Ionized calcium: analytical challenges and clinical relevance
Introduction
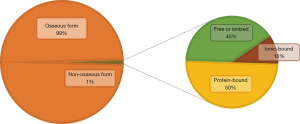
Calcium is the fifth most abundant element in the organism (1). Calcium level depends primarily on dietary intake (2-5). This divalent cation, present in two forms (bound or free) in the body, plays an indispensable role in multiple biological functions, the most important of which is bone mineralization. Indeed, the vast majority of the body calcium (>99%) is stored in bones, as hydroxyapatite crystals, thus responsible for the biomechanical properties of the skeleton (6).
Non-osseous calcium (<1% of body calcium storage) is very finely regulated by a complex interaction between several actors. Its homeostasis is based on a permanent communication between the hormonal system (PTH, 1,25-dihydroxyvitamin D) and the receptors located on three peripheral organs (digestive tract, kidneys and bones) (7,8). This non-osseous calcium thus comprises protein-bound calcium (mainly albumin and globulins in the serum and calmodulin in the cells), free (or ionized) calcium and ionic-bound calcium (complexes of phosphate, carbonate and calcium oxalate), representing respectively about 50%, 40% and 10% of the circulating calcium (Figure 1). This calcium form plays many important roles: from its involvement in several intra- and extracellular signaling pathways, to its implication in nerve impulse transmission and muscle contraction (1,7). This pool of non-osseous calcium is closely regulated in order to maintain total serum calcium level in a physiological window between 2.20 and 2.60 mmol/L. This regulation is even finer regarding ionized calcium levels (between 1.10 and 1.35 mmol/L), given its potentially severe toxicity outside this physiological range. Dehydration, digestive disorders, confusion and arrhythmia mainly in case of hypercalcemia; numbness/tingling in the extremities, muscle spasm, confusion, hallucination and weakness in case of hypocalcemia (9-11).
Calcium is involved in many physiological functions; its dosage and monitoring are essential in many clinical situations. The question about choosing between total or ionized calcium testing has been arising for many years (12-14), and is still debated even though some evidence has recently emerged in favor of routine ionized calcium measurement (15). Indeed, there are a number of factors that influence the value of serum calcium, most notably protein and parathormone serum levels. Thus, the main risk of systematically using the total calcium level is the overestimation of serum calcium status.
The aim of this article is first to discuss about the measurement and estimation of ionized calcium in laboratories, and then to describe its applications in clinical practice.
Ionized calcium and its estimation: an analytical challenge
Calcium is distributed in three forms, an important fraction of it being complexed with proteins (mainly albumin). As previously described, the free form of calcium is the one that plays a major role in the body (13,16-18). This free fraction is very closely modulated by endocrine functions (1,8,16). However, its dosage is limited by constraints which are reducing its routinely use (19,20). Indeed, its precise dosage requires both strict conditions of sample handling as well as taking into account many pre-analytical conditions (21).
Sample handling requirements and ionized calcium measurements
For an accurate determination of the calcium status based on ionized calcium level, it is essential to take all necessary precautions regarding the type of specimen collection and the choice of container collection. The type of tube collection plays a key role in this analysis given the presence of common anticoagulants (EDTA, citrate, oxalate), whose principal function is precisely the ability to complex with free calcium. Not taking this element into account would lead to a significant underestimation of ionized calcium level (22). The International Federation of Clinical Chemistry (IFCC) guidelines recommend heparin as the anticoagulant of choice for the measurement of ionized calcium (23). Heparin chelates a moderate but significant portion of ionized calcium, depending on its type and dosage quantity in the tube. The international recommendations do not particularly prefer sodium/lithium or calcium titrated heparin, as long as the concentrations do not exceed 15 and 50 IU/mL blood respectively (19,21). The assay can thus be performed on whole blood sample with heparin, heparinized plasma or serum.
According to the Clinical Laboratory Standards Institute (CLSI) guidelines, measurement of ionized calcium with plasma should be avoided and use of whole blood specimens should be preferred (in syringes) (24). However, evacuated blood collection tubes containing lithium heparin and gel separator material are used very frequently in clinical laboratories nowadays. The use of heparin lithium tubes has certain advantages, notably that of allowing a rapid result (latency time of approximately 30 minutes for the gel separator tubes), sometimes better corresponding to the clinician’s expectations. The other advantage of lithium heparin tubes is the possibility of using the entire sample volume for analysis. Although not recommended, if the sample has been taken in an evacuated tube, care should be applied to keep it sealed until analysis, in order to avoid any pH change caused by CO2 leakage. It is also important to treat the samples as soon as possible to limit the potential effects of anaerobic metabolism-induced glycolysis on erythrocytes and leucocytes (25). Of course, the use of a serum sample remains the ideal solution, avoiding in particular any bias related to erythrocytes or heparin but this requires a larger sample volume and a longer process time. Regarding the sample stability, whole blood samples (especially for blood gas analysis) need to be treated very quickly (within half an hour) whereas samples taken into centrifugation to obtain serum/plasma may offer longer stability ranging from several hours (at room temperature) to several days (in refrigerated environment) as long as the tubes remain sealed (26,27). Although many methods, more or less complex, have been described to measure ionized calcium, it seems that its measurement has recently been democratized by the advent of ion-selective electrodes (ISE) (28,29). Indeed, this method currently recommended by the IFCC guidelines is based on a simple principle: the measurement of a potential across a membrane only allowing selective calcium transit, this potential being mathematically correlated to the ionized calcium concentration in the sample tested. To the best of our knowledge, there is currently no large automated instrument for the specific measurement of ionized calcium; however, the vast majority of blood-gas analyzers currently allow a direct ISE assessment of ionized calcium level.
Physiological and pre-analytical issues
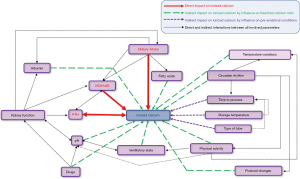
In the literature, a number of situations have been identified as potentially responsible for ionized calcium level variations (24,30). We present below the details of the main factors to consider when measuring and interpreting ionized calcium levels.
- Physical activity: by an increase in the lactate level, a drop in pH and bicarbonates, an increase in ionized calcium may occur after a moderate-intensity effort. In a previous work, Ljunghall et al. observed a 5–10% change in ionized calcium levels in healthy volunteers after bicycling exercise (65% of theorical maximal effort) (31).
- Dietary intake: it has been shown that a moderate decrease (approximately 5%) in ionized calcium levels is usually observed within 2 hours of dietary intake (2,3,32). This decrease is probably the consequence of an increased complexation of calcium with proteins and other anions such as phosphates/bicarbonates. Briefly, ionized calcium levels may also be impacted in the same way by all factor which could hinder the calcium-protein complex production (pyruvate, sulfate, beta-hydroxybutyrate).
- Ortho- or clinostatism: indeed, it has been demonstrated that the patient posture at the time of sampling could have an effect on ionized calcium levels, in particular by substantial variations in protein/albumin levels, as well as an increase of both muscle tonus and hydrostatic pressure during the change of position (26,33).
- Circadian rhythm: there is a strong association between circadian rhythm and ionized calcium dosage; indeed, the study of Markowitz et al. revealed significant variations (up to 10%) in ionized calcium levels during a 24 hour-cycle, the nadir being most often reached in the late afternoon. Interestingly, the daily evolution curves for ionized and total calcium levels were not superimposable (34). These differences are probably explained by dietary intakes and variations in acid-base balance during the circadian cycle.
- pH and by extension the ventilatory state: on a biological level, it has been clearly demonstrated that pH fundamentally influences ionized calcium levels by its impact on the complexation with proteins and other anions (16,35-37). Thus, a rise in sample pH promotes the formation of calcium-albumin complexes and causes a decrease in ionized calcium levels. Conversely, a drop in pH may cause an increase in the free fraction of blood calcium and therefore an increase in ionized calcium levels. Thus, the occurrence of hyperventilation-induced respiratory alkalosis a few minutes before sampling may result in a significant decrease of the ionized calcium value (38,39). Overall, there is a strong correlation between ionized calcium and pH, quantified as around 5% calcium level modification for each 0.1 pH unit variation. This relationship is only verified for pH values ranging between 7.20 and 7.60. Given this predictable and well-described relationship, the use of a correction formula when reporting results is discussed (16,40): on the one hand, the effect of pH on ionized calcium is relevant in vivo, so not applying the correction seems consistent if one seeks to measure ionized calcium in the clinical situation where the sample was taken. In other situations where the pH appears distorted by post-analytical manipulation and when the patient has no apparent reason to present any acid-base disorder, this formula may be of interest.
- Other factors potentially affecting the calcium complexing ability: in the first place, we should of course mention albumin, the main protein to which calcium binds and whose variations have a major impact on ionized calcium levels. Briefly, we could also mention here the effects of heparin, fatty acid and bilirubin levels, certain drugs and temperature variations (26).
In conclusion, the realization and interpretation of ionized calcium measurement are challenging as the conditions and constraints that can affect its serum level before, during and after the sampling are numerous and diverse (Figure 2). These elements added to the significant cost of the technique represent all obstacles to its systematic use in current practice (41,42).
The unachieved quest of a perfect calcium estimation formula
Although its dosage is currently more accessible thanks to the ISE technology allowing its determination on blood-gas analyzers, many institutions continue to use total calcium assay in their daily practice, test that is otherwise recommended in the international guidelines (43). In this context, a very large number of formulas have been developed for nearly a century with two main objectives: to estimate the calcium status reported to a normal albumin level or to estimate the ionized calcium level from total calcium and a number of biological parameters such as albumin, protein, pH and anion gap. Historically, the first estimation formula is attributed to the nomogram developed by McLean and Hastings in the 1930s (18). Nevertheless, its tedious application has given way to the most used estimation formula: the Payne correction equation (44). Indeed, this very simple and easy-to-use formula is based on the strong link between total serum calcium and albumin levels (calcium = total calcium − 0.025× albumin +1). According to data from Vancouver Coastal Health and Providence Health Care Laboratories in 2018, total calcium and albumin were ordered concomitantly to biological samples in almost three out of four cases, suggesting that for the vast majority of clinicians, the interpretation of one cannot be fulfilled without the value of the other (45).
The literature concerning these estimation formulas is extremely rich, as illustrated by the work of Dickerson et al. (46). This study evaluated calcium estimation methods in a population of critically ill multiple trauma patients receiving specialized nutrition support. Interestingly, they did a literature review of all available estimation formulas and found 22 different ones, either trying to determine a “corrected” total serum calcium concentration, or to estimate ionized calcium concentration. The results are eloquent, as the set of formulas clearly failed to accurately assess calcium status: Dickerson et al. thus observed a very low average sensitivity of these formulas, for both hypocalcemia and hypercalcemia (25% and 15% respectively). The recent work of Mateu-de Antonio et al. focused on ionized calcium prediction formulas developed in adults, out of any pathology: eleven formulas have been highlighted, including the classic formulas of McLean-Hastings and Payne (47). Again, these formulas showed very poor predictive abilities in two cohorts of hospitalized patients for the detection of hypocalcemia (18% and 26% sensitivity respectively for McLean-Hastings and Payne formulas). In total, at least 30 different formulas have been created or derived from the oldest ones, and the literature has regularly reported their limits in practice (48) (Table 1).
Table 1
| References | Formulas | Population based | Evaluation |
|---|---|---|---|
| Dent et al. (49) 1962 | TCa (mg/dL) − 0.675× [Protein (g/dL) −7.2] | ||
| Orrell (50) 1971 | TCa (mmol/L) + 0.0176× [34 − albumin (mmol/L)] | Derived from 954 patient samples | External validation cohort on 558 geriatric hospital patients and 254 patient samples (51,52) |
| Payne et al. (44) 1973 | TCa (mmol/L) + 0.025× [40 − albumin (g/L)] | Derived from 200 patient samples | Multiple external validation cohort (53-57) |
| Parfitt et al. (58) 1974 | TCa (mmol/L)/[0.55 + (protein (g/L)/160)] | ||
| Kelly et al. (59) 1976 | (TCa (mg/dL) − 6)/[0.5× Protein (g/dL)] | Derived from 2,340 patient samples | |
| Walker et al. (60) 1979 | TCa (mg/dL) + 0.92× [4 − albumin (mg/dL)] | Derived from 6 normal volunteers and 15 patients with myocardial infarction | External validation cohort on 25 patient samples and 17 healthy subjects (61) |
| Thode et al. (62) 1989 | TCa (mg/dL) ×2.7/[1.7 + (albumin (mg/dL)/4.2)] | Derived from 1,213 patient samples | External validation cohort on 6,549 patient samples (56) |
| Clase et al. (63) 2000 | TCa (mmol/L) + 0.018×[35 − albumin (g/L)] | Derived from 50 stable hemodialysis patients | External validation cohort on 237 stable hemodialysis patients and 5,055 patient samples (55,64) |
| Jain et al. (64) 2008 | TCa (mmol/L) + 0.01×[30 − albumin (g/L)] | Derived from 60 hemodialysis patients | Internal validation cohort on 237 stable hemodialysis patients; External validation cohort on 5,055 patient samples and 2,503 samples (53,55) |
| James et al. (65) 2008 | TCa (mmol/L) + 0.012×[39.9 − albumin (g/L)] | Derived from 4,613 outpatient samples | Internal validation cohort on 1,538 outpatient samples; External validation cohort on 6,549 patient samples (56) |
| Ferrari et al. (66) 2009 | TCa (mg/dL) + 1.6×[4 − albumin (g/dL)] + 0.56×[1.5 − 0.32× protein (mg/dL)] | Derived from 82 hemodialysis patients | External validation cohort on 2,503 samples from 942 patients (53) |
| Kaku et al. (53) 2016 | TCa (mg/dL) = TCa + 0.25×(4 − albumin) + 4×(7.4 − pH) + 0.1×(6 − phosphate) + 0.3 | Derived from 2,503 samples from 942 patients | |
| Ridefelt et al. (67) 2017 | TCa (mmol/L) − 0.0135× albumin (g/L) + 0.4525 | Derived from 3,106 samples | Internal validation cohort on 16,897 samples; External validation cohort on 5,055 patient samples (55) |
Different hypotheses to explain the limits of theses estimation formulas
The strong correlation between serum calcium and albumin levels is unquestionable, and justifies the common use of these estimation formulas in absence of pH or serum protein abnormalities. Nevertheless, as previously described, the correlation between total and ionized calcium may collapse in the presence of a wide variety of factors: acid-base imbalance, albumin abnormality, protein-bound drugs or fatty acids, or the presence of an excess circulating immunoglobulin for example (20,22,26).
An important hypothesis made about the relation between albumin and calcium is that a decrease of the first induces a decrease of albumin-bound calcium fraction. This would lead to a decrease of total calcium while ionized calcium levels remain normal. In fact, this hypothesis has been dismantled since there is some evidence that in hypoalbuminemic states, the calcium affinity for albumin increases significantly (68). Starting from the Payne formula, the most used in practice, it is not surprising to see a risk of ionized calcium overestimation: indeed, the starting postulate relies on a constant albumin-calcium relationship, regardless of albumin levels. Thus, several studies have since abounded in this direction, even demonstrating that in situations of severe hypo-albuminemia, the correction formula is less efficient than the uncorrected total calcium level (46,63). Payne formula estimation was constructed from 200 patients but was not validated in a second cohort (44). The main goal of this formula was to deduce, in hypoalbuminemic patients, the calcium value it would have been if albumin level was normal. The patients included were not suspected for any ionized calcium abnormality. Recently, Payne himself has addressed a letter to the editor in which he reminds that the formula was not intended to be universally used, being for example not at all suitable to the chronic kidney disease population (69). Moreover, this formula was produced from the data of the time, based on the analytical methods applied to biological measurements at that time, which implies that it should be updated according to the methods used nowadays in clinical laboratories. Another major hypothesis, demonstrated in a recent work, is the need of taking into account the pH in these estimation formulas (53-55). Indeed, it now seems clear that pH is one of the most important adjustment variables when trying to estimate a patient’s calcium status. Indeed, a retrospective study of 5,055 samples confirmed the lack of added value of several correction formulas (including Payne’s) compared to total calcium measurement, in order to accurately estimate the calcium status. Taking into account the pH value has radically improved the prediction performances in our own derived formula (55), especially in patients presenting alkalosis. pH-adjusted formulas are thus rare in the literature, partly explaining the overall poor performance of these estimation equations (53,55). More recently, to overcome this limitation, some authors suggested to also use the anion gap to better estimate the calcium status when ionized calcium is unavailable (70). Moreover, these formulas would be of limited interest since pH determination and ionized calcium measurement require actually the same strict pre-analytical conditions and may be performed under the same conditions on blood-gas analyzers.
Some authors propose to avoid the reproducibility issue of such formulas by producing their own formula directly derived from the measurements carried out in their laboratory (65). On the basis of their work on a cohort of over 4,600 outpatients, James et al. showed a significant improvement in the patients’ calcium status assessment using their own local adjusted equation. Other researchers seem to go further in their reasoning: for Lian et al., the problem lies in the mathematical conception of the regression equations used to define the various estimation formulas (56). Indeed, for them, it would be advisable to take into account, beyond total calcium and albumin levels, other explanatory variables including albumin, ionized calcium value as well sex, age, kidney function, serum phosphate level, and the in- or outpatient status. Although the hypothesis appears convincing, it seems that even a formula derived from its own dataset and resulting from an enlarged number of parameters fails doing better than unadjusted total calcium levels. This may explain why the question of an indisputable formula is still unachieved: this may actually be an unsolvable problem.
Clinical relevance of ionized calcium measurement
We aimed to discern, from the literature data, the situations in which the ionized calcium estimation formulas produce unreliable results, which may negatively impact patient management and justify the systematic use of ionized calcium dosage.
Thyroid, parathyroid and malignancy-related calcium disorders
Calcium regulation relies above all on a complex and integrated regulation by the endocrine system, based on two essential hormones: PTH and active vitamin D. The diagnosis of primary hyperparathyroidism is based on the concomitant presence of abnormally high or normal PTH levels and hypercalcemia with a particular relevance of ionized calcium (71-80). Indeed, the diagnosis of hyperparathyroidism is based primarily on the detection of hypercalcemia, and the frequency of hyperparathyroidism revealed by an isolated increased ionized serum calcium level should make us wary of the sole dosage of total calcium (72-74,76,77,79,80). In a retrospective study of 268 patients treated with parathyroidectomy for primary hyperparathyroidism, ionized calcium levels were found to be more sensitive than total serum calcium levels for the detection of hypercalcemia. In addition, a strong linear relationship between ionized calcium and parathyroid adenoma size has also been demonstrated, a clearer association than with total serum calcium levels (74). In some international guidelines, the dosage of ionized calcium is recommended for the diagnosis of hyperparathyroidism, its routine use being only hampered by its wider accessibility (81). In parallel with hyperparathyroidism, the other major cause of hypercalcemia in humans is malignancy, related to various mechanisms such as bone resorption mediated by PTH related peptide (PTHrp) in case of paraneoplastic syndrome or by the presence of bone metastases (82,83). In the population of cancer patients, studies confirm a significant discrepancy between total and ionized calcium values for the diagnosis of malignancy-related hypercalcemia (84,85). The question of an association between ionized calcium and the cancer-related mortality risk remains debated (84), although several recent prospective studies seem to emphasize this link, especially in prostate and ovarian cancers (86-89). More interestingly, the ionized calcium testing could also detect occult cancer pathologies, as suggested by a prospective study conducted on 6,707 men with more than 15-year follow-up (88). To those situations, we could also add several case reports of pseudo-hypercalcemia (total calcium) related to multiple myeloma, which have led to misdiagnosis and injudicious care (90,91), while ionized calcium levels were normal.
The added value of ionized calcium has also been advanced in the context of hypocalcemia, a frequent and sometimes serious complication of thyroidectomy. Although ionized calcium measurement does not seem to bring any obvious benefit in the detection of postoperative hypocalcemia compared to total serum calcium levels (90), its preoperative and early postoperative values may have predictive capacities regarding the occurrence of symptomatic post-operative hypocalcemia (91,92). This could also apply to all other forms of primary or secondary hypocalcemia, but data are still lacking.
Calcium monitoring and kidney dysfunction
For the follow-up of patients with moderate or advanced chronic kidney disease (including end-stage kidney disease), the monitoring of serum calcium levels is essential. Indeed, these patients are at high cardiovascular risk, mainly mediated by the chronic kidney disease—mineral bone disorder (CKD-MBD) (93,94). This syndrome begins in the early stages of CKD and worsens as kidney function deteriorates. This complex pathology involves the progressive increase in serum phosphorus, PTH and fibroblast growth factor 23 (FGF23) levels in parallel with decreased 1α-hydroxylase activity, calcium uptake and serum calcium levels. This leads to specific abnormalities in the bone turnover, which can reach the stage of adynamic bone disease (95). Clinically, we observe a renal osteodystrophy syndrome, responsible for increased bone fragility, as well as accelerated vessels and soft tissues calcification, thus explaining the high cardiovascular risk in the CKD population (96). This explains the need to closely monitor the mineral metabolism parameters in CKD patients.
Nevertheless, at the most advanced CKD stages, pH modifications and serum albumin fluctuations do not guarantee an adequate estimation of calcium status by total serum calcium measurements (97), although the 2017 ‘Kidney Disease: Improving Global Outcome’ (KDIGO) recommendations do not exclude total calcium monitoring, after albumin adjustment (43). However, reports confirm the relevance of ionized calcium testing for this population. Indeed, there may be a significant risk of misdiagnosing a CKD patient as hypercalcemic with total calcium measurement although ionized calcium level is in the standard range (63,97-100). These results may thus have a direct impact on these patients care, regarding the prescription of calcium or vitamin D supplementation or the use of calcimimetics. Surprisingly, a study of more than 100 CKD stage 3 to 5 patients demonstrated that albumin-adjusted serum calcium levels did not perform better than unadjusted total serum calcium for the detection of ionized calcium abnormalities (100). Similarly, the study of Clase et al. on 50 stable hemodialysis patients emphasized a better agreement between ionized and uncorrected total serum calcium levels than with all formulas for adjusted calcium estimations (63).
After kidney transplantation, calcium monitoring is essential, since hypercalcemia affects more than 10% of this population, even after the first-year post-transplantation (101). This phenomenon is most often related to persistent hyperparathyroidism. For these patients as well, the performance of total calcium dosage seems very limited, in the same way as for CKD patients. Indeed, in a study involving 268 kidney transplant recipients, the diagnosis of hypercalcemia would be missed in 80% of cases if the clinicians would have stuck only to total serum calcium measurements (102). This result is probably related to the high prevalence of metabolic acidosis in the population of kidney transplant patients (10% to 50%, depending on post-transplantation kidney function) (103-105), prevalence much higher than in CKD population before transplantation (106).
Serum calcium levels and cardiovascular outcomes
The extracellular calcium concentration has a direct impact on cells with calcium-sensing receptors on their surface (parathyroid, renal tubule) but also on excitable cells by influencing their membrane potential. This concerns particularly the heart, for which calcium homeostasis is directly involved in myocardial contraction. There is a link between ionized calcium levels and electrocardiogram modifications (107-111), illustrated by few case reports of severe arrhythmias caused by abrupt changes in ionized calcium levels (111,112). Calcium levels also interact with the smooth muscle fibers present in vessel walls, thus explaining the observed link between ionized calcium and blood pressure (113,114). Furthermore, in addition to enhancing calcium deposits in the soft tissues and vascular walls, chronic exposure to high levels of circulating calcium may increase the risk of cardiovascular events (96). This may also be mediated by the association of calcium serum levels and some specific cardiovascular risk factors such as high blood pressure, carbohydrate intolerance and dyslipidemia (115-118). While several studies have shown a link between high levels of total calcium and the occurrence of cardiovascular complications (96,119-121), the work of Ogard et al. on 974 patients followed for nearly 20 years, could not confirm this relationship with ionized calcium levels (122).
Calcium disturbances in critically ill patients
The population of patients admitted to intensive care units (ICU) is very often subject to hypocalcemia (60% to 90% of patients) (123,124), favored by various mechanisms: increased fecal and urinary excretion, vitamin D deficiency and decreased calcium intake, pH modifications, catecholamine-mediated intracellular shift, citrate anticoagulation for continuous veno-venous hemodiafiltration or plasmapheresis, and parenteral nutrition (125-127). Among these critically ill patients, it has been clearly demonstrated that total serum calcium and correction formulas are poor estimators of ionized calcium levels as they failed to accurately classify calcium status in up to 40% of this particular population (46,126,128,129). Although some studies have found a link between the depth of hypocalcemia and the risk of in-hospital or early mortality, the predictive value of ionized calcium levels at ICU admission is still debated (124,130-133). The studies of Choi et al. (133) and Hastbacka et al. (134), including 255 and 993 patients respectively, demonstrated limited predictive capacities with an estimated area under curve inferior to 0.70.
Nevertheless, ionized calcium assay appears to be more strongly associated with mortality risk in some specific ICU patients, such as those experiencing acute kidney injury (135), and those requiring renal replacement therapy (136,137). Moreover, the literature has highlighted some discrepancies between total and ionized calcium levels in patients admitted for acute pancreatitis (138,139) or for severe liver dysfunction requiring transplantation (140). Finally, a recently conducted prospective study on 111 patients hospitalized for hypertensive intracerebral hemorrhage showed that low ionized calcium was an independent risk factor for early expansion of intracerebral hematoma, as well as poor short-term prognosis (141). The use of extracorporeal circulation, and more specifically plasmapheresis, justifies the close monitoring of ionized calcium level, given the risk of its sudden drop due to citrate anticoagulation and the common substitution by fresh plasma (142-144).
Other potential leads for clinical usefulness of ionized calcium testing
The use of ionized calcium dosage has also been the subject of some other sparse studies, which may suggest a broader indication of its measurement. Indeed, in a study involving 1,248 men with fertility disorders, a link was found between ionized calcium levels and semen quality, as well as sex steroid levels (145). Some works have also reported a relationship between ionized calcium levels and the occurrence of preeclampsia (146,147). A study in elderly patients followed for depression also described a link between elevated levels of ionized calcium and white matter hemorrhages detected on brain MRI (148). Finally, the use of the ionized calcium assay also seems useful when administering drugs and chemotherapies known to be associated with ionized calcium disturbances (cisplatin, leucovorin-5-fluorouracil, bisphosphonates for example) (149-151). Some recent reports, however, have drawn attention to the risk of calcium status misinterpretation by ionized calcium testing in patients under Leflunomide treatment, due to analytical interferences (152,153).
Towards a rationale and reasonable prescription of ionized calcium testing in daily practice
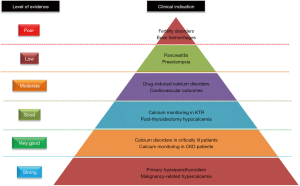
In conclusion, the current state of the art seems to insist on the added value of routine ionized calcium measurement for specific populations, such as patients suspected of hyperparathyroidism, suffering from cancer, admitted to intensive care units, or followed for CKD (Figure 3). For the other conditions mentioned above, we currently lack data to justify the broadening of ionized calcium monitoring indications.
Although its routine use seems increasingly justified in many situations (15), some authors have recently pointed out the risk of drifting to an inappropriate over-prescribing of ionized calcium testing (16,41,154). Indeed, these studies describe the setting of an educational intervention regarding the appropriateness of ionized calcium measurement, which has allowed an up to 75% decrease of its institutional prescription. In connection with these previous works concerning the overuse of ionized calcium, we may distinguish two main situations of interest: first, the specific cases previously detailed and the situations of moderate dyscalcemia. Indeed, the discrepancies between total and ionized calcium almost never concern the situations of frank hyper- or hypocalcemia.
This confirms the need for a thinking on precise clinical indications of ionized calcium testing and their integration into international guidelines (12,13).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-60). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010;5:S23-30. [Crossref] [PubMed]
- Ireland P, Fordtran JS. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest 1973;52:2672-81. [Crossref] [PubMed]
- Spencer H, Lewin I, Fowler J, et al. Influence of dietary calcium intake on Ca47 absorption in man. Am J Med 1969;46:197-205. [Crossref] [PubMed]
- Heaney RP, Saville PD, Recker RR. Calcium absorption as a function of calcium intake. J Lab Clin Med 1975;85:881-90. [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011.
- Osterhoff G, Morgan EF, Shefelbine SJ, et al. Bone mechanical properties and changes with osteoporosis. Injury 2016;47:S11-20. [Crossref] [PubMed]
- Cheng HP, Wei S, Wei L, et al. Calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin 2006;27:767-72. [Crossref] [PubMed]
- Goltzman D, Mannstadt M, Marcocci C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front Horm Res 2018;50:1-13. [Crossref] [PubMed]
- Turner JJO. Hypercalcaemia - presentation and management. Clin Med (Lond) 2017;17:270-3. [Crossref] [PubMed]
- Fong J, Khan A. Hypocalcemia. Can Fam Physician 2012;58:158-62. [PubMed]
- Cooper MS, Gittoes NJL. Diagnosis and management of hypocalcaemia. BMJ 2008;336:1298-302. [Crossref] [PubMed]
- Calvi LM, Bushinsky DA. When Is It Appropriate to Order an Ionized Calcium? J Am Soc Nephrol 2008;19:1257-60. [Crossref] [PubMed]
- Forman DT, Lorenzo L. Ionized calcium: its significance and clinical usefulness. Ann Clin Lab Sci 1991;21:297-304. [PubMed]
- Ladenson JH, Lewis JW, Boyd JC. Failure of total calcium corrected for protein, albumin, and pH to correctly assess free calcium status. J Clin Endocrinol Metab 1978;46:986-93. [Crossref] [PubMed]
- Glendenning P. It is time to start ordering ionized calcium more frequently: preanalytical factors can be controlled and postanalytical data justify measurement. Ann Clin Biochem 2013;50:191-3. [Crossref] [PubMed]
- Baird GS. Ionized calcium. Clin Chim Acta 2011;412:696-701. [Crossref] [PubMed]
- Boden SD, Kaplan FS. Calcium homeostasis. Orthop Clin North Am 1990;21:31-42. [PubMed]
- McLean FC, Hastings AB. The State of Calcium in the Fluids of the Body I. the Conditions Affecting the Ionization of Calcium. J Biol Chem 1935;108:285-321.
- Jafri L, Khan AH, Azeem S. Ionized Calcium Measurement in Serum and Plasma by Ion Selective Electrodes: Comparison of Measured and Calculated Parameters. Indian J Clin Biochem 2014;29:327-32. [Crossref] [PubMed]
- Kallner A. Preanalytical procedures in the measurement of ionized calcium in serum and plasma. Eur J Clin Chem Clin Biochem 1996;34:53-8. [PubMed]
- Boink AB, Buckley BM, Christiansen TF, et al. Recommendation on sampling, transport, and storage for the determination of the concentration of ionized calcium in whole blood, plasma, and serum. IFC Scientific Division, Working Group on Ion-Selective Electrodes (WGSE). J Int Fed Clin Chem 1992;4:147-52. [PubMed]
- Haverstick DM, Brill LB, Scott MG, et al. Preanalytical variables in measurement of free (ionized) calcium in lithium heparin-containing blood collection tubes. Clin Chim Acta 2009;403:102-4. [Crossref] [PubMed]
- Boink AB, Buckley BM, Christiansen TF, et al. IFCC recommendation on sampling, transport and storage for the determination of the concentration of ionized calcium in whole blood, plasma and serum. J Automat Chem 1991;13:235-9. [Crossref] [PubMed]
- D’Orazio P, Toffaletti JG, Wandrup J, National Committee for Clinical Laboratory Standards. Ionized calcium determinations: precollection variables, specimen choice, collection, and handling: approved guideline. Wayne, PA: National Committee for Clinical Laboratory Standards; 2001.
- Grzych G, Roland E, Beauvais D, et al. Leukocytosis interference in clinical chemistry: shall we still interpret test results without hematological data? J Med Biochem 2019;1. [Epub ahead of print]. [PubMed]
- Ferreira-Junior M, Lichtenstein A, Sales MM, et al. Rational use of blood calcium determinations. Sao Paulo Med J 2014;132:243-8. [Crossref] [PubMed]
- Toffaletti J, Blosser N, Kirvan K. Effects of storage temperature and time before centrifugation on ionized calcium in blood collected in plain vacutainer tubes and silicone-separator (SST) tubes. Clin Chem 1984;30:553-6. [Crossref] [PubMed]
- Bowers GN, Brassard C, Sena SF. Measurement of ionized calcium in serum with ion-selective electrodes: a mature technology that can meet the daily service needs. Clin Chem 1986;32:1437-47. [Crossref] [PubMed]
- Burnett RW, Christiansen TF, Covington AK, et al. IFCC recommended reference method for the determination of the substance concentration of ionized calcium in undiluted serum, plasma or whole blood. Clin Chem Lab Med 2000;38:1301-14. [Crossref] [PubMed]
- Lopez J. Carl A. Burtis, Edward R. et al. Tietz Textbook of Clinical Chemistry and Molecular Diagnosis (5th edition). Indian J Clin Biochem 2013;28:104-5.
- Ljunghall S, Joborn H, Benson L, et al. Effects of physical exercise on serum calcium and parathyroid hormone. Eur J Clin Invest 1984;14:469-73. [Crossref] [PubMed]
- Haynes S, Hickson E, Linden J, et al. Dietary citrate and plasma ionized calcium: Implications for platelet donors. J Clin Apher 2018;33:222-5. [Crossref] [PubMed]
- Dixon M, Paterson CR. Posture and the composition of plasma. Clin Chem. 1978;24:824-6. [Crossref] [PubMed]
- Markowitz ME, Arnaud S, Rosen JF, et al. Temporal interrelationships between the circadian rhythms of serum parathyroid hormone and calcium concentrations. J Clin Endocrinol Metab 1988;67:1068-73. [Crossref] [PubMed]
- Kancir CB, Petersen PH, Madsen T, et al. In vivo and in vitro ionized calcium variations induced by acute respiratory acid base disturbances. Clin Chim Acta 1988;175:307-13. [Crossref] [PubMed]
- Shore AC, Booker J, Sagnella GA, et al. Serum ionized calcium and pH: effects of blood storage, some physiological influences and a comparison between normotensive and hypertensive subjects. J Hypertens 1987;5:499-505. [Crossref] [PubMed]
- Peoples JB. The role of pH in altering serum ionized calcium concentration. Surgery 1988;104:370-4. [PubMed]
- Moon HS, Lee SK, Chung JH, In CB. Hypocalcemia and hypokalemia due to hyperventilation syndrome in spinal anesthesia -A case report-. Korean J Anesthesiol 2011;61:519-23. [Crossref] [PubMed]
- Watchko J, Bifano EM, Bergstrom WH. Effect of hyperventilation on total calcium, ionized calcium, and serum phosphorus in neonates. Crit Care Med 1984;12:1055-6. [Crossref] [PubMed]
- Thode J, Holmegaard SN, Transbøl I, et al. Adjusted ionized calcium (at pH 7.4) and actual ionized calcium (at actual pH) in capillary blood compared for clinical evaluation of patients with disorders of calcium metabolism. Clin Chem 1990;36:541-4. [Crossref] [PubMed]
- Baird GS, Rainey PM, Wener M, et al. Reducing Routine Ionized Calcium Measurement. Clin Chem 2009;55:533-40. [Crossref] [PubMed]
- Toffaletti JG. Is Ionized Calcium Always Right and Total Calcium Always Wrong? Clinical Laboratory News [Internet). 2011 Sep [cited 2019 Oct 15). Available onlibe: https://www.jiscmail.ac.uk/cgi-bin/webadmin%3FA3%3Dind1211%26L%3DACB-CLIN-CHEM-GEN%26E%3Dbase64%26P%3D4304049%26B%3D--_004_1F6326A289C50C408617B39F4439CAEF0530CBD593RANEML23lanse_%26T%3Dapplication%252Fpdf%3B%2520name%3D%2522Tofaletti%2520on%2520corr%2520Ca%2520formulas%2520CLN2011.pdf%2522%26N%3DTofaletti%2520on%2520corr%2520Ca%2520formulas%2520CLN2011.pdf%26attachment%3Dq%26XSS%3D3
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) 2017;7:1-59.
- Payne RB, Little AJ, Williams RB, et al. Interpretation of Serum Calcium in Patients with Abnormal Serum Proteins. Br Med J 1973;4:643-6. [Crossref] [PubMed]
- Simons J. Correcting the Myth of Calcium Correction [Internet). This changed my practice (UBC CPD). 2019 [cited 2019 Oct 10). Available online: https://thischangedmypractice.com/myth-of-calcium-correction/
- Dickerson RN, Alexander KH, Minard G, et al. Accuracy of methods to estimate ionized and “corrected” serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. JPEN J Parenter Enteral Nutr 2004;28:133-41. [Crossref] [PubMed]
- Mateu-de Antonio J. New Predictive Equations for Serum Ionized Calcium in Hospitalized Patients. Med Princ Pract 2016;25:219-26. [Crossref] [PubMed]
- Gidenne S, Vigezzi J, Delacour H, et al. Direct determination or estimated value of plasma ionized calcium: indications and limits. Ann Biol Clin (Paris) 2003;61:393-9. [PubMed]
- Dent CE. Some Problems of Hyperparathyroidism. Br Med J 1962;2:1495-500. [Crossref] [PubMed]
- Orrell DH. Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta 1971;35:483-9. [Crossref] [PubMed]
- Sorva A, Elfving S, Pohja P, et al. Assessment of calcaemic status in geriatric hospital patients: Serum ionized calcium versus albumin-adjusted total calcium. Scand J Clin Lab Invest 1988;48:489-94. [Crossref] [PubMed]
- Mir AA, Goyal B, Datta SK, et al. Comparison Between Measured and Calculated Free Calcium Values at Different Serum Albumin Concentrations. J Lab Physicians 2016;8:71-6. [Crossref] [PubMed]
- Kaku Y, Ookawara S, Miyazawa H, et al. New Method for the Approximation of Corrected Calcium Concentrations in Chronic Kidney Disease Patients. Ther Apher Dial 2016;20:46-52. [Crossref] [PubMed]
- Grzych G, Pekar JD, Durand G, et al. Albumin-Adjusted Calcium and Ionized Calcium for Assessing Calcium Status in Hospitalized Patients. Clin Chem 2019;65:703-5. [Crossref] [PubMed]
- Pekar JD, Grzych G, Durand G, et al. Calcium state estimation by total calcium: the evidence to end the never-ending story. Clin Chem Lab Med 2020;58:222-31. [Crossref] [PubMed]
- Lian IA, Åsberg A. Should total calcium be adjusted for albumin? A retrospective observational study of laboratory data from central Norway. BMJ Open 2018;8:e017703 [Crossref] [PubMed]
- Smith JD, Wilson S, Schneider HG. Misclassification of Calcium Status Based on Albumin-Adjusted Calcium Studies in a Tertiary Hospital Setting. Clin Chem 2018;64:1713-22. [Crossref] [PubMed]
- Parfitt AM. Letter: Correction of plasma calcium measurements. Br Med J 1974;1:520. [Crossref] [PubMed]
- Kelly A, Munan L. Use of values for calcium and protein in serum, and of a derived index obtained from a probability population sample. Clin Chem 1976;22:1723-7. [Crossref] [PubMed]
- Walker BE, Payne RB. Adjusted calcium conflict resolved? Differing effects on plasma total calcium of changes in plasma albumin after venous stasis or myocardial infarction. J Clin Pathol 1979;32:488-91. [Crossref] [PubMed]
- Pain RW, Phillips PJ, Duncan BM. Corrected calcium conflict continues. J Clin Pathol 1980;33:413. [Crossref] [PubMed]
- Thode J, Juul-Jørgensen B, Bhatia HM, et al. Comparison of serum total calcium, albumin-corrected total calcium, and ionized calcium in 1213 patients with suspected calcium disorders. Scand J Clin Lab Invest 1989;49:217-23. [Crossref] [PubMed]
- Clase CM, Norman GL, Beecroft ML, et al. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant 2000;15:1841-6. [Crossref] [PubMed]
- Jain A, Bhayana S, Vlasschaert M, et al. A formula to predict corrected calcium in haemodialysis patients. Nephrol Dial Transplant 2008;23:2884-8. [Crossref] [PubMed]
- James MT, Zhang J, Lyon AW, et al. Derivation and internal validation of an equation for albumin-adjusted calcium. BMC Clin Pathol 2008;8:12. [Crossref] [PubMed]
- Ferrari P, Singer R, Agarwal A, et al. Serum phosphate is an important determinant of corrected serum calcium in end-stage kidney disease. Nephrology (Carlton) 2009;14:383-8. [Crossref] [PubMed]
- Ridefelt P, Helmersson-Karlqvist J. Albumin adjustment of total calcium does not improve the estimation of calcium status. Scand J Clin Lab Invest 2017;77:442-7. [Crossref] [PubMed]
- Besarab A, Caro JF. Increased absolute calcium binding to albumin in hypoalbuminaemia. J Clin Pathol 1981;34:1368-74. [Crossref] [PubMed]
- Payne RB. Albumin-Adjusted Calcium and Ionized Calcium. Clin Chem 2019;65:705-6. [Crossref] [PubMed]
- Yap E, Roche-Recinos A, Goldwasser P. Predicting Ionized Hypocalcemia in Critical Care: An Improved Method Based on the Anion Gap. J Appl Lab Med 2020;5:4-14. [Crossref] [PubMed]
- Hoang TD, Jani AG, Mai VQ, et al. Associations of serum ionized calcium, phosphate, and PTH levels with parathyroid scan in primary hyperparathyroidism. Endocr Pract 2019;25:16-22. [Crossref] [PubMed]
- Ong GSY, Walsh JP, Stuckey BGA, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab 2012;97:3138-45. [Crossref] [PubMed]
- Forster J, Monchik JM, Martin HF. A comparative study of serum ultrafiltrable, ionized, and total calcium in the diagnosis of primary hyperparathyroidism in patients with intermittent or no elevation in total calcium. Surgery 1988;104:1137-42. [PubMed]
- Nordenström E, Katzman P, Bergenfelz A. Biochemical diagnosis of primary hyperparathyroidism: Analysis of the sensitivity of total and ionized calcium in combination with PTH. Clin Biochem 2011;44:849-52. [Crossref] [PubMed]
- Shepherd JJ, Teh BT, Parameswaran V, et al. Hyperparathyroidism with normal albumin-corrected total calcium in patients with multiple endocrine neoplasia type 1. Henry Ford Hosp Med J 1992;40:186-90. [PubMed]
- Tee MC, Holmes DT, Wiseman SM. Ionized vs serum calcium in the diagnosis and management of primary hyperparathyroidism: which is superior? Am J Surg 2013;205:591-6; discussion 596. [Crossref] [PubMed]
- Monchik JM, Gorgun E. Normocalcemic hyperparathyroidism in patients with osteoporosis. Surgery 2004;136:1242-6. [Crossref] [PubMed]
- Ladenson JH, Lewis JW, McDonald JM, et al. Relationship of free and total calcium in hypercalcemic conditions. J Clin Endocrinol Metab 1979;48:393-7. [Crossref] [PubMed]
- Larsson L, Ohman S. Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism. Clin Chem 1978;24:1962-5. [Crossref] [PubMed]
- McLeod MK, Monchik JM, Martin HF. The role of ionized calcium in the diagnosis of subtle hypercalcemia in symptomatic primary hyperparathyroidism. Surgery 1984;95:667-73. [PubMed]
- Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 2017;28:1-19. [Crossref] [PubMed]
- Ramos REO, Perez Mak M, Alves MFS, et al. Malignancy-Related Hypercalcemia in Advanced Solid Tumors: Survival Outcomes. J Glob Oncol 2017;3:728-33. [Crossref] [PubMed]
- Mirrakhimov AE. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. N Am J Med Sci 2015;7:483-93. [Crossref] [PubMed]
- Riancho JA, Arjona R, Sanz J, et al. Is the routine measurement of ionized calcium worthwhile in patients with cancer? Postgrad Med J 1991;67:350-3. [Crossref] [PubMed]
- Ijaz A, Mehmood T, Qureshi AH, et al. Estimation of ionized calcium, total calcium and albumin corrected calcium for the diagnosis of hypercalcaemia of malignancy. J Coll Physicians Surg Pak 2006;16:49-52. [PubMed]
- Skinner HG, Schwartz GG. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 2009;18:575-8. [Crossref] [PubMed]
- Schwartz GG, Skinner HG. Prospective studies of total and ionized serum calcium in relation to incident and fatal ovarian cancer. Gynecol Oncol 2013;129:169-72. [Crossref] [PubMed]
- Schwartz GG, Skinner HG. A prospective study of total and ionized serum calcium and time to fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 2012;21:1768-73. [Crossref] [PubMed]
- George GP, Ramesh V, Mittal RD. Impact of total and ionized serum calcium on prostate cancer risk in North Indian men. Asian Pac J Cancer Prev 2011;12:1257-60. [PubMed]
- Del Rio P, Arcuri MF, Cataldo S, et al. Can we use ionized calcium in the evaluation of post-thyroidectomy hypocalcemia? Minerva Endocrinol 2009;34:289-93. [PubMed]
- de Andrade Sousa A, Salles JMP, Soares JMA, et al. Course of ionized calcium after thyroidectomy. World J Surg 2010;34:987-92. [Crossref] [PubMed]
- Tartaglia F, Giuliani A, Sgueglia M, et al. Is ionized calcium a reliable predictor of hypocalcemia after total thyroidectomy? A before and after study. G Chir 2014;35:27-35. [PubMed]
- Hruska KA, Seifert M, Sugatani T. Pathophysiology of the Chronic Kidney Disease - Mineral Bone Disorder (CKD-MBD). Curr Opin Nephrol Hypertens 2015;24:303-9. [PubMed]
- Hruska KA, Sugatani T, Agapova O, et al. The chronic kidney disease - Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017;100:80-6. [Crossref] [PubMed]
- Massy Z, Drueke T. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol 2017;30:629-34. [Crossref] [PubMed]
- Reid IR, Birstow SM, Bolland MJ. Calcium and Cardiovascular Disease. Endocrinol Metab (Seoul) 2017;32:339-49. [Crossref] [PubMed]
- Gøransson LG, Skadberg Ø, Bergrem H. Albumin-corrected or ionized calcium in renal failure? What to measure? Nephrol Dial Transplant 2005;20:2126-9. [Crossref] [PubMed]
- Gouri A, Dekaken A. A comparison of corrected serum calcium levels to ionized calcium levels in haemodialysis patients. Ann Biol Clin (Paris) 2012;70:210-2. [Crossref] [PubMed]
- Burritt MF, Pierides AM, Offord KP. Comparative studies of total and ionized serum calcium values in normal subjects and patients with renal disorders. Mayo Clin Proc 1980;55:606-13. [PubMed]
- Gauci C, Moranne O, Fouqueray B, et al. Pitfalls of Measuring Total Blood Calcium in Patients with CKD. J Am Soc Nephrol 2008;19:1592-8. [Crossref] [PubMed]
- Torres A, Lorenzo V, Salido E. Calcium Metabolism and Skeletal Problems after Transplantation. J Am Soc Nephrol 2002;13:551-8. [PubMed]
- Evenepoel P, Bammens B, Claes K, et al. Measuring total blood calcium displays a low sensitivity for the diagnosis of hypercalcemia in incident renal transplant recipients. Clin J Am Soc Nephrol 2010;5:2085-92. [Crossref] [PubMed]
- Ansell D, Udayaraj UP, Steenkamp R, et al. Chronic Renal Failure in Kidney Transplant Recipients. Do They Receive Optimum Care?: Data from the UK Renal Registry. Am J Transplant 2007;7:1167-76. [Crossref] [PubMed]
- Fernando ME, Jayanivash J, Srinivasaprasad ND, et al. Post-Renal Transplant Metabolic Acidosis: A Neglected Entity. Indian J Nephrol 2019;29:116-21. [PubMed]
- Park S, Kang E, Park S, et al. Metabolic Acidosis and Long-Term Clinical Outcomes in Kidney Transplant Recipients. J Am Soc Nephrol 2017;28:1886-97. [Crossref] [PubMed]
- Moranne O, Froissart M, Rossert J, et al. Timing of Onset of CKD-Related Metabolic Complications. J Am Soc Nephrol 2009;20:164-71. [Crossref] [PubMed]
- Gardner JD, Calkins JB, Garrison GE. ECG diagnosis: The effect of ionized serum calcium levels on electrocardiogram. Perm J 2014;18:e119-20. [Crossref] [PubMed]
- El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J 2011;18:233-45. [PubMed]
- Deo M, Weinberg SH, Boyle PM. Calcium Dynamics and Cardiac Arrhythmia. Clin Med Insights Cardiol 2017;11:1179546817739523 [Crossref] [PubMed]
- Rumancik WM, Denlinger JK, Nahrwold ML, et al. The QT interval and serum ionized calcium. JAMA 1978;240:366-8. [Crossref] [PubMed]
- Pepe J, Cipriani C, Curione M, et al. Reduction of arrhythmias in primary hyperparathyroidism, by parathyroidectomy, evaluated with 24-h ECG monitoring. Eur J Endocrinol 2018;179:117-24. [Crossref] [PubMed]
- Mosebach CM, Kluger J. Probable Hypocalcemia Induced Ventricular Fibrillation and Torsades de Pointes following Blood Product Administration. Cureus 2018;10:e3765 [PubMed]
- Kawano Y, Yoshimi H, Matsuoka H, et al. Calcium supplementation in patients with essential hypertension: assessment by office, home and ambulatory blood pressure. J Hypertens 1998;16:1693-9. [Crossref] [PubMed]
- van Mierlo LAJ, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens 2006;20:571-80. [Crossref] [PubMed]
- Jorde R, Sundsfjord J, Fitzgerald P, et al. Serum calcium and cardiovascular risk factors and diseases: the Tromsø study. Hypertension 1999;34:484-90. [Crossref] [PubMed]
- Walsh JP, Divitini ML, Knuiman MW. Plasma calcium as a predictor of cardiovascular disease in a community-based cohort. Clin Endocrinol (Oxf) 2013;78:852-7. [Crossref] [PubMed]
- Hagström E, Lundgren E, Lithell H, et al. Normalized dyslipidaemia after parathyroidectomy in mild primary hyperparathyroidism: population-based study over five years. Clin Endocrinol (Oxf) 2002;56:253-60. [Crossref] [PubMed]
- Wareham NJ, Byrne CD, Carr C, et al. Glucose intolerance is associated with altered calcium homeostasis: a possible link between increased serum calcium concentration and cardiovascular disease mortality. Metab Clin Exp 1997;46:1171-7. [Crossref] [PubMed]
- Yarmohammadi H, Uy-Evanado A, Reinier K, et al. Serum Calcium and Risk of Sudden Cardiac Arrest in the General Population. Mayo Clin Proc 2017;92:1479-85. [Crossref] [PubMed]
- Reid IR, Gamble GD, Bolland MJ. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J Intern Med 2016;279:524-40. [Crossref] [PubMed]
- Grandi NC, Brenner H, Hahmann H, et al. Calcium, phosphate and the risk of cardiovascular events and all-cause mortality in a population with stable coronary heart disease. Heart 2012;98:926-33. [Crossref] [PubMed]
- Ogard CG, Petersen J, Jørgensen T, et al. Serum ionised calcium and cardiovascular disease in 45-years old men and women followed for 18 years. Eur J Epidemiol 2006;21:123-7. [Crossref] [PubMed]
- Zivin JR, Gooley T, Zager RA, et al. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis 2001;37:689-98. [Crossref] [PubMed]
- Zhang Z, Xu X, Ni H, et al. Predictive Value of Ionized Calcium in Critically Ill Patients: An Analysis of a Large Clinical Database MIMIC II. PLoS One 2014;9:e95204 [Crossref] [PubMed]
- Zaloga GP, Willey S, Tomasic P, et al. Free fatty acids alter calcium binding: a cause for misinterpretation of serum calcium values and hypocalcemia in critical illness. J Clin Endocrinol Metab 1987;64:1010-4. [Crossref] [PubMed]
- Zaloga GP, Chernow B, Cook D, et al. Assessment of calcium homeostasis in the critically ill surgical patient. The diagnostic pitfalls of the McLean-Hastings nomogram. Ann Surg 1985;202:587-94. [Crossref] [PubMed]
- Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J Intensive Care Med 2013;28:166-77. [Crossref] [PubMed]
- Slomp J, van der Voort PHJ, Gerritsen RT, et al. Albumin-adjusted calcium is not suitable for diagnosis of hyper- and hypocalcemia in the critically ill. Crit Care Med 2003;31:1389-93. [Crossref] [PubMed]
- Byrnes MC, Huynh K, Helmer SD, et al. A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Am J Surg 2005;189:310-4. [Crossref] [PubMed]
- Steele T, Kolamunnage-Dona R, Downey C, et al. Assessment and clinical course of hypocalcemia in critical illness. Crit Care 2013;17:R106. [Crossref] [PubMed]
- Cherry RA, Bradburn E, Carney DE, et al. Do early ionized calcium levels really matter in trauma patients? J Trauma 2006;61:774-9. [Crossref] [PubMed]
- Egi M, Kim I, Nichol A, et al. Ionized calcium concentration and outcome in critical illness. Crit Care Med 2011;39:314-21. [Crossref] [PubMed]
- Choi YC, Hwang SY. The value of initial ionized calcium as a predictor of mortality and triage tool in adult trauma patients. J Korean Med Sci 2008;23:700-5. [Crossref] [PubMed]
- Hästbacka J, Pettilä V. Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anaesthesiol Scand 2003;47:1264-9. [Crossref] [PubMed]
- Wang B, Li D, Gong Y, et al. Association of serum total and ionized calcium with all-cause mortality incritically ill patients with acute kidney injury. Clin Chim Acta 2019;494:94-9. [Crossref] [PubMed]
- Link A, Klingele M, Speer T, et al. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit Care 2012;16:R97. [Crossref] [PubMed]
- Bakker AJ, Boerma EC, Keidel H, et al. Detection of citrate overdose in critically ill patients on citrate-anticoagulated venovenous haemofiltration: use of ionised and total/ionised calcium. Clin Chem Lab Med 2006;44:962-6. [Crossref] [PubMed]
- Croton RS, Warren RA, Stott A, et al. Ionized calcium in acute pancreatitis and its relationships with total calcium and serum lipase. Br J Surg 1981;68:241-4. [Crossref] [PubMed]
- Ahmed A, Azim A, Gurjar M, et al. Hypocalcemia in acute pancreatitis revisited. Indian J Crit Care Med 2016;20:173-7. [Crossref] [PubMed]
- Scheinin B, Orko R, Lalla ML, et al. Significance of ionized calcium during liver transplantation. Acta Anaesthesiol Belg 1989;40:101-5. [PubMed]
- Zhang Y-B, Zheng S-F, Yao P-S, et al. Lower Ionized Calcium Predicts Hematoma Expansion and Poor Outcome in Patients with Hypertensive Intracerebral Hemorrhage. World Neurosurg 2018;118:e500-4. [Crossref] [PubMed]
- Sulemanji DS, Bloom JD, Dzik WH, et al. New insights into the effect of rapid transfusion of fresh frozen plasma on ionized calcium. J Clin Anesth 2012;24:364-9. [Crossref] [PubMed]
- Roberts WH, Domen RE, Walters MI. Changes in calcium distribution during therapeutic plasmapheresis. Arch Pathol Lab Med 1984;108:881-3. [PubMed]
- Silberstein LE, Naryshkin S, Haddad JJ, et al. Calcium homeostasis during therapeutic plasma exchange. Transfusion 1986;26:151-5. [Crossref] [PubMed]
- Blomberg Jensen M, Gerner Lawaetz J, Andersson A-M, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod 2016;31:1875-85. [Crossref] [PubMed]
- Seely EW, Wood RJ, Brown EM, et al. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J Clin Endocrinol Metab 1992;74:1436-40. [PubMed]
- Kisters K, Barenbrock M, Louwen F, et al. Membrane, intracellular, and plasma magnesium and calcium concentrations in preeclampsia. Am J Hypertens 2000;13:765-9. [Crossref] [PubMed]
- Payne ME, Pierce CW, McQuoid DR, et al. Serum ionized calcium may be related to white matter lesion volumes in older adults: a pilot study. Nutrients 2013;5:2192-205. [Crossref] [PubMed]
- Kido Y, Okamura T, Tomikawa M, et al. Hypocalcemia associated with 5-fluorouracil and low dose leucovorin in patients with advanced colorectal or gastric carcinomas. Cancer 1996;78:1794-7. [Crossref] [PubMed]
- Oronsky B, Caroen S, Oronsky A, et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol 2017;80:895-907. [Crossref] [PubMed]
- Do WS, Park J-K, Park M-I, et al. Bisphosphonate-induced Severe Hypocalcemia - A Case Report -. J Bone Metab 2012;19:139. [Crossref] [PubMed]
- Verhoeven Y, Kemperman H, Abrahams AC. Falsely decreased ionized calcium levels in kidney transplant recipients with polyomavirus-associated nephropathy treated with leflunomide. Transpl Int 2015;28:874-5. [Crossref] [PubMed]
- Hubeek I, Abrahams AC, de Bruin M, et al. Falsely decreased ionized calcium results due to analytical interference by teriflunomide, the active metabolite of leflunomide (Arava®). Clin Chem Lab Med 2012;50:755-6. [Crossref] [PubMed]
- Newman DB, Siontis KC, Chandrasekaran K, et al. Intervention to Reduce Inappropriate Ionized Calcium Ordering Practices: A Quality-Improvement Project. Perm J 2015;19:49-51. [Crossref] [PubMed]
Cite this article as: Hamroun A, Pekar JD, Lionet A, Ghulam A, Maboudou P, Mercier A, Brousseau T, Grzych G, Glowacki F. Ionized calcium: analytical challenges and clinical relevance. J Lab Precis Med 2020;5:22.