
The hateful eight: serological testing in pregnancy
Introduction
The chance of transmitting infections from the infected pregnant woman or mother to the fetus or to the newborn has been postulated since a long time. In 1941, the Australian ophthalmologist Norman Gregg first described congenital defects and malformations of the heart, eye and hearing apparatus in babies born to women who had acquired rubella virus (RV) during the first trimester of pregnancy (1), later defined as the “Gregg’s congenital rubella syndrome”. Several other congenital infections in the neonate can lead to significant morbidity and mortality. In 1971 the acronym “ToRCH” was proposed (2) to include perinatal infections by Toxoplasma gondii (T. gondii), RV, human cytomegalovirus (CMV) and herpes simplex virus (HSV). Afterwards, the “o” has become an “O” to expand the list to other pathogens and more recently CHEAP TORCHES have been suggested (3) to include other viruses that infect the placenta during a maternal infection—chicken pox, hepatitis E virus, parvovirus B19 (ParvoB19)—(4) and other that may be transmitted “in utero” or in the perinatal period—HSV 1 & 2, hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV). Regardless of the number and type of pathogens included in this definition, there is an absolute need of assessing the risk of vertical transmission in pregnancy and diagnosing early this occurrence to reduce the incidence and improve the prognosis whenever infections occur (5).
Vertical transmission of pathogens may occur already in utero, in the peripartum period (perinatal transmission, through the contact of maternal blood and vaginal secretions), or postnatally through breast feeding or the repeat contact with other body fluids. The time of transmission bears a clinical relevance, as the clinical manifestations of the infection (Table 1) (6) vary according to that in many instances. The diagnostic process should ideally begin with the detection of the infection and its staging (acute or chronic) in women before pregnancy, or at least early during pregnancy so to adopt adequate preventive measures to reduce the likelihood/frequency of transmission both in utero and at delivery.
Table 1
| Clinical findings | Possible congenital infections |
|---|---|
| Intrauterine growth retardation | Rubella, CMV, T. gondii |
| Microcephaly | ZIKV, CMV, T. gondii, rubella |
| Cerebral calcifications | T. gondii (distributed), CMV (mostly periventricular), ParvoB19, rubella, HIV |
| Hydrocephalus | T. gondii, CMV |
| Hearing loss (generally progressive) | Rubella, CMV, T. gondii |
| Ocular findings | CMV, T. gondii, Rubella, Syphilis, ParvoB19 |
| Bone lesions | Rubella |
| Anemia with hydrops | ParvoB19, CMV, T. gondii |
| Congenital heart disease | Rubella |
| Hydrops, ascites, pleural effusions | ParvoB19, CMV, T. gondii |
| Hepatosplenomegaly | CMV, Rubella, T. gondii, ParvoB19 |
| Jaundice with or without thrombocytopenia | CMV, T. gondii, rubella, HBV, HCV |
| Progressive hepatic disease, hepatic failure and clotting abnormalities | T. gondii, HBV, HCV |
| Maculopapular rash | Rubella, ParvoB19 |
| Purpura (usually on first day after birth) | CMV, T. gondii, rubella, ParvoB19 |
Modified from Ford-Jones, 1999 (6). Infections are listed in order of likelihood to be linked with the clinical signs or symptoms. CMV, cytomegalovirus; ZIKV, Zika virus; ParvoB19, parvovirus B19; HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; T. gondii, Toxoplasma gondii.
The aim of this short review is to provide an updated summary on the frequency of transmission, the recommended procedures for maternal screening, the eventual pharmacological treatment during pregnancy and the available vaccines. On these purposes, this review deals with eight major infections—seven viral and T. gondii—whose main features are reported in Table 2.
Table 2
| Pathogen | Family or Subfamily | Genus | Genome | Reservoir | Routes of transmission (besides vertical) | Treatment during pregnancy | Vaccine |
|---|---|---|---|---|---|---|---|
| RV | Togaviridae | Rubivirus | RNA | Human | Aerosols | Not available | Available |
| CMV | Betaherpesvirinae | CMV | DNA | Human | Secretions, urine | Not available | Early development |
| ParvoB19 | Parvoviridae | Erythrovirus | DNA | Human | Aerosols, blood | Not available | Not available |
| HBV | Hepadnaviridae | Ortohepadnavirus | DNA | Human | Blood, sexual, parenteral | Recommended | Available |
| HCV | Flaviviridae | Hepacivirus | RNA | Human | Blood, parenteral, sexual | Available, partially recommended | Not available |
| HIV | Retroviridae | Lentivirus | RNA | Human | Blood, sexual | Strongly recommended | Under evaluation |
| ZIKV | Flaviviridae | Flavivirus | RNA | Human, mosquitos | Mosquitos, sexual | Not available | Under evaluation |
| T. gondii | Sarcocystidae | Toxoplasma | DNA | Cats | Water, food, cat stools | Recommended | Not available |
Modified from Pereira, 2018 (4). RV, rubella virus; CMV, cytomegalovirus; ParvoB19, parvovirus B19; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; ZIKV, Zika virus; T. gondii, Toxoplasma gondii.
Rubella
RV belongs to the Togaviridae family and in immunocompetent hosts it causes a benign systemic illness that bears resemblance with a mild form of measles, and therefore was initially named “German measles”. Main symptoms are a cutaneous rash, lymphadenopathy and fever; infected adults, and especially women, may also develop arthritis in up to 70% of cases (7). The main health risk for rubella is a congenital infection, that may lead to the aforementioned congenital rubella syndrome (CRS) in up to 85% of newborns, together with a risk of develop late onset sequelae, including diabetes and possibly autism. Fetal damage caused by intrauterine rubella infection tends to occur only when infection occurs in the first 8 to 16 weeks of gestation and in general, the earlier the onset of infection, the more severe are the malformations (7,8).
Overall, acute rubella cases showed a huge decrease but in poorer countries rubella and CRS remain common and in the late 1990s it was estimated to be between 44 and 275 cases per 100,000 live births (8). Immunization with live attenuated RV vaccine has the demonstrated ability to prevent infection and CRS but the initial vaccination policies that targeted only adolescent girls proved unsuccessful. As an example, during a rubella epidemic in 2013 in Japan that involved more than 11,000 cases and at least 13 CRS, seventy percent of the cases occurred among males 20 to 39 years old (7). For this reason, vaccination is now offered or mandated to for both males and females.
In most countries, it is recommended to screen childbearing age women (if not vaccinated) for RV-specific immunoglobulin G (RV-IgG) to identify susceptible women and offer them vaccination before pregnancy or after delivery, since the rubella vaccine cannot be administered during pregnancy (7). International Standard preparations for RV-IgG are available since 1970s and since the 1980s all commercial immunoassays (CIAs) report results in international units per milliliter (IU/mL). However, depending on the type of assay used, wide variation of calculated IU values to establish the “minimum immune titer” (below which individuals should be vaccinated) were reported. Nowadays, CIAs are widely used and are calibrated against the WHO International Standard, reporting results in IU/mL and with a cutoff for immunity set at 10 IU/mL, following the recommendations of the WHO (9). This assumes that results on the same sample by different assays would be equivalent or at least the interpretation identical. Unfortunately, RV-IgG results may be significantly different and even discordant, depending on the assay used, highlighting that comparability of results even by use of standard preparations is not effective (9), especially at low RV-IgG titers (10). It is therefore safer to use RV-IgG assays and/or thresholds that will be able to guarantee a high predictive value for a susceptible status (11), since a “false negative” will lead to possibly unnecessary vaccine while a “false positive” may have severe consequences if infection occurs in pregnancy, and necessary to use the same assay throughout pregnancy.
Testing for immunoglobulin M (IgM), as in all infection, has value to assist the diagnosis of acute infections; unfortunately, also in this case there is no 100% concordance and IgM may persist for quite a long time after the acute episode, and resurface in case of reinfection (12). A useful tool to discriminate acute/recent infections is the evaluation of RV-IgG avidity, and some tests have now been developed and may be useful when a first serum sample is collected months after symptoms, as low avidity anti-rubella IgG suggests recent infection (12). However, avidity tests are not widely available and vary in performance (9). Due to this, testing for RV-IgG on paired samples collected a couple of weeks from each other to look for a significant increase of IgG levels is still a common practice in cases with a “dubious” IgM result.
CMV
Human CMV belongs to the large family of Herpesviridae, huge DNA viruses that share some common features, such as to be prevalent in humans in every area of the world and to persist indefinitely in the infected host, which raised the famous aphorism: “What’s the difference between love and herpes? Herpes in forever!”. The life-long persistence of HCMV in the infected host and its intermittent shedding in saliva, breast milk, and genital secretions provide an efficient mode of spread throughout populations. CMV infection has a low prevalence in northern Europe and non-urban US, whereas prevalence of 90% or higher are reported in Africa, southern Asia, South America, many Mediterranean countries and huge urban areas in the USA. A peculiar characteristic of congenital CMV infection is that its prevalence increases as the rate of infection in the maternal population increases whereas for rubella once the rate of immunity in mothers exceeds 85% the incidence of congenital rubella drops vertically. Congenital infection by CMV is the most frequently reported viral infection in the newborn infant, with a prevalence ranging from 2/1,000 to as high as 20/1,000—the wide variations depending on the characteristics of specific maternal population such as race, age, economic status, and co-existing infections (13).
In contrast to the usually asymptomatic infection in pregnant women, intrauterine transmission to the developing fetus can result in devastating consequences, including termination of pregnancy by fetal loss, that occurs mainly when the infection is acquired early during pregnancy (14). Approximately 85–90% of infants born with CMV are asymptomatic at birth, while out of the 10–15% that are symptomatic, 40–60% will develop permanent sequelae including cerebral palsy, cognitive impairment, microcephaly and neurosensorial hearing loss (15). The infants who are born asymptomatic are also at risk of developing sequelae, mainly hearing loss that occurs in 6–23% cases. Overall, CMV is the leading non-genetic cause of neurosensorial hearing loss (15). Furthermore, as many as 5% of infants without CMV-associated sequelae at birth may develop microcephaly or neurodevelopmental deficits within the first year of life.
Placental transmission of CMV occurs mainly if a mother acquires a primary CMV infection during pregnancy, with a frequency of 30–50%, but also in mothers with have a prior natural immunity to CMV and are therefore chronically infected, with an estimated frequency of 1–2%. In case of a chronic CMV infection in the mother congenital CMV infections result from either reactivation of the virus from a previous infection or from a reinfection, most likely with different strains, and in the latter case placental transmission has been reported to be as high as 3.4% (16).
The gold standard of serological diagnosis of primary CMV infection is maternal seroconversion for anti-CMV antibodies in women that tested negative previously or the presence of serum anti-CMV specific IgM antibodies combined with low avidity anti-CMV IgG antibodies. In the first instance the diagnosis is straightforward, but requires women to be tested not too much before pregnancy, which unfortunately does not occur often so diagnosis via seroconversion is only occasionally achieved. Testing for IgM anti-CMV is the most widely used and appropriate procedure for screening pregnant women (17). Specific IgM are a good indicator of acute or recent infection but are not always correlated with primary infection because IgM antibodies may be produced also during reactivations or reinfections. Furthermore, IgM may persist for months after natural infection and have been detected in some cases up to 6–9 months after the acute phase. False positive results are also common in patients with other viral infections, such as Epstein-Barr virus or ParvoB19, or autoimmune diseases, and depend also from the laboratory method employed. CMV IgM testing is then just the first step in the diagnostic process for a putative acute CMV infection, and CMV IgG avidity test is currently the most reliable procedure, with several commercial options available, to identify primary infection in pregnant women. Low avidity CMV-IgG antibodies last for approximately 16–18 weeks and thus a low avidity index indicates an acute or recent primary CMV infection, while a high avidity index during the first 12–16 weeks of gestation could be considered a good indicator of past infection (17). The limitations of CMV IgG avidity testing are that the kinetics of antibody affinity maturation depends on the reagents used and, for some assays, on the levels of CMV IgG (18). Difficulties arise also when the avidity assays yield a gray zone result that indicates a “moderate” avidity and does not allow a clear dating of the infection. This occurrence is limited when IgG avidity is tested for only on samples that are positive for both IgG and IgM (17) but shall lead to a repetition of the whole testing process in a couple of weeks and shall be correctly reported to the patient and her physician for appropriate counseling. A negative result for both CMV IgG and IgM before or at the beginning of pregnancy indicates that the woman is susceptible to CMV infection and should therefore be counseled on how to prevent getting infected and checked again for IgG and IgM at regular intervals during pregnancy. On the other side, if only CMV IgG are detectable the woman is “immune”, e.g., is chronically infected and this immunity shall help preventing transmission to the fetus in case of reactivation (13). The process of CMV serological screening in pregnancy is described in Figure 1.
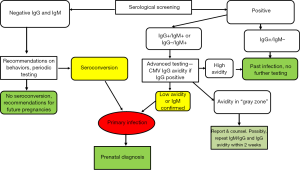
Besides screening, efforts to prevent or limit CMV congenital disease focus on pharmacological treatment and immune prophylaxis. Antiviral drugs to treat CMV-related disease are available and frequently used in patients at high risk of severe or fatal outcome, such as patients undergoing stem cell transplantation or other severely immunodepressed other causes, including HIV infection (19), but antiviral treatment is currently not validated to prevent vertical transmission during pregnancy.
Passive immunoprophylaxis does not represent a viable option. An initial study showing the efficacy on limiting “in utero” transmission by administering CMV hyperimmune immunoglobulins has not been confirmed by a randomized trial (20) and has therefore not been pursued. Thus, a maternal vaccine to prevent congenital CMV continues to be a top public health priority, but no licensed vaccine is available so far. To develop this, one huge unmet need is to understand the correlates between maternal immune status and protection (13), which is critical to design an efficacious maternal vaccine. The current view is that, as we have seen before, natural immunity does not completely prevent reinfection and transmission, but the huge medical and social burden of congenital CMV calls for an extensive evaluation of the vaccines currently in the pipeline (21).
ParvoB19
ParvoB19 has been discovered by chance in 1975 in asymptomatic patients during a screening for hepatitis B. It belongs to the Parvoviridae family and has a worldwide distribution, with prevalence increasing from 2–20% in early infancy up to 80% in the elderly (4). In infants ParvoB19 causes infectious erythema, also called 5th disease. In susceptible adults, the clinical manifestations include malaise, a reticular rash, and joint pain/swelling that may last from days to months. Approximately 20% to 30% of patients have no symptoms at all (22). The linkage with vertical transmission happened in 1984, when ParvoB19 infection was causally associated with hydrops fetalis, a severe condition caused by anemia (23). Serological data show that 26–44% of women of childbearing age are not immune, and the major risk factor for acquiring ParvoB19 during pregnancy is contact with children. The seroconversion rate during pregnancy is around 1% but may increase up to 13% during epidemics, which occur every 4 to 5 years (24). When ParvoB19 is acquired during the first trimester and there is a huge viral load, the rate of intrauterine transmission has been reported to range between 24% and 39% and the risk of fetal hydrops and death caused by infection is 4–10% when transmission occurs before 20 weeks of gestation (25). Screening for ParvoB19 in pregnant women is not performed, but due to the high risk of transmission and fetal disease following a potential exposure, pregnant women should be tested for specific IgG and IgM antibodies. IgG usually persist for life and are protective, whereas IgM class antibodies disappear within a few months. So, if IgG are positive and IgM are absent the patient can be reassured on the absence of risk for prenatal infection. In case of a positivity for IgM, testing for IgG avidity may assist the diagnosis of a recent infection (26). If both IgG and IgM are negative, the patient is susceptible to infection and out of precaution both tests should be repeated in 3 to 4 weeks. If they remain negative, the patient remains susceptible to the disease without evidence of infection. Since there is no vaccine or medical treatment for ParvoB19, susceptible pregnant women shall be counseled to careful and continued hand-washing and avoidance of possible sources of infection, such as working in primary schools or as daycare providers (24). Whenever there is evidence of seroconversion with positive titers of IgG and IgM ultrasound surveillance of the fetus should be initiated, focusing on ascites, pleural or pericardial effusions, or scalp edema. Recently, middle cerebral artery (MCA) Doppler has been recommended to evaluate for fetal anemia, which may be detected before fetal hydrops.
HBV
HBV, a DNA virus with an incomplete double-stranded DNA genome of approximately 3,200 base pairs, belongs to the Hepadnaviridae family that comprises similar viruses that infect a broad variety of mammals and other warm-blood animals. The distinctive characteristics of this family are that the viral DNA polymerase has also a reverse transcriptase activity and viral DNA is synthetized from one of the two RNA filaments that are initially translated, and that the replication cycle encompasses the production of circular covalently closed DNA (cccDNA) that represents a functional reservoir of the virus in all infected cells. The presence of this episomic, replication-competent form of HBV DNA in the liver and/or of HBV DNA in the blood of people who test negative for hepatitis B surface antigen (HBsAg) by currently available assays is the most recent consensus definition of the occult HBV infection, or OBI (27).
According to the most recent estimates (28) approximately 2 billion people of the world population have been infected by HBV and 257 million people are living with an overt HBV infection, that is defined by the positive finding in serum or plasma of the hepatitis B surface antigen, called HBsAg. Although many of this people remain lifelong asymptomatic carriers, the evolution to chronic hepatitis B (CHB) is frequent and HBV is one of the leading causes of liver cirrhosis, hepatocellular carcinoma (HCC), and liver-related disease worldwide (29). HBV only causes disease in humans, is transmitted through blood and apparent or inapparent parenteral routes, by sexual contact, and vertically from mother to child. In the latter case infectivity is high (30) and transmission is perinatal, as “in utero” transmission is controversial, and most cases occurs at delivery. The infected newborns develop a chronic infection at a very high rate—80% to 90%—unlike adults (30) and often develop serious long-term health complications such as cirrhosis, liver failure, HCC, and death (31). Hepatitis B screening in pregnancy has been a standard of care for more than 30 years and aims to identify women at risk of transmitting the infection to their infants by testing them for HBsAg. Universal screening is important because known risk factors are present in only 35% to 65% of HBV-positive pregnant women (32). Currently available assays detect as low as 20 mIU/mL of HBsAg, that correspond to about 100 IU/mL of the nucleic acid of the virus (HBV-RNA) (33). Although this procedure is quite safe, the new insights on OBI have raised concerns that, since HBV infection persists indefinitely also in HBsAg-negative individuals, the risk of transmission may persist also in part of those cases. Indeed, next-generation assays for HBsAg that will soon be available have been demonstrated to reach a sensitivity of 5 mU/mL (33) and to unveil HBsAg in more than 20% of cases previously classified as OBI (34).
Currently, therapy regimens for chronic, HBV-related liver diseases envision either the use of pegylated interferon or of nucleos(t)ide analogues (NUCs), primarily Tenofovir and Entecavir, and there is also a rich pipeline of novel drugs, such as entry or capsid inhibitors (30). Since HBV persistence is guaranteed by cccDNA even if replication is suppressed, the current goal is to achieve a sustained clearance of HBV-DNA and HBsAg, thereby decreasing progression to cirrhosis and HCC. According to the recent guidelines (35) the use of antiviral therapy to reduce the risk of perinatal transmission is recommended for HBsAg-positive pregnant women with an HBV DNA level >200,000 IU/mL. Tenofovir seems to be a preferred choice due to the high antiviral potency and minimum risk of resistance compared with other drugs. In a recent experience on 208 children (36), those in the tenofovir group had a rate of HBV-DNA positivity at birth of 5.22% compared to 30.11% among the untreated and a rate of HBsAg seropositivity at 6 months of 1.74% compared to 11.83%. Unfortunately, less than 1% of mothers with a high viral load had received antiviral therapy to reduce mother-to-child transmission (28).
Perinatal vaccination of infants born to mothers with HBV prevents transmission in almost all cases if the first dose of vaccine is administered together with hyperimmune gamma globulins (HBIG) (35,37). The most recent estimate is that 46% of babies born to HBV-infected mothers have received timely birth-dose vaccination, and only 13% have been given HBIG along with the full vaccination regimen. Universal vaccination of children (using the recombinant anti-HBV vaccines) is the most effective intervention to control HBV globally; up to now, 87% of infants worldwide have received the three-dose HBV vaccination in the first year of life (30).
HCV
HCV is an enveloped, positive-sense single-stranded RNA virus belonging to the Flaviviridae family and is currently classified in into 8 genotypes (38). Humans are the only natural host of HCV and the global prevalence of viremic HCV is estimated to be 1.0% (95% uncertainty interval 0.8–1.1) in 2015, corresponding to 71.1 (62.5–79.4) million infections (39). HCV is transmitted from person to person mainly through the blood borne route. The principal modes of transmission include the use of contaminated needles in persons who inject drugs, parenteral exposure during esthetic treatment and exposure to contaminated medical equipment (30). Sexual transmission may occur especially in people coinfected by other agents, namely HIV. Less than 15% of patients with a primary HCV infection develop an acute disease but HCV persists in about 85% of all newly infected. Viral clearance occurs naturally in about 15% of these, whereas the remainder are at risk of developing a chronic liver disease state. About one-third of the latter will develop cirrhosis over the subsequent 30 years. Once cirrhosis occurs, there is an annual risk 1% to 5% of developing HCC, for which HCV is the leading cause worldwide (30,39).
Vertical transmission occurs in about 3% to 10% of children born to HCV-infected mothers and the rate of transmission is higher from mothers with a more severe HCV-related disease, higher viral load and/or coinfected by HIV. In the latter group, HCV vertical transmission may be as high as 19% (40,41). Perinatal transmission is thought to be the leading cause of mother to baby transmission (42), but both intrauterine and intrapartum transmission are possible. It is estimated that up to one-third of the infected children acquire the infection in utero, as evidenced by a positive result for HCV-RNA by PCR within the first 3 days of life, and up to one-half as late intrauterine or intrapartum as babies are PCR positive after 3 days of life (41).
Despite many efforts, a viable HCV vaccine is not available yet. Routine testing of pregnant women for HCV infection is currently not recommended (43) but may be envisioned in women at particularly high risk of transmitting HCV vertically, due to the availability of directly acting antiviral (DAA) drugs that may be administered during pregnancy (44). The diagnosis of HCV infection starts with testing for anti-HCV antibodies but only 50–70% of anti-HCV positive individuals have an active infection, e.g., are positive also for HCV-RNA (45). The screening cascade shall then include testing for HCV-RNA or, as an alternative, for the HCV core antigen (HCVAg). The latter test, though less sensitive than HCV-RNA, has been validated on the purpose of diagnosing active HCV infections when HCV-RNA is not available or affordable (43) as is indicated for the diagnosis of chronic HCV infections (44,46). A recent review of HCV cascade to care in pregnant women in the USA has shown that 85% of women have been assessed for HCV, but only 72% of the anti-HCV positives have been tested for HCV-RNA an ultimately only 41% of the women eligible were linked to care (47). Testing for HCVAg may help improving the screening yield also when HCV-RNA assays are available because it may reduce turnaround time and streamline the process (48).
HIV
HIV belongs to the Retroviridae family and is classified as HIV-1 or HIV-2. HIV genome consists of two identical single-stranded RNA molecules within the core of the virus particle. Proviral DNA is generated by reverse transcription of the viral RNA genome into DNA, RNA degradation, and integration of the double-stranded HIV DNA into the human genome, making then not possible to eliminate HIV from the infected individuals.
In 2018, approximately 37.9 million people in the world were living with HIV (49). While the annual number of new HIV infections worldwide has declined by 16% since 2010, the rate of decline is too slow to meet the UNAIDS goal to decrease the number of new HIV infections worldwide each year to fewer than 500,000 by the year 2020. About 50% of HIV-infected people are women of childbearing age (>15 years), and this adds to the at-risk infant population since HIV is transmitted through body fluids, including blood, semen, and vaginal secretions. Transmission from mother to child may occur during pregnancy, perinatally, or even postnatally through breast milk. Transmission during pregnancy has been shown as early as at 12 weeks of gestation; however, over 90% of prenatal transmission occurs during the third trimester, usually during labor and delivery (50).
HIV testing for fertile women shall be undertaken ideally before pregnancy, as part of the overall recommendations to reduce HIV incidence. If this does not happen, since antiretroviral treatment during pregnancy is both available and recommended HIV testing shall be actively proposed as early as possible during the first trimester (51). HIV testing in pregnant women, as in all other people, shall be performed by 4th generation assays that simultaneously detect viral p24 antigen in addition to HIV-1 and HIV-2 antibodies (52). Detection thresholds for p24 antigen during acute infection correspond to approximately 30,000 viral RNA copies per milliliter (53) and will reduce the window period from infection by a median of 5 days compared to 3rd generation HIV immunoassays who recognize only anti-HIV antibodies (53). However, viral variability may affect the sensitivity for p24 by several assays (54,55), thus widening the window period and limiting the clinical usefulness. Repeat testing in the third trimester is recommended for pregnant women with negative initial HIV test result and in those who are at increased risk of acquiring HIV (51). When a 4th generation assay yields a repeat reactive result, the HIV positivity is first assessed by confirming the presence of HIV antibodies by an immunoblot or another HIV-1/HIV-2 antibody differentiation assay. Reactive results on either confirmatory assay will identify most HIV infections, that are positive for HIV antibodies. If antibody positivity is not confirmed, the third step is testing for HIV-1 RNA or as an alternative testing for the p24 antigen (53).
Antiretroviral treatment during pregnancy is recommended and the same regimens used for other adult groups may be used, although caution should be used when considering the use of regimens that contain drugs with little or no pregnancy data for evaluation (51). Early initiation of treatment lowers significantly the rate of mother-to-child transmission of HIV. If treatment is initiated before conception the rate is approximately 0.2%, compared with first-trimester initiation at 0.4%, second-trimester initiation at 0.9%, and third-trimester initiation at 2.2%. In the absence of interventions during the perinatal or delivery period, the rate of transmission is approximately 25% (50). Treatment should be initiated at diagnosis and should be continued throughout pregnancy and thereafter. The risk of transmission is almost eliminated in cases of ongoing viral load suppression (HIV-RNA not detectable in serum/plasma) at the time of conception and throughout pregnancy (56).
Zika virus (ZIKV)
ZIKV belongs to Flaviviridae family, which comprised other major human viruses such as HCV, West Nile virus, Dengue virus and the viruses causing yellow fever and Japanese encephalitis. The genome is a positive-sense, single-stranded RNA coding for a single polypeptide that is processed in structural (three) and non-structural (five) proteins. The main route of transmission is through the bite of Aedes aegypti mosquito, very frequent in urban and peri-urban areas and the first outbreak has been reported in 2007 (57). The infection is mostly inapparent as only about 20% of infected people will develop a self-limiting illness with symptoms common to many other acute viral infections such as rash, fever, conjunctivitis, and/or arthralgia/myalgia. The incubation period is less than 1 week, and symptom persist for 1 week or less as well. Though the viremic phase is limited to the initial phase and clears after the onset of symptoms, the virus may be isolated from semen for several weeks after infection and ZIKV RNA may be detected in blood or vaginal secretions for many weeks or even months (58). So, if ZIKV infection occurs during pregnancy it may be transmitted to the fetus, and a congenital infection is a major concern as it may result with a high frequency in microcephaly and other birth defects and neurodevelopmental problems.
The recommendations for laboratory diagnosis of ZIKV infection are the detection of viral-RNA by molecular methods, detection of ZIKV-specific IgM and IgG antibodies. IgM testing remains useful for diagnosing congenital Zika syndrome but is no longer recommended in evaluating asymptomatic pregnant women in non-endemic areas, since a positive test is supportive but not definitive for ZIKV infection, and confirmatory neutralization may be required (58).
The duration of IgM response is unclear, but it can persist beyond 12 weeks, thus limiting the reliability of this assay to clearly define a recent ZIKV infection. IgG against ZIKV may be detectable after 10–14 days and supposedly lasts for years, as with other flaviviruses. The kinetics of the IgG response have direct bearing on performance, as IgG assays may not reach peak sensitivity until after the first 10 to 15 days post onset (DPO) of symptoms. Also, the magnitude and abundance of cross-reactive Ab may be highest early after infection, compromising assay specificity until the late convalescent period and IgG testing is not part of CDC recommendations for ZIKV diagnosis. Molecular diagnostics mostly by RT-PCR have the advantage of being highly specific for ZIKV and can be performed on noninvasive specimens, such as urine and saliva. Sensitivity may decline as early as a few DPO of symptoms, and therefore RT-PCR is generally not used beyond 14 DPO. This method is not appropriate for the surveillance or detection of inapparent or prior infection, with the exception that RT-PCR testing is recommended for detecting inapparent ZIKV infection during pregnancy when there is ongoing exposure. An accurate laboratory diagnosis requires combining serologic data to molecular testing, as well as clinical and epidemiological criteria, especially for pregnant women and children born with Zika congenital syndromes.
Currently, there is no pharmacological treatment available for ZIKV infection. Vaccination may represent a viable option in highly endemic areas, but tough many promising candidate vaccines have been developed, an approved vaccine against ZIKV remains an unmet goal (59).
T. gondii
Toxoplasmosis is caused by the T. gondii, an obligate intracellular protozoan belonging to the Sarcocystidae family whose cysts can infect any warm-blooded animal or may be found in soil, while its definitive host is the cat. This microorganism completes its sexual cycle in feline intestinal epithelial cells and the resulting oocysts are shed in the feces for several weeks. Oocysts may infect humans from contaminated water, fruits and vegetables or soil and from cat feces (24). T. gondii infection is also acquired by ingestion of cysts in undercooked meat (60).
The global prevalence of toxoplasmosis is hard to estimate because it is not a reportable infection and huge differences exist among nations and geographical areas, mostly due to different lifestyles (60). In developed countries seroprevalence among pregnant women is low. For instance, in the USA Toxo IgG antibodies were detected in 15% (24) and in Italy in 21% (61) in similar periods, so a substantial proportion of women of childbearing age are susceptible to this infection and may then transmit T. gondii if the infection is acquired during pregnancy. Vertical transmission from a pregnant woman to her fetus can cause congenital toxoplasmosis, with consequences including stillbirth, chorioretinitis, deafness, microcephaly, and developmental delay (62). Vertical transmission of infection to the fetus is most likely to occur with a primary infection during pregnancy. However, immunocompromised women with chronic infection may also transmit the disease (63). More than 90% of pregnant women who acquire a primary infection are asymptomatic, as are 85% of neonates born with congenital toxoplasmosis. Transmission frequency increases over the time of pregnancy: in the first trimester is approximately 15%, 30% in the second trimester, and 60% in the third trimester (24). Exposure to infection acquired in the first trimester causes more severe congenital toxoplasmosis, with fetuses exposed in the third trimester most likely to be asymptomatic. These infants require treatment to prevent manifestations later in life.
Diagnosis can be made by testing maternal serum for specific IgG and IgM antibodies. There is a wide panel of commercial assays available on this purpose and automated immunoassays with high throughput and good levels of sensitivity and specificity are the most frequently used. If both IgM and IgG are negative the infection has not occurred and women shall be counseled on lifestyle—especially alimentary—and followed up serologically. A negative IgM result with a positive IgG during the first or second trimester often indicates an infection that predated the pregnancy, and this is also likely in the third trimester but the infection could have occurred early in pregnancy with the IgM decreased to undetectable levels (64). A positive IgM result with or without IgG shall indicate a very recent infection by T. gondii but requires further testing because of a possible false-positive result for IgM or a chronic or past infection, as IgM may persist for 1 year or more after the onset (24,65). Due to the low prevalence and incidence the positive predictive value for a recent infection of a positive IgM result is low and up to 60% of patients positive for IgM have results inconsistent with recent infection. Confirmation of a recent T. gondii infection is then imperative and may be achieved by a complete serologic profile, performed at reference laboratories, that includes the employ of the Sabin-Feldman test, the differential agglutination (AC/HS) test, IgG avidity, and additional enzyme-linked immunosorbent assays for specific IgM, IgE, and IgA (24,47). This approach is methodologically sound but does not cope well with the need of obtaining a complete diagnostic profile within a reasonable time, especially during pregnancy. The current recommendations are then to perform testing for T. gondii IgG avidity on pregnant women who test positive for IgM and IgG. High avidity requires at least 3 months to develop, and so helps to rule out acute infection. On the other side, IgG avidity maturation taking such a long time does not allow to employ the low avidity results to diagnose accurately a very recent infection (66), though attempts have been made to use an immunoblot for IgG avidity on that purpose (67). Another issue is the lack of concordance of T. gondii IgG assays, that may cause problems in assessing a previous immunity when low IU/mL are detected. As for rubella, even if most assays the calibrators are based on the WHO international standard —the fourth human anti-Toxoplasma Ig IS (IS 13/132; 160 IU of Ig/ampoule) has been established in 2015—and the result unit for the IgG assay is reported in IU/mL, no perfect correlation is achieved and even large differences in anti-Toxoplasma IgG titers on samples from individual patients may be observed (25).
Vaccination towards T. gondii is not available. Vertical transmission of newly acquired infection during early pregnancy at 18 weeks or more can be prevented by administration of oral spiramycin, 1 g every 8 hours. This macrolide antibiotic does not cross the placenta, so is not appropriate for fetal treatment. In the absence of signs of fetal infection, spiramycin should be continued until delivery. Spiramycin does not seem to have any fetal effects, however a small percentage of women may develop gastrointestinal side effects (68).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giuseppe Lippi, Martina Montagnana and Zhi-De Hu) for the series “Laboratory Medicine in Pregnancy” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm.2019.12.05). The series “Laboratory Medicine in Pregnancy” was commissioned by the editorial office without any funding or sponsorship. The author is currently employed by Abbott Diagnostics as the Global Associate Medical Director for Infectious Diseases and owns Abbott shares.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gregg NM. Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust 1941;3:35-46.
- Nahmias AJ, Walls KW, Stewart JA, et al. The ToRCH complex-perinatal infections associated with toxoplasma and rubella, cytomegol-and herpes simplex viruses. Pediatr Res 1971;5:405-6. [Crossref]
- Ford-Jones EL, Kellner JD. “CHEAP TORCHES”: a better acronym when considering congenital and perinatal infections. Pediatr Infect Dis 1995;14:638-40. [Crossref] [PubMed]
- Pereira L. Congenital viral infection: traversing the uterine-placental interface. Annu Rev Virol 2018;5:273-99. [Crossref] [PubMed]
- European Centre for Disease Prevention and Control. Antenatal screening for HIV, hepatitis B, syphilis and rubella susceptibility in the EU/EEA – addressing the vulnerable populations. Stockholm: ECDC, 2017.
- Ford-Jones EL. An approach to the diagnosis of congenital infections. Paediatr Child Health 1999;4:109-12. [Crossref] [PubMed]
- Lambert N, Strebel P, Orenstein W, et al. Rubella. Lancet 2015;385:2297-307. [Crossref] [PubMed]
- Mawson AR, Croft AM. Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int J Environ Res Public Health 2019; [Crossref] [PubMed]
- Vauloup-Fellous C. Standardization of rubella immunoassays. J Clin Virol 2018;102:34-8. [Crossref] [PubMed]
- Huzly D, Hanselmann I, Neumann-Haefelin D, et al. Performance of 14 rubella IgG immunoassays on samples with low positive or negative haemagglutination inhibition results. J Clin Virol 2016;74:13-8. [Crossref] [PubMed]
- Charlton CL, Laic FY, Dover DC. How to determine protective immunity in the post-vaccine era. Hum Vaccin Immunother 2016;12:903-6. [Crossref] [PubMed]
- Wandinger KP, Saschenbrecker A, Steinhagen K, et al. Diagnosis of recent primary rubella virus infections: significance of glycoprotein-based IgM serology, IgG avidity and immunoblot analysis. J Virol Methods 2011;174:85-93. [Crossref] [PubMed]
- Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses 2018; [Crossref] [PubMed]
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 1986;256:1904-8. [Crossref] [PubMed]
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 2013;57:S178-81. [Crossref] [PubMed]
- Simonazzi G, Curti A, Cervi F, et al. Perinatal outcomes of non-primary maternal cytomegalovirus infection: a 15-year experience. Fetal Diagn Ther 2018;43:138-42. [Crossref] [PubMed]
- Lazzarotto T, Guerra B, Gabrielli L, et al. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011;17:1285-93. [Crossref] [PubMed]
- Revello MG, Genini E, Gorini G, et al. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J Clin Virol 2010;48:255-9. [Crossref] [PubMed]
- Britt WJ, Prichard MN. New therapies for human cytomegalovirus infections. Antiviral Res 2018;159:153-74. [Crossref] [PubMed]
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370:1316-26. [Crossref] [PubMed]
- Permar SR, Schleiss MR, Plotkin SA. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol 2018; [Crossref] [PubMed]
- Chorba T, Coccia P, Holman RC, et al. The role of parvovirus B19 in anaplastic crisis and erythema infectiosum (fifth disease). J Infect Dis 1986;154:383-93. [Crossref] [PubMed]
- Brown T, Anand A, Ritchie LD, et al. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet 1984;2:1033-4. [Crossref] [PubMed]
- Feldman DM, Timms D, Borgida AF. Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy. Clin Lab Med 2010;30:709-20. [Crossref] [PubMed]
- Zhang K, Lin G, Han Y, et al. The standardization of 5 immunoassays for anti-Toxoplasma immunoglobulin G(IgG). Clin Chim Acta 2017;472:20-5. [Crossref] [PubMed]
- Enders M, Klingel K, Weidner A, et al. Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection. J Clin Virol 2010;49:163-8. [Crossref] [PubMed]
- Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol 2019;71:397-408. [Crossref] [PubMed]
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383-403. [Crossref] [PubMed]
- Likhitsup A, Lok AS. Understanding the natural history of hepatitis B virus infection and the new definitions of cure and the endpoints of clinical trials. Clin Liver Dis 2019;23:401-16. [Crossref] [PubMed]
- Lanini S, Ustianowski A, Pisapia R, et al. Viral hepatitis: etiology, epidemiology, transmission, diagnostics, treatment, and prevention. Infect Dis Clin North Am 2019;33:1045-62. [Crossref] [PubMed]
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67:1-31. [Crossref] [PubMed]
- Henderson JT, Webber EM, Bean SI. Screening for hepatitis B infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019;322:360-2. [Crossref] [PubMed]
- Lou S, Taylor R, Pearce S, et al. An ultra-sensitive Abbott ARCHITECT® assay for the detection of hepatitis B virus surface antigen (HBsAg). J Clin Virol 2018;105:18-25. [Crossref] [PubMed]
- Kuhns MC, Holzmayer V, McNamara AL, et al. Improved detection of early acute, late acute, and occult Hepatitis B infections by an increased sensitivity HBsAg assay. J Clin Virol 2019;118:41-5. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [Crossref] [PubMed]
- Chang K-C, Chang M-H, Lee C-N, et al. Decreased neonatal hepatitis B virus (HBV) viremia by maternal tenofovir treatment predicts reduced chronic HBV infection in children born to highly viremic mothers. Aliment Pharmacol Ther 2019;50:306-16. [Crossref] [PubMed]
- Spada E, Tosti ME, Zuccaro O, et al. Evaluation of the compliance with the protocol for preventing perinatal hepatitis B infection in Italy. J Infect 2011;62:165-71. [Crossref] [PubMed]
- World Health Organization. Global hepatitis report, 2017. Geneva: World Health Organization, 2017.
- The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161-76. [Crossref] [PubMed]
- Zanetti AR, Tanzi E, Newell ML. Mother-to-infant transmission of hepatitis C virus. J Hepatol 1999;31:96-100. [Crossref] [PubMed]
- Arshad M, El-Kamary SS, Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period--are they opportunities for treatment? J Viral Hepat 2011;18:229-36. [Crossref] [PubMed]
- Indolfi G, Resti M. Perinatal transmission of hepatitis C virus infection. J Med Virol 2009;81:836-43. [Crossref] [PubMed]
- World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva: World Health Organization, 2018.
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461-511. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013;62:362-5. [PubMed]
- World Health Organization. Guidelines on hepatitis B and C testing. Geneva: World Health Organization, 2017.
- Epstein RL, Sabharwal V, Wachman EM, et al. Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr 2018;203:34-40.e1. [Crossref] [PubMed]
- Jülicher P, Chulanov VP, Pimenov NN, et al. Streamlining the screening cascade for active hepatitis C in Russia: a cost-effectiveness analysis. PLoS One 2019;14:e0219687 [Crossref] [PubMed]
-
UNAIDS data 2019 . Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf - Lynch NG, Johnson AK. Congenital HIV: prevention of maternal to child transmission. Adv Neonatal Care 2018;18:330-40. [Crossref] [PubMed]
- Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal hiv transmission in the United States. Available online: https://aidsinfo.nih.gov/guidelines/html/3/perinatal/224/whats-newin-the-guidelines
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: updated recommendations 2014. Available online: https://stacks.cdc.gov/view/cdc/23447
- Branson BM. HIV diagnostics: current recommendations and opportunities for improvement. Infect Dis Clin North Am 2019;33:611-28. [Crossref] [PubMed]
- Ly TD, Plantier JC, Leballais L, et al. The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. J Clin Virol 2012;55:121-7. [Crossref] [PubMed]
- Stone M, Bainbridge J, Sanchez AM, et al. Comparison of detection limits of fourth- and fifth-generation combination HIV antigen-antibody, p24 antigen, and viral load assays on diverse HIV isolates. J Clin Microbiol 2018; [Crossref] [PubMed]
- Mandelbrot L, Tubiana R, Le Chanadec J, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 2015;61:1715-25. [PubMed]
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536-43. [Crossref] [PubMed]
- Collins MH. Serologic tools and strategies to support intervention trials to combat zika virus infection and disease. Trop Med Infect Dis 2019. doi:
10.3390/tropicalmed4020068 . - Thomas SJ, L’Azou M, Barrett AD, et al. Fast-track zika vaccine development—is it possible? N Engl J Med 2016;375:1212-16. [Crossref] [PubMed]
- Dard C, Fricker-Hidalgo H, Brenier-Pinchart MP, et al. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol 2016;32:492-506. [Crossref] [PubMed]
- De Paschale M, Agrappi C, Clerici P, et al. Seroprevalence and incidence of Toxoplasma gondii infection in the Legnano area of Italy. Clin Microbiol Infect 2008;14:186-9. [Crossref] [PubMed]
- McClure EM, Goldenberg RL. Infection and stillbirth. Semin Fetal Neonatal Med 2009;14:182-9. [Crossref] [PubMed]
- Lopez A, Dietz VJ, Wilson M, et al. Preventing congenital toxoplasmosis. MMWR Recomm Rep 2000;49:59-68. [PubMed]
- Montoya JG, Rosso F. Diagnosis and management of toxoplasmosis. Clin Perinatol 2005;32:705-26. [Crossref] [PubMed]
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2012;25:264-96. [Crossref] [PubMed]
- Remington JS, Tulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol 2004;42:941-5. [Crossref] [PubMed]
- Ali-Heydari S, Keshavarz H, Shojaee S, et al. Diagnosis of antigenic markers of acute toxoplasmosis by IgG avidity immunoblotting. Parasite 2013;20:18. [Crossref] [PubMed]
- Cook GC. Use of antiprotozoan and anthelmintic drugs during pregnancy: side-effects and contra-indications. J Infect 1992;25:1-9. [Crossref] [PubMed]
Cite this article as: Galli C. The hateful eight: serological testing in pregnancy. J Lab Precis Med 2020;5:14.


