low-grade chronic inflammation, such as cytokine IL-6 and acute-phase protein CRP, establishing, quantifying and understanding biomarkers that reflect tissue-specific inflammatory processes and pathways are needed. Further strengths of our study include our relatively large sample size and the evaluation of reliability in both sexes according to specific phenotypic subgroups.
Limitations of our study should nevertheless be considered. Despite our large sample for a validation study, our results may not be generalizable because we measured apparently healthy older-aged individuals living in a specific geographic region. Future studies should take into account repeated samplings per time point, storage time, age range, and the health of the individuals at the time of measurement. We did not have the data to control for infections or other factors that may have influenced the inflammation state of the participants at the time of the measurement. Furthermore, we measured the biomarkers in different seasons whilst seasonality may influence biomarkers. Improvements would be to take samples in each season to detect changes, or repeat samples in the same season to measure if reliability is improved. Lastly, a small proportion (10%) of individuals was non-fasting at the time of measurement. When stratifying for fasting status, however, we found no influence on biomarker levels.
In conclusion, our findings suggest serum calprotectin and lactoferrin as reliable biomarkers in human research. Our analysis clearly showed lipocalin-2 as the main predictor of their serum concentrations, and this comes in support of their role in impaired microbiota and inflammation in the gut. These results may be applied when designing studies in epidemiological research evaluating activity and size of an inflammatory process in the gut.
Table S1
| Selected biomarkers | Calprotectin | Lactoferrin | |||||
|---|---|---|---|---|---|---|---|
| ρa | 95% CIb | P value | ρa | 95% CIb | P value | ||
| Lactoferrin | 0.55 | 0.43, 0.65 | <0.0001 | ||||
| BMIc | 0.14 | 0.00, 0.27 | 0.053 | 0.16 | 0.00, 0.30 | 0.046 | |
| Waist circumference | 0.16 | 0.02, 0.29 | 0.0226 | 0.10 | −0.06, 0.25 | 0.2297 | |
| hsCRP | 0.41 | 0.26, 0.53 | <0.0001 | 0.21 | 0.05, 0.36 | 0.0093 | |
| FABP-4 | 0.12 | −0.01, 0.26 | 0.0787 | 0.01 | −0.15, 0.16 | 0.9349 | |
| IL-6 | 0.34 | 0.21, 0.46 | <0.0001 | 0.31 | 0.16, 0.44 | <0.0001 | |
| IL-8 | −0.1 | −0.24, 0.04 | 0.1459 | 0.05 | −0.10, 0.20 | 0.5182 | |
| TNF-α | 0.08 | −0.05, 0.22 | 0.2274 | 0.06 | −0.09, 0.22 | 0.4253 | |
| SuPAR | 0.10 | −0.05, 0.25 | 0.1919 | 0.13 | −0.03, 0.28 | 0.1195 | |
| MCP1 | −0.01 | −0.15, 0.13 | 0.86 | 0.08 | −0.08, 0.23 | 0.3277 | |
| Clusterin | 0.04 | −0.11, 0.19 | 0.5625 | 0.17 | 0.02, 0.32 | 0.0298 | |
| Lipocalin-2 | 0.49 | 0.38, 0.59 | <0.0001 | 0.75 | 0.67, 0.81 | <0.0001 | |
| Procalcitonin | 0.14 | 0.01, 0.28 | 0.0406 | 0.12 | −0.04, 0.27 | 0.1297 | |
a, Spearman partial correlation coefficient. b, based on Fisher’s z transformation. c, adjusted for age and sex only. BMI, body mass index; hsCRP, high sensitivity C-reactive protein; IL, interleukin; TNFa, tumor necrosis factor alpha; MCP1, monocyte chemoattractant protein 1; SuPAR, soluble urokinase-type plasminogen activator receptor.
Table S2
| Model | Biomarker | Added variance (%) | Total adjusted R2 (%) |
|---|---|---|---|
| BMI, waist circumference | Calprotectin | +3.9 | 3.9 |
| Lactoferrin | 0 | 0 | |
| + hsCRP, IL-6 | Calprotectin | +3.7 | 7.6 |
| Lactoferrin | 0 | 0 | |
| + clusterin | Calprotectin | 0 | 7.1 |
| Lactoferrin | +6.3 | 6.3 | |
| + lipocalin-2 | Calprotectin | +21.7 | 28.8 |
| Lactoferrin | +49.6 | 55.9 | |
| + procalicitonin | Calprotectin | +0.1 | 28.9 |
| Lactoferrin | +0.8 | 56.7 |
Percentages of explained variance (represented by adjusted R2) of calprotectin and lactoferrin concentrations. Basic model consists of BMI and waist circumference, and additional variables are added including CRP and IL-6, clusterin, lipocalin-2, and procalcitonin. Calculations are based on cross-sectional data obtained from first measurement with available hsCRP levels (n=151). Adjusted R2 was derived from linear regression models with calprotectin or lactoferrin as dependent variables and anthropometric and additional biomarker concentrations as independent variables. BMI, body mass index; WC, waist circumference; IL-6, interleukin 6; hsCRP, high sensitivity C-reactive protein.
Table S3
| Reference | N | Disease state | Variable | Assay | Correlation measure | Outcome | Mean ± SD or median [IQR] serum calprotectin concentration |
|---|---|---|---|---|---|---|---|
| Carlsen et al. 2019 (37) | 19* | Ulcerative colitis | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: r (P) | R=0.01 (P=0.96) | 1,350 [240–194,600] ng/mL |
| McCann et al. 2017 (38) | 109 | Gastrointestinal | Faecal calprotectin vs. serum calprotectin | ELISA | ICC (95% CI) | 0.10 (−0.09, 0.29) | 6,670 [1,060–24,000] ng/mL |
| Meuwis et al. 2013 (39) | 79 | Crohn’s | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: r (P) | R=0.27 (P=0.018) | 8,892 [410–125,000] ng/mL |
| Hare et al. 2013 (40) | 45 | Acute severe ulcerative colitis | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: R2 (P) | R2=0.02 (P=0.45) | |
| Fukunaga et al. 2018 (41) | 54 | IBD (ulcerative colitis and Crohn’s) | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: R2 (P) | R2=0.1013 (P=0.47) | |
| Cypers et al. 2016 (42) | 58 | Patients with spondyloarthritis | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: R2 (P) | P=0.38 | 3,948 [782–17,246] ng/mL |
| Boschetti et al. 2015 (43) | 32 | Crohn’s | Faecal calprotectin vs. serum calprotectin | ELISA | Spearman: R2 (P) | R2=0.18 (P=0.50) | 12,700±6,500 ng/mL |
*, patients <18 years old [median age 13 years (7–17 years)]. ICC, intra-class correlation; IQR, interquartile range; CI, confidence interval.
Acknowledgments
We express thanks to Katrin Ritter (DIfE) for her technical assistance and Human Study Centre (DIfE) for data collection and logistics. Special thanks to Manuela Bergmann for leading the data generation, to Silke Navia Fruth and Herbert Piechot for their assistance with biosample management, and to Ellen Kohlsdorf for data management. Finally, thanks to all EPIC-Potsdam participants for contributing to the study.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.12.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Medical Association of Brandenburg approved the study procedures. Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505-22. [Crossref] [PubMed]
- Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Curr Opin Immunol 2014;29:23-8. [Crossref] [PubMed]
- Biragyn A, Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol 2018;19:e295-304. [Crossref] [PubMed]
- Thevaranjan N, Puchta A, Schulz C, et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018;23:570. [Crossref] [PubMed]
- Roth J, Burwinkel F, van den Bos C, et al. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993;82:1875-83. [Crossref] [PubMed]
- Rezniczek GA, Forster C, Hilal Z, et al. Calprotectin in pregnancy and pregnancy-associated diseases: a systematic review and prospective cohort study. Arch Gynecol Obstet 2019;299:1567-77. [Crossref] [PubMed]
- De Rycke L, Baeten D, Foell D, et al. Differential expression and response to anti-TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 2005;206:17-27. [Crossref] [PubMed]
- Frosch M, Ahlmann M, Vogl T, et al. The myeloid-related proteins 8 and 14 complex, a novel ligand of toll-like receptor 4, and interleukin-1beta form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2009;60:883-91. [Crossref] [PubMed]
- Hammer HB, Odegard S, Fagerhol MK, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis 2007;66:1093-7. [Crossref] [PubMed]
- Kruzliak P, Novak J, Novak M, et al. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev 2014;25:67-75. [Crossref] [PubMed]
- Bouma G, Lam-Tse WK, Wierenga-Wolf AF, et al. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes 2004;53:1979-86. [Crossref] [PubMed]
- Arai K, Takano S, Teratani T, et al. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets 2008;8:243-52. [Crossref] [PubMed]
- Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a Context of Inflammation-Induced Pathology. Front Immunol 2017;8:1438. [Crossref] [PubMed]
- Kruzel ML, Actor JK, Boldogh I, et al. Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 2007;61:261-7. [PubMed]
- Mayeur S, Veilleux A, Pouliot Y, et al. Plasma Lactoferrin Levels Positively Correlate with Insulin Resistance despite an Inverse Association with Total Adiposity in Lean and Severely Obese Patients. PLoS One 2016;11:e0166138 [Crossref] [PubMed]
- Moreno-Navarrete JM, Ortega FJ, Bassols J, et al. Association of circulating lactoferrin concentration and 2 nonsynonymous LTF gene polymorphisms with dyslipidemia in men depends on glucose-tolerance status. Clin Chem 2008;54:301-9. [Crossref] [PubMed]
- Mayeur S, Spahis S, Pouliot Y, et al. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid Redox Signal 2016;24:813-36. [Crossref] [PubMed]
- Hao L, Shan Q, Wei J, et al. Lactoferrin: Major Physiological Functions and Applications. Curr Protein Pept Sci 2019;20:139-44. [Crossref] [PubMed]
- Boeing H, Korfmann A, Bergmann MM. Recruitment procedures of EPIC-Germany. European Investigation into Cancer and Nutrition. Ann Nutr Metab 1999;43:205-15. [Crossref] [PubMed]
- InterAct C. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol 2012;27:15-25. [Crossref] [PubMed]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [Crossref] [PubMed]
- Kalla R, Kennedy NA, Ventham NT, et al. Serum Calprotectin: A Novel Diagnostic and Prognostic Marker in Inflammatory Bowel Diseases. Am J Gastroenterol 2016;111:1796-805. [Crossref] [PubMed]
- Ehrchen JM, Sunderkotter C, Foell D, et al. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 2009;86:557-66. [Crossref] [PubMed]
- Oda H, Wakabayashi H, Yamauchi K, et al. Lactoferrin and bifidobacteria. Biometals 2014;27:915-22. [Crossref] [PubMed]
- Mastromarino P, Capobianco D, Campagna G, et al. Correlation between lactoferrin and beneficial microbiota in breast milk and infant's feces. Biometals 2014;27:1077-86. [Crossref] [PubMed]
- Lylloff L, Bathum L, Madsbad S, et al. S100A8/A9 (Calprotectin), Interleukin-6, and C-Reactive Protein in Obesity and Diabetes before and after Roux-en-Y Gastric Bypass Surgery. Obes Facts 2017;10:386-95. [Crossref] [PubMed]
- Mortensen OH, Nielsen AR, Erikstrup C, et al. Calprotectin--a novel marker of obesity. PLoS One 2009;4:e7419 [Crossref] [PubMed]
- Calcaterra V, De Amici M, De Silvestri A, et al. Serum Calprotectin Level in Children: Marker of Obesity and Its Metabolic Complications. Ann Nutr Metab 2018;73:177-83. [Crossref] [PubMed]
- Castaner O, Goday A, Park YM, et al. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol 2018;2018:4095789 [PubMed]
- Ortega FJ, Sabater M, Moreno-Navarrete JM, et al. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur J Endocrinol 2012;167:569-78. [Crossref] [PubMed]
- Genre F, Rueda-Gotor J, Remuzgo-Martinez S, et al. Association of circulating calprotectin with lipid profile in axial spondyloarthritis. Sci Rep 2018;8:13728. [Crossref] [PubMed]
- Satija J, Punjabi N, Mishra D, et al. Plasmonic-ELISA: expanding horizons. RSC Advances 2016;6:85440-56. [Crossref]
- Whitehead SJ, French J, Brookes MJ, et al. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem 2013;50:53-61. [Crossref] [PubMed]
- Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J 2014;2:30-7. [Crossref] [PubMed]
- Markova M, Koelman L, Hornemann S, et al. Effects of plant and animal high protein diets on immune-inflammatory biomarkers: A 6-week intervention trial. Clin Nutr 2019; [Epub ahead of print]. [PubMed]
- Ohlsson B, Roth B, Larsson E, et al. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed Rep 2017;6:411-22. [Crossref] [PubMed]
- Carlsen K, Malham M, Hansen LF, et al. Serum calprotectin in adolescents with inflammatory bowel disease-a pilot investigation. J Pediatr Gastroenterol Nutr 2019;68:669-75. [Crossref] [PubMed]
- McCann RK, Smith K, Gaya DR. A prospective single centre pilot evaluation of a serum calprotectin assay in unselected gi patients. Clin Biochem 2017;50:533-6. [Crossref] [PubMed]
- Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis 2013;7:e678-83. [Crossref] [PubMed]
- Hare NC, Kennedy NA, Kingstone K, et al. Pth-082 serum calprotectin: A novel biomarker to predict outcome in acute severe ulcerative colitis? Gut 2013;62:A244-5. [Crossref]
- Fukunaga S, Kuwaki K, Mitsuyama K, et al. Detection of calprotectin in inflammatory bowel disease: Fecal and serum levels and immunohistochemical localization. Int J Mol Med 2018;41:107-18. [PubMed]
- Cypers H, Varkas G, Beeckman S, et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis 2016;75:1357-62. [Crossref] [PubMed]
- Boschetti G, Garnero P, Moussata D, et al. Accuracies of serum and fecal s100 proteins (calprotectin and calgranulin c) to predict the response to tnf antagonists in patients with Crohn's disease. Inflamm Bowel Dis 2015;21:331-6. [Crossref] [PubMed]
Cite this article as: Koelman L, Grune T, Pfeiffer AFH, Rudovich NN, di Giuseppe R, Aleksandrova K. In pursuit of novel biomarkers reflecting intestinal inflammation: temporal variability and phenotypic characterisation of serum calprotectin and lactoferrin. J Lab Precis Med 2020;5:11.


