
Aspartate aminotransferase and cardiovascular disease—a narrative review
Historic perspective
Aminotransferases (also called transaminases) are ubiquitous pyridoxal-5’-phosphate-dependent enzymes that catalyze reversible transfer of amino group from amino acids to α-keto acids. These enzymes play a key role in the metabolism of amino acids in all species. Transamination reaction was discovered in muscle tissue in 1937 by Braunstein and Kritzmann (1). Subsequent studies by Braunstein and Kritzmann (2), Cohen (3,4) and Green et al. (5) characterized the transaminase reaction and identified 2 aminotransferases as most metabolically active - aspartate aminotransferase [AST; EC 2.6.1.1; also known as serum glutamic oxaloacetic transaminase (SGOT or GOT)] and alanine aminotransferase [ALT; EC 2.6.1.2; also known as serum glutamic pyruvic transaminase (SGPT or GPT)]. In early 1950s various methods for aminotransferase measurements were developed and indications for diagnostic testing (mostly for liver disease) were introduced (6,7). In 1951 Cammarata and Cohen (8) introduced a spectrophotometric method for studying the kinetics of aminotransferase reactions. In 1954, Karmen et al. (9) demonstrated the presence of AST and ALT in serum and blood using paper chromatography. However, the method was impractical and extremely time-consuming (10). In the same year, Karmen (11) developed an accurate and simple spectrophotometric method that was used by Ladue et al. to demonstrate for the first time an increase in AST activity in the serum of patients with acute myocardial infarction (12). Historically, this represents the first biochemical test used for the diagnosis of acute myocardial infarction. The coupled enzyme reaction for AST measurement developed by Karmen was further improved by Henry et al. (13) and standardized and adapted for use on many automatic analyzers (14). The method served as a basis for many modern assays. The AST measurement was used for decades for the diagnosis of acute myocardial infarction until it was substituted by more sensitive diagnostic tests such as enzymes [lactate dehydrogenase, creatine kinase (total and myocardial band isoenzyme)] and more recently cardiac troponins. Although ALT and AST are commonly assayed together, the enzymes differ with respect to protein structure and gene location, kinetic characteristics and substrate specificity, tissue distribution and specificity. It is believed that ALT is more specific for liver disease whereas AST is more specific for myocardium. AST and ALT may also differ with respect to the pattern of association with cardiovascular disease (CVD). In this narrative review, we aimed to summarize the existing knowledge on the association between AST and CVD. The AST to ALT ratio and the association of AST with other diseases (inflammatory liver disease) are not covered. We present the following article in accordance with the Narrative Review Checklist (available at http://dx.doi.org/10.21037/jlpm-20-93).
AST structure, function and circulating levels
AST structure is similar across various species. In humans, AST exists as two genetically and immunologically distinct isoenzymes: cytoplasmic AST (cAST or GOT1) and mitochondrial AST (mAST or GOT2) (15). Both isoenzymes catalyze the same reaction albeit with different kinetics, share a sequence homology of ~45% and are thought to have evolved from a common ancestral gene (via gene duplication) (16). The enzyme consists of two identical dimers where each dimer consists of a large and a small domain (17). Each monomer of cytoplasmic AST represents a polypeptide chain of 413 amino acid residues with a secondary structure consisting of α-helices and β-strands and a molecular weight of approximately 45 kD (18,19). Each dimer has an identical binding site for pyridoxal-5'-phosphate which is located in the dimer interface (Figure 1). Pyridoxal-5'-phosphate is stabilized by a number of surrounding amino acid residues. The human mitochondrial preAST contains 430 amino acid residues (deduced from nucleotide sequence analysis of cDNAs) including N-terminal 29-amino acid sequence which is required for enzyme entry in mitochondria (20-22). Cytoplasmic AST is more acidic (isoelectric point ~5.5) compared with mitochondrial AST (isoelectric point >8) (23,24). While mitochondrial AST exists as a single form, cytoplasmic AST exists in 3 to 5 isoforms detected by isoelectric focusing (15). In the liver, 80% of AST activity is found in mitochondria and 20% in cytoplasm (6). Mitochondrial AST accounts for about 65% of the total AST activity in human cardiac tissue (25). AST protein is cleared from the circulation predominantly by sinusoid cells in the liver (26) The mean plasma half-life is 17 hours for total AST and 87 hours for mitochondrial AST (27). AST activity was detected across almost all human tissues. Distribution of AST activity in human tissues expressed as a ratio [in parentheses] to the activity found in serum is as follows: heart [7,700], liver [7,000], skeletal muscle [4,800], kidney [4,500], brain [2,500], pancreas [1,400], spleen [700], lung [500] and erythrocytes [40] (6).
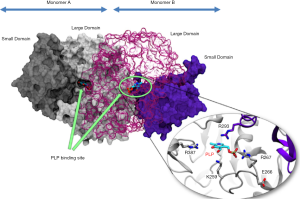
Studies in tweens have suggested that serum AST activity is influenced by both genetic and environmental factors (28,29). A study in tweens by Whitfield et al. (28). showed that after adjusting for the effect of age and sex, AST activity was heritable with additive genetic effects accounting for 32% variation in the AST activity. Another study in tweens showed that additive genetic effects accounted for 40% in the variation of AST activity (29). It was also suggested that 28% of the AST variance could be explained by additive genetic factors, 15% by non-additive genetic factors and 57% by non-shared environmental factors (for both men and women) (30). The cytosolic AST gene is localized on chromosome 10 at the interface of bands q241-q251 and it has 9 exons. Mitochondrial AST is characterized by a multigene family located on chromosomes 12 (p131-p132), 16 (q21), and 1 (p32-p33 and q25-q31) (31). However, only gene located on chromosome 16 is functional whereas genes found in other locations are pseudogenes with unknown functions (31). The gene located on chromosome 16 has 10 exons. Messenger RNA for mitochondrial AST is translated on free ribosomes and the pre-protein product is transported in mitochondria via N-terminal leader sequence (and other regions), which is thereafter cleaved by a specific endopeptidase in mitochondrial matrix to produce mature enzyme (32). Although, regulation of expression of AST genes remains largely unknown, expression of hepatic cytoplasmic AST is under hormonal control. Glucocorticoid hormones induce AST gene expression at transcriptional level and the effect is potentiated by cAMP and inhibited by insulin (33). However, the expression of AST gene is tissue specific.
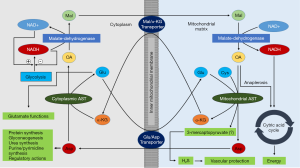
AST has multiple metabolic functions (Figure 2). First, the most important metabolic function of AST is maintenance of the nicotinamide adenine dinucleotide/reduced nicotinamide adenine dinucleotide (NAD+/NADH) ratio in cells. This function is realized by AST participation in the malate-aspartate shuttle—a closed cyclic pathway that enables transfer of NADH from cytoplasm to mitochondria which is oxidized by mitochondrial respiratory chain. Since the inner mitochondrial membrane is impermeable to NADH (34), reducing energy of this agent is transferred in mitochondria via substrates (shuttle) (35). In the malate-aspartate shuttle, cytoplasmic and mitochondrial AST cooperate with cytoplasmic and mitochondrial malate dehydrogenase mediating net transport of NADH from cytoplasm to mitochondria (Figure 2). Second, the AST reaction product—aspartate—is a highly active amino acid participating in numerous metabolic functions including synthesis of purine and pyrimidine bases, urea synthesis, protein synthesis and gluconeogenesis. Third, α-keto acids produced by AST reaction—alpha-ketoglutarate and oxaloacetate increase the amount of Krebs cycle intermediates (anaplerotic action) contributing to maintenance of oxidative capacity of the cells and participate in gluconeogenesis depending on the metabolic needs of the cells. AST has also other less well-known physiological functions including participation in the glyceroneogenesis (synthesis of glycerol) (36) and serving as a translocase for trans-membrane fatty acid transport (37). Mitochondrial AST was found in plasma membranes and suggested to participate in the trans-membrane transport of fatty acids (38,39). It has been suggested that cytoplasmic AST and the vesicular glutamate transporters (VGLUT) play a role in the β-cell glutamate signaling for incretin-induced insulin secretion (40). Since cysteine aminotransferase (CAT) is identical with cytosolic AST (41), AST may contribute to production of hydrogen sulfide (H2S) via CAT/3-mercaptopyruvate sulfurtransferase pathway (42). CAT has been found in vascular endothelium of the thoracic aorta and lysates of vascular endothelial cells produced H2S from cysteine and α-ketoglutarate (43). The CAT/3-mercaptopyruvate sulfurtransferase pathway exists in cytoplasm and mitochondria (44). H2S is a signaling molecule with an important role in vascular biology exerting a vascular protection action.
Circulating levels of AST are influenced by multiple factors. Four different mechanisms have been postulated to explain the elevation of AST (and ALT) in circulation (7). First, direct tissue damage (plasma membrane damage with protein leakage or cell necrosis caused by various noxious agents or stimuli) or apoptosis (in conditions of physiological cellular renewal or increased apoptotic stimuli) is the most common cause of increased activity of aminotransferases (including AST) in circulation. Reasonably, large organs with highest AST activity contribute mostly to AST elevation in circulation. Elevated AST levels are commonly related to inflammatory liver disease (viral hepatitis), alcoholic liver disease, cirrhosis, cholestatic syndromes, drug toxicity, acute myocardial infarction, septic shock and skeletal muscle injury/trauma. Severe myocardial ischemia or myocardial cell necrosis occurring in the setting of acute myocardial infarction is a common cause of increased serum AST activity. However, a poor correlation between liver cell damage and plasma amino transferases has been shown (45). Moreover, mild cell damage releases the enzymes in the soluble (cytoplasmic) fraction only whereas severe necrotic lesions release enzymes from cytoplasm and mitochondria (46). Second, plasma membrane blebs (following decoupling of plasma membrane from the cytoskeleton) that bud off from the cell membranes releasing confined cytoplasmic content (including AST) are formed in conditions of increased stress on cells. Membrane blebs have been demonstrated during ischemia-reperfusion injury in liver (47) and myocardium (48). Third, increased AST gene expression may contribute to increased AST activity levels in circulation. Experiments in mice treated with carbon tetrachloride suggested that increased AST gene expression leads to increased AST levels in circulation (49). Furthermore, peroxisome-proliferator-activated receptor-α (PPARα) agonists like fenofibrate in mice (50), antidiabetic drug and PPARγ agonist rosiglitazone in adipocytes (36) and up-regulation of the IRE1α/c-Jun signaling pathway (51) contribute to increased synthesis and release of cytoplasmic AST (and ALT). Although microsomal enzyme-inducing drugs, including alcohol increase circulating levels of aminotransferases (including AST), whether this occurs as a result of increased expression remains unclear. Exposure of cultured HepG2 hepatoma cells to 24-exposure to 0, 20, 40, or 80 mmol/L ethanol was associated with a 13-fold increase of messenger RNA for mitochondrial AST without any significant change in the cellular AST content (37). Moreover, an increase in messenger RNA for mitochondrial AST without change in cellular content of the enzyme has been reported in liver biopsies from patients with alcohol-related liver disease (52). Fourth, macroenzymes (macroAST)—high-molecular-weight compounds that are formed by polymerization or association of enzymes with other serum proteins (typically with immunoglobulins)—represent another mechanism of asymptomatic enzyme elevation linked with prolonged clearance of AST from the serum (53). Macroenzyme formation may involve autoimmunity with immunoglobulins targeting enzymes as antigens via molecular mimicry (54). Macro-AST is a type I macroenzyme with a molecular weight of ~250 kDa that is produced by association of AST with immunoglobulins A or G (or both) (55). One study has suggested that ~13% of cases with isolated AST elevation (i.e., without concomitant ALT elevation) are due to macro-AST (56).
The frequency and metabolic consequences of hereditary deficiency of AST are largely unknown. A genome-wide association study of AST activity in 866 Amish participants showed a significant association of AST activity with a cluster of single nucleotide polymorphisms located on chromosome 10q24.1. Sequencing of GOT1 gene revealed an in-frame deletion of three nucleotides encoding asparagine at position 389 in the GOT1 gene. Only heterozygote carriers of this deletion were found. The deletion carriers had serum AST activity levels that were approximately one-half that of normal homozygotes (10.0±2.8 versus 18.8±5.2 U/L) suggesting that the deletion resulted in a complete loss of enzymatic function of cytoplasmic AST. However, no association between the deletion and metabolic traits such as serum fasting glucose or insulin, fasting and post-meal lipids, inflammatory markers, or sub-clinical markers of CVD were observed (57). The authors commented that AST is not a rate-limiting step in intermediary metabolism and that a single functional allele is sufficient for normal metabolic function. Acquired low AST levels are relatively rare and may be related to vitamin B6 deficiency occurring in elderly, alcoholics or people with liver, kidney or inflammatory conditions (58). Low serum AST activity is seen in patients with uremia (59,60). and is attributed to various factors (discussed later in this review). Hormone replacement therapy decreases AST levels (61).
Epidemiological evidence for an association between AST and CVD and mortality
Following the first report of increased AST activity in patients with acute myocardial infarction (12), measurement of serum AST was routinely used for the diagnosis of this disease. Numerous studies over the subsequent 2 decades investigated the AST value for the diagnosis, quantification of ischemic damage (necrosis) and risk stratification of patients with acute myocardial infarction. In acute myocardial infarction, AST start raising 6 to 8 hours after the symptom onset, reaches the peak level at 24 to 36 hours and returns to normal in 3 to 7 days. Reperfusion by thrombolysis or balloon angioplasty shortens the time to AST peak value. The widespread distribution of AST across human tissues (particularly in liver and skeletal muscle) and relatively late increase in serum activity following coronary occlusion are disadvantages of this test with respect to the diagnosis of myocardial infarction. Since more sensitive biomarkers of myocardial ischemia/necrosis became available, AST is no longer used for the diagnosis of acute myocardial infarction. Although AST activity is higher in myocardium compared with ALT or gamma-glutamyl transferase (GGT), AST has drawn less epidemiological interest with respect to the association with CVD compared with other enzymes. In this narrative review, the existing epidemiological evidence will be assessed without time or type of the study restriction. AST was mostly investigated in the setting of the association of liver enzymes with CVD. As a consequence, evidence on the association between AST and CVD remains limited (Table 1).
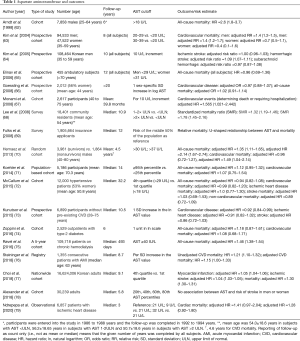
Full table
In 1998, Arndt et al. (62) investigated the association between liver enzymes (GGT, ALT and AST) and all-cause mortality or vocational disability in male construction workers undergoing occupational health examinations in southern Germany from 1986 to 1988. Over approximately 6 years of follow-up, subjects with an AST level >18 U/L (11.9% of the participants) had a two-fold risk of early retirement and a 2.6-fold higher risk of mortality compared with subjects with lower AST values after adjustment for age, nationality, occupational group, smoking, body mass index and alcohol consumption. A large prospective cohort Korean study comprising 94,533 men and 47,522 women aged 35–59 years showed a strong association between AST activity and mortality from all-causes, CVD, cancer, liver and digestive system. In men, for AST values 20–29, 30–39, 40–49, 50–99 and ≥100 U/L, the risk ratios for the association between AST and all-cause (CVD-related) mortality were: 1.3 (1.4), 1.7 (1.4), 2.7 (1.8), 5.4 (2.6) and 8.6 (3.3), respectively, compared with AST values <20 U/L. The risk ratios were adjusted for age, body mass index, smoking status, alcohol consumption, plasma glucose, total cholesterol, blood pressure and family history of liver disease. In women, the association was less evident. Only AST values exceeding 50 U/L were associated with all-cause mortality [adjusted risk ratio =2.9 (1.4 to 5.8)] whereas no association was found with CVD-related mortality (due to limited CVD-related deaths in women) (63). Another study in Korean population investigated the association between aminotransferases and the risk for ischemic stroke, hemorrhagic stroke, subarachnoid hemorrhage and intracerebral hemorrhage in men over 10 years of follow-up. After adjustment for age, body mass index, blood pressure, fasting glucose, total cholesterol, smoking and alcohol consumption AST remained associated with the risk for hemorrhagic stroke but not with the risk for ischemic stroke or subarachnoid hemorrhage (64). A small study in ambulatory subjects >70 years did not find an association between AST and all-cause mortality over 12 years of follow-up (65).
A number of studies published in 2008 assessed the association between AST and mortality or other outcomes. The Framingham Offspring Heart Study did not show an association between AST and all-cause or CVD-related mortality over 20 years of follow-up in the overall sample or subjects with AST within normal range after adjustment for age, sex, systolic blood pressure, antihypertensive and lipid-lowering drug therapy, smoking, body mass index, diabetes mellitus, total and high-density lipoprotein cholesterol and alcohol use. However, AST correlated with the risk for developing diabetes in overall sample [adjusted OR =1.33 (1.17–1.53)] and subjects with AST within normal range [adjusted OR =1.24 (1.04–1.48), both calculated for one sex-specific standard deviation (SD) increase in the log AST value] (66). The Firenze Bagno a Ripoli (FIBAR) study assessed the association between liver enzymes (GGT, ALT and AST) in subjects free of diabetes or CVD and without liver disease except for nonalcoholic fatty liver disease (NAFLD). After adjustment for age, sex, alcohol, smoking and fasting plasma glucose, AST remained associated with the risk for incident CVD but not diabetes [adjusted HR =0.990 (0.722–1.356)]. An AST value exceeding the 40 U/L cutoff was associated with the risk of CVD with an age and sex-adjusted HR of 6.52 (1.51–28.12) (67). A population-based epidemiological study conducted in Olmsted County, Minnesota showed that abnormal values of AST were associated with reduced survival (significantly increased standardized mortality ratio) with a dose response relationship. Subjects in whom AST was not assayed and those with normal AST values had a better survival than expected [standardized mortality ratio of 0.79 (0.75–0.83) and 0.95 (0.91–0.98), respectively] (68). The association between liver enzymes and the risk of mortality was assessed also in a study of >1.9 million insurance applicants between 1993 and 1997. There were 50,174 deaths over a median follow-up of 12 years. Using the risk of the middle 50% of the population by distribution as a reference, relative mortality was increased at lower and higher AST levels demonstrating a U-shaped relationship between AST and mortality (69).
A 2013 study assessed the association between liver enzymes and mortality (from all-causes, CVD, cancer and hepatocellular carcinoma) in survivors (3,961 subjects) and nonsurvivors (n=1,864 subjects) selected from 54,751 Taiwanese males 40–80 years of age free of cancer at entry over 5.8±2.5 years of follow-up. Continuous AST (entered as natural logarithm of AST values) was not associated with all-cause or CVD mortality. However, an AST level >37 U/L was associated with the risk of all-cause and CVD mortality in the hepatitis B surface antigen-negative subjects after adjustment for age, body mass index, smoking, alcohol consumption, education, physical activity, diabetes, total cholesterol, high-density lipoprotein, systolic and diastolic blood pressure and C-reactive protein (70). A 2014 study embedded in the Rotterdam Study—a large prospective population-based cohort—assessed the association of liver enzymes with all-cause and cause-specific mortality in subjects ≥55 years of age up to 19.5 years of follow-up. Subjects with an AST between >25th percentile and <95th percentile had a lower all-cause mortality risk [adjusted HR =0.85 (0.77–0.95), adjusted HR =0.76 (0.69–0.85) and adjusted HR =0.81 (0.72–0.90) for AST >25th to 50th percentile, AST >50th to 75th percentile and AST >75th to 95th percentile, respectively] compared with ASP <25th percentile. The association of AST with CVD mortality was not significant for any of the comparisons. In this study the association between AST and all-cause mortality was U-shaped. The risk estimates were adjusted for age, sex, education, smoking status, alcohol intake, hypertension, diabetes mellitus, body mass index and total cholesterol (71). In one study of 12,000 hypertensive patients who were followed up for 35 years, AST was not associated independently with mortality from all causes, CVD (or non-CVD) or stroke after adjustment for age, sex, body mass index, smoking, alcohol use, baseline and final achieved values of systolic and diastolic blood pressure, year of first visit, cholesterol, diabetes and liver tests (except for ALT) (72). Of interest was the finding that AST showed a U-shaped relationship with mortality in distinction from ALT, which showed a linear relationship. Finally, adding AST in a multivariable model alongside baseline demographic and clinical variables did not improve discrimination power of the model for mortality at 35 or 20 years of follow-up. However, AST improved risk discrimination for mortality at 10 years of follow-up (72). The PREVEND prospective cohort study that included 6,899 participants 28–75 year of age without pre-existing CVD reported 729 CVD events during 10.5 years of follow-up. AST was approximately log-linearly associated with CVD risk. However, adding AST to the CVD risk prediction model containing established risk factors did not improve the C-index or net reclassification regarding mortality (73). A cohort study of 2,529 outpatients with diabetes mellitus followed up for 6 years did not show an association between AST and all-cause or CVD mortality, either unadjusted or after adjustment for age, sex and body mass index. However, the AST to ALT ratio was strongly associated with both outcomes (74). A large cohort study of patients studied in dialysis clinics in the US showed a linear association between serum AST and mortality. For AST levels exceeding 20 U/L, AST was incrementally and almost linearly associated with the risk of mortality at all levels of adjustment. In fully adjusted models, an AST ≥40 IU/L was associated with 46% higher adjusted hazard for mortality. Patients with AST level between 15 and 20 U/L had the best survival (75). A recent registry of patients with acute myocardial infarction showed an association between AST and CVD mortality which was attenuated to the level of borderline statistical significance after adjustment and a strong association between the AST to ALT ratio with this outcome (CVD mortality) (76). The largest study to date—a population-based, nationwide cohort database of >16 million Korean adults—showed a significant association between AST and the risk of myocardial infarction, ischemic stroke or mortality over a median follow-up of 9.1 years. Thus calculated for AST quartile 4 versus quartile 1, the risk for myocardial infarction, ischemic stroke or mortality increased by 5%, 4% and 30%, respectively, after adjusting for age, sex, body mass index, smoking, alcohol, exercise, diabetes, hypertension, and dyslipidemia (77). AST showed a U-shaped relationship with mortality. In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort of 30,239 American adults, AST was not associated with the risk of ischemic stroke in either sex (78). Finally, our group assessed the association between AST and all-cause and CVD mortality in patients with confirmed ischemic heart disease over a 3-year follow-up. In univariable analysis AST was associated with all-cause and CVD mortality. After adjustment, the association was attenuated for both CVD and all-cause mortality. The association with CVD mortality was U-shaped with lowest mortality observed for AST value between 21.1 and 23.1 U/L. In this study, body mass index, hypercholesterolemia, acute coronary syndrome, extent of coronary atherosclerosis, glomerular filtration rate, fasting glucose and high-density lipoprotein correlated positively with the AST levels. Conversely, diabetes mellitus, history of myocardial infarction, C-reactive protein, low-density lipoprotein and left ventricular ejection fraction correlated significantly but inversely with AST level (79).
Sustainability of the AST elevation seems also to affect mortality. A recent study of subjects without cancer, CVD, CVD risk factors or liver disease participating in the national health screening in Korea who had 2 measurements of aminotransferases (n=68,431 men) showed that only subjects having persistently high AST levels had higher mortality over 8 to 9 years of follow-up. Thus, only men who had sustained AST elevation [adjusted HR =1.95 (1.07–3.56)] but not those with AST normalization [adjusted HR =1.52 (0.82–2.81)] or new elevation [adjusted HR =1.27 (0.66–2.44)] had significantly higher CVD mortality compared with subjects with sustained normal AST levels (80).
Meta-analyses of epidemiological studies assessing the association between AST and mortality or CVD have been inconclusive. One meta-analysis of four epidemiological studies reported an insignificant 23% increase [RR =1.23 (0.80–1.88)] in the risk for all-cause mortality in subjects with AST in the top compared with the bottom third of levels (81). The cubic spline model that included two studies indicated a non-linear (J-shaped) association between AST and all-cause mortality. Another more recent meta-analysis including four studies showed that AST (highest versus lowest categories) was not significantly associated with the increased risk of CVD mortality [HR =1.20 (0.83−1.73)]. However, heterogeneity across the studies was significant (P for heterogeneity =0.024) (82).
In aggregate, evidence on the association between AST and CVD or mortality remains controversial with positive, neutral, inverse or U (or J)-shaped relationships between AST and CVD or mortality having been reported. Although, a positive association between AST and CVD was more common in epidemiological studies in Asian population whether there are geographic or racial differences in the association between AST and CVD remains unknown.
Mechanisms of association of AST with CVD
The underlying mechanisms of the association between AST and the risk for CVD or mortality are mostly hypothetical. It is important to note that there is no conclusive evidence that AST activity in serum parallels AST activity within the cells. Although, transamination is a fundamental reaction in the metabolism, a direct link between metabolic derangement associated with high or low AST levels and CVD remains largely unexplored. Furthermore, there are no known specific physiological function of AST in circulation outside the function as constituent of plasma proteins. Thus in order to explain the risk associated with AST, attention should be focused on underlying morbid conditions that are associated with high or low AST levels. For ease of presentation, putative mechanisms linking high and low AST levels with CVD or mortality are analyzed separately.
An association between elevated AST level and the risk for CVD may be explained by at least four mechanisms. First, the most common cause of AST elevation in circulation is liver disease. The most relevant liver disease with respect to the association with CVD is NAFLD. NAFLD is a progressive liver disease occurring in the absence of excessive alcohol consumption that is characterized by intrahepatic triglyceride accumulation and inflammation and hepatocyte injury progressing gradually to liver fibrosis, cirrhosis and hepatocellular carcinoma (83). NAFLD is the most common chronic liver diseases, affecting approximately 25% of adult European population (84,85). CVD is the most common cause of death in patients with NAFLD (86). Large epidemiological studies have demonstrated that NAFLD is associated with prevalent CVD even after adjustment for most important demographic, clinical and metabolic confounders (87,88). Numerous studies have reported an association between NAFLD and various aspects of CVD including coronary artery disease, carotid artery intima-media thickness, carotid artery atherosclerotic plaques, increased arterial wall stiffness, impaired endothelium-dependent flow-mediated vasodilatation and coronary artery calcium, left ventricular diastolic dysfunction, impaired coronary blood flow, impaired myocardial energetics, left ventricular hypertrophy, increased oxidative stress, valvular heart disease (aortic valve sclerosis and mitral annulus calcification), excessive epicardial fat deposition, accelerated atherosclerosis and cardiac arrhythmia (83,86,89). However, the association of NAFLD with atherosclerosis and its manifestations is clinically most relevant. Mechanistically, the association of NAFLD with CVD may be explained by close association between NAFLD and traditional (metabolic syndrome, abdominal obesity, hypertension, dyslipidemia and insulin resistance/dysglycemia syndrome) and nontraditional (hyperuricaemia, hypoadiponectinemia and hypovitaminosis D) cardiovascular risk factors. Moreover, NAFLD is associated with pro-inflammatory and procoagulant states related to increased synthesis of various pro-inflammatory and procoagulant factors, likely promoted by non-alcoholic steatohepatitis component of NAFLD (83,86,89). Reasonably, this proatherogenic and dysfunctional milieu offers a pathophysiological basis for an association between NAFLD and increased risk for CVD. A recent study by Sookoian et al. (90) reported a significant correlation between systolic blood pressure and GOT2 messenger RNA in liver cells and a significant association between the rs6993 polymorphism located in the 3’ untranslated region of the GOT2 locus and features of metabolic syndrome. The authors suggested that fatty liver is associated with a deregulated liver expression of aminotransferases and that serum aminotransferase levels are a signature of liver metabolic perturbations, particularly at the level of amino acid metabolism and Krebs cycle. They advanced the hypothesis that increased levels of circulating aminotransferases reflect an increase in hepatic expression of aminotransferases and rates of transamination reactions to compensate for liver metabolic derangement associated with greater gluconeogenesis and insulin resistance (90). A recent Genome-Wide Association Study (GWAS) of liver enzymes provided evidence that PNPLA3 gene is associated with liver enzymes (ALT and AST) and NAFLD. The PNPLA3 gene is located on chromosome 22. It is expressed in adipocytes and hepatocytes and its protein product is involved in triacylglycerol hydrolysis in adipocytes (91).
Second, an elevated AST level is associated with cardiovascular risk factors, particularly, metabolic syndrome, abdominal obesity, insulin resistance and diabetes. In the Framingham Heart Study, elevated liver enzymes (ALT and AST) were associated with increased risk for arterial hypertension, diabetes, metabolic syndrome, impaired fasting glucose and insulin resistance estimated by HOMA-IR. The associations between liver enzymes and metabolic disorders persisted after adjustment for visceral fat and insulin resistance. However, the association was stronger for ALT than AST (92). In the Framingham Offspring Heart Study, AST was associated with the risk for developing incident diabetes, but not metabolic syndrome, over >20 years of follow-up (66). Conversely, in the FIBAR study AST was not associated with the risk of diabetes over nearly 40 months of follow-up (67). A recent study in adult Koreans reported a significant association between AST and metabolic syndrome and its components. Thus, subjects with an elevated AST level had a significantly higher prevalence of metabolic syndrome [adjusted OR =3.81 (3.10–4.74)] and its components (abdominal obesity, high blood pressure, triglyceride and glucose levels) (93). Another recent study in obese adolescents showed a strong association between increased body mass index, adolescent age, male sex, liver echogenicity and liver enzymes (AST and ALT). There was a significant correlation between AST (or ALT) >50 U/L and impaired glucose metabolism and between AST (or ALT) >25 U/L and arterial hypertension (94). In summary, ample evidence suggests an association between elevated liver enzymes (including AST) and cardiometabolic risk. However, whether this association is independent of NAFLD remains unknown. In general, studies in Asian population reported positive associations between AST and metabolic syndrome more often compared with studies in other populations.
Third, elevated AST may reflect chronic alcoholism. A thorough discussion of cardiovascular effects of alcohol is outside the scope of this review. Although it has been repeatedly reported that moderate alcohol consumption may have salutary effects on coronary arteries (and consequently on the risk for coronary artery disease) any amount of alcohol is toxic to myocardium. Chronic alcohol consumption is associated with increased oxidative stress, lipid peroxidation, acetaldehyde toxicity and increased local (hepatic and myocardial) and systemic inflammation (particularly increased expression of tumor-necrosis factor alpha, and inflammatory cytokines) (95,96). Via these and other mechanisms chronic alcohol consumption promotes apoptosis and hepatic or myocardial cellular damage leading to increased AST levels in circulation. While moderate alcohol consumption may reduce the risk for coronary artery disease and related mortality (97) chronic alcohol consumption (particularly chronic heavy drinking in subjects with alcohol use disorder) is associated with increased risk for acute myocardial infarction (98) and coronary artery disease (99). Thus, an elevated AST level may serve as a marker of risk induced by myocardial or hepatic cellular damage occurring in the setting of chronic alcoholism.
Fourth, elevated AST level may be a marker of structural CVD. Release of AST from myocardium in conditions of increased stress (such as stress imposed by cardiovascular risk factors) or acute ischemia/necrosis (acute coronary syndromes) or reperfusion-related injury may also explain the increased cardiovascular risk associated with higher AST levels. Historically, AST was the first biochemical test used for diagnosis of acute myocardial infarction (12) and the biomarker was used in clinical setting for diagnosis of this condition until substituted by more sensitive tests. Since AST has the highest activity in myocardium, myocardial necrosis leads to considerable increase in AST in serum. In patients with acute myocardial infarction peak AST correlates closely with creatine kinase myocardial band (CK-MB) area under the curve (100), congestive heart failure assessed by Killip’s classification (101) and acute hypoxic liver injury (102). The Atherosclerosis Risk in the Communities (ARIC) study showed that higher levels of AST, even within the normal range, were significantly and independently associated with detectable (>3 ng/L) or elevated (≥14 ng/L) concentrations of high-sensitivity cardiac troponin T (103). AST is also increased in patients with congestive heart failure, likely due to liver injury caused by reduced forward blood flow to liver or passive backward congestion occurring in the setting of reduced cardiac output (104). However nonhemodynamic factors may play a role. As elegantly demonstrated by Killip et al. (105) very high AST activity found in patients with congestive heart failure or cardiogenic shock may be caused by acute hepatic central necrosis secondary to a drop in cardiac output and reduced hepatic blood flow. Thus, cardiovascular risk associated with elevated AST in these conditions reflects underlying structural CVD.
In aggregate, an elevated AST level not occurring in the setting of inflammatory liver disease may signify an increased cardiovascular risk related to NAFLD, cardiometabolic risk factors, chronic alcoholism or structural heart disease.
The association between low AST and increased risk of CVD or mortality has been demonstrated but is hardly explainable. Considering how the reference range for AST is defined, a small proportion of healthy population may have AST levels lower than the lower limit of reference range meaning that low AST levels are uncommon in the general population. Low AST levels may be due to vitamin B6 deficiency (58). Low plasma pyridoxal 5’-phosphate (active vitamin B6) is associated with the risk of CVD (106). In addition, low plasma pyridoxal 5’-phosphate is associated with elevated alkaline phosphatase (58) which correlates with cardiovascular risk (107). In clinical setting, vitamin B6 deficiency is more likely to be found in elderly subjects and patients with advanced kidney, liver and inflammatory diseases (58). Lower AST levels have been reported in patients with chronic kidney disease which became lower with the increase in disease severity even before reaching the end stage renal disease (59). In one study of patients with predialysis chronic kidney disease AST levels decreased in proportion to the progression of the disease and correlated negatively with serum creatinine and positively with glomerular filtration rate (60). Low AST levels in patients with chronic kidney disease are explained by vitamin B6 deficiency, hemodilution, and hyperhomocysteinemia (108-110). Other factors such as a high level of uremic toxins, decreased synthesis and inhibition of release of AST from hepatic cells and accelerated clearance from the serum (59) or presence of inhibitory substances which are removable by dialysis (111) have been suggested. Since chronic kidney disease is strongly associated with CVD, accelerated atherosclerosis and CVD-related mortality, a low AST level occurring in the setting of chronic kidney disease may reflect metabolic disarrangements and increased CVD risk associated with this syndrome. Finally, if AST and ALT share common factors underlying their low activities as recently suggested (112), then putative factors underlying low ALT levels may also underlie low AST levels and association with CVD.
Concluding remarks
This narrative review summarized the existing knowledge on the association between AST and CVD. AST activity in serum is routinely assayed and used for the assessment of liver disease. In addition, AST is a reliable marker of general health and high and low levels of this biomarker have clinical meaning. In this regard, abnormal values of AST activity in serum not occurring in the setting of clinically overt inflammatory liver disease may be a harbinger of health problems that need to be investigated. An elevated AST level (outside inflammatory liver disease) may indicate an increased CVD risk. The increased risk for CVD and related mortality may be explained by association of elevated AST with an array of conditions such as, NAFLD, cardiometabolic risk factors, chronic alcoholism or structural heart disease. Although epidemiological studies support an association between an elevated AST and increased cardiovascular risk, the evidence remains limited and not entirely assertive. Low AST levels may also indicate an increased risk for CVD related to advanced liver disease, vitamin B6 deficiency, kidney disease with chronic uremia or inflammatory diseases. Future dedicated epidemiological, clinical, experimental and genetic studies are needed to better clarify the association of AST with CVD and explain the underlying mechanisms.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review Checklist reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-20-93
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-93). Gjin Ndrepepa serves as an unpaid editorial board member of Journal of Laboratory and Precision Medicine from July 2020 to July 2022. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braunstein AE, Kritzmann MG. Decomposition and synthesis of amino acids by conversion of amines; studies on muscle tissue. Enzymologia 1937;2:129-46.
- Braunstein AE, Kritzmann MG. On the specifity range of the process of amino nitrogen transfer. IV. communication on the formation and breakdown of amino-acids by interrnolekular transfer of amino groups. Biokhymiya 1938;3:590-602.
- Cohen PP. Transamination in pigeon breast muscle. Biochem J 1939;33:1478-87. [Crossref] [PubMed]
- Cohen pp. Kinetics of transaminase activity. J Biol Chem 1940;136:585-601. [Crossref]
- Green DE, Leloir LF, Nocito V. Transaminases. J Biol Chem 1945;161:559-82. [Crossref] [PubMed]
- Rej R. Aminotransferases in disease. Clin Lab Med 1989;9:667-87. [Crossref] [PubMed]
- McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J 2016;15:817-28. [PubMed]
- Cammarata PS, Cohen PP. Spectrophotometric measurement of transamination reactions. J Biol Chem 1951;193:45-52. [Crossref] [PubMed]
- Karmen A, Wroblewski F, Ladue JS. Transaminase Activity in Human Blood. J Clin Invest 1955;34:126-31. [Crossref] [PubMed]
- Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med 2016;4:194. [Crossref] [PubMed]
- Karmen A. A note on the spectrophotometric assay of glutamic-oxaloacetic transaminase in human blood serum. J Clin Invest 1955;34:131-3. [PubMed]
- Ladue JS, Wroblewski F, Karmen A. Serum Glutamic Oxaloacetic Transaminase Activity in Human Acute Transmural Myocardial Infarction. Science 1954;120:497-9. [Crossref] [PubMed]
- Henry RJ, Chiamori N, Golub OJ, et al. Revised Spectrophotometric Methods for the Determination of Glutamic-Oxalacetic Transaminase, Glutamic-Pyruvic Transaminase, and Lactic Acid Dehydrogenase. Am J Clin Pathol 1960;34:381-98. [Crossref] [PubMed]
- Wilkinson JH, Baron DN, Moss DW, et al. Standardization of Clinical Enzyme Assays - a Reference Method for Aspartate and Alanine Transaminases. J Clin Pathol 1972;25:940-4. [Crossref] [PubMed]
- Panteghini M. Aspartate-Aminotransferase Isoenzymes. Clin Biochem 1990;23:311-9. [Crossref] [PubMed]
- Hayashi H, Wada H, Yoshimura T, et al. Recent Topics in Pyridoxal 5'-Phosphate Enzyme Studies. Annu Rev Biochem 1990;59:87-110. [Crossref] [PubMed]
- Mcphalen CA, Vincent MG, Picot D, et al. Domain Closure in Mitochondrial Aspartate-Aminotransferase. J Mol Biol 1992;227:197-213. [Crossref] [PubMed]
- Doyle JM, Schinina ME, Bossa F, et al. The Amino-Acid-Sequence of Cytosolic Aspartate-Aminotransferase from Human Liver. Biochem J 1990;270:651-7. [Crossref] [PubMed]
- Lee J, Gokey T, Ting DL, et al. Dimerization misalignment in human glutamate-oxaloacetate transaminase variants is the primary factor for PLP release. PLoS One 2018;13:e0203889. [Crossref] [PubMed]
- Martini F, Angelaccio S, Barra D, et al. The Primary Structure of Mitochondrial Aspartate-Aminotransferase from Human-Heart. Biochimica Et Biophysica Acta 1985;832:46-51. [Crossref] [PubMed]
- Pol S, Bousquetlemercier B, Pavepreux M, et al. Nucleotide-Sequence and Tissue Distribution of the Human Mitochondrial Aspartate-Aminotransferase Messengerrna. Biochem Biophys Res Commun 1988;157:1309-15. [Crossref] [PubMed]
- Schatz G, Butow RA. How Are Proteins Imported into Mitochondria. Cell 1983;32:316-8. [Crossref] [PubMed]
- Rej R. Multiple Molecular-Forms of Human Cytoplasmic Aspartate-Aminotransferase. Clin Chim Acta 1981;112:1-11. [Crossref] [PubMed]
- Leung FY, Henderson AR. Isolation and Purification of Aspartate-Aminotransferase Isoenzymes from Human-Liver by Chromatography and Isoelectric-Focusing. Clin Chem 1981;27:232-8. [Crossref] [PubMed]
- Vanderlaarse A, Dijkshoorn NJ, Hollaar L, et al. The (Iso) Enzyme-Activities of Lactate-Dehydrogenase, Alpha-Hydroxybutyrate Dehydrogenase, Creatine-Kinase and Aspartate-Aminotransferase in Human Myocardial Biopsies and Autopsies. Clin Chim Acta 1980;104:381-91. [Crossref] [PubMed]
- Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma-Clearance of Intravenously Injected Aspartate-Aminotransferase Isozymes - Evidence for Preferential Uptake by Sinusoidal Liver-Cells. Hepatology 1985;5:367-75. [Crossref] [PubMed]
- Dufour DR, Lott JA, Nolte FS, et al. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000;46:2027-49. [Crossref] [PubMed]
- Whitfield JB, Zhu G, Nestler JE, et al. Genetic covariation between serum gamma-glutamyltransferase activity and cardiovascular risk factors. Clin Chem 2002;48:1426-31. [Crossref] [PubMed]
- Rahmioglu N, Andrew T, Cherkas L, et al. Epidemiology and Genetic Epidemiology of the Liver Function Test Proteins. PLoS One 2009;4:e4435. [Crossref] [PubMed]
- van Beek JH, de Moor MH, de Geus EJ, et al. The Genetic Architecture of Liver Enzyme Levels: GGT, ALT and AST. Behav Genet 2013;43:329-39. [Crossref] [PubMed]
- Pol S, Bousquetlemercier B, Pavepreux M, et al. Chromosomal Localization of Human Aspartate-Aminotransferase Genes by Insitu Hybridization. Hum Genet 1989;83:159-64. [Crossref] [PubMed]
- Giannattasio S, Marra E, Vacca RA, et al. Import of Mutant Forms of Mitochondrial Aspartate-Aminotransferase into Isolated-Mitochondria. Arch Biochem Biophys 1992;298:532-7. [Crossref] [PubMed]
- Aggerbeck M, Garlatti M, Feilleuxduche S, et al. Regulation of the Cytosolic Aspartate-Aminotransferase Housekeeping Gene Promoter by Glucocorticoids, Camp, and Insulin. Biochemistry 1993;32:9065-72. [Crossref] [PubMed]
- Lehninger AL. Phosphorylation Coupled to Oxidation of Dihydrodiphosphopyridine Nucleotide. J Biol Chem 1951;190:345-59. [Crossref] [PubMed]
- Scholz TD, Koppenhafer SL. Reducing Equivalent Shuttles in Developing Porcine Myocardium - Enhanced Capacity in the Newborn Heart. Pediatr Res 1995;38:221-7. [Crossref] [PubMed]
- Tordjman J, Leroyer S, Chauvet G, et al. Cytosolic aspartate aminotransferase, a new partner in adipocyte glyceroneogenesis and an atypical target of thiazolidinedione. J Biol Chem 2007;282:23591-602. [Crossref] [PubMed]
- Zhou SL, Gordon RE, Bradbury M, et al. Ethanol up-regulates fatty acid uptake and plasma membrane expression and export of mitochondrial aspartate aminotransferase in HepG2 cells. Hepatology 1998;27:1064-74. [Crossref] [PubMed]
- Berk PD, Wada H, Horio Y, et al. Plasma-Membrane Fatty Acid-Binding Protein and Mitochondrial Glutamic-Oxaloacetic Transaminase of Rat-Liver Are Related. Proc Natl Acad Sci U S A 1990;87:3484-8. [Crossref] [PubMed]
- Isola LM, Zhou SL, Kiang CL, et al. 3t3 Fibroblasts Transfected with a Cdna for Mitochondrial Aspartate-Aminotransferase Express Plasma-Membrane Fatty-Acid-Binding Protein and Saturable Fatty-Acid Uptake. Proc Natl Acad Sci U S A 1995;92:9866-70. [Crossref] [PubMed]
- Murao N, Yokoi N, Honda K, et al. Essential roles of aspartate aminotransferase 1 and vesicular glutamate transporters in β-cell glutamate signaling for incretin-induced insulin secretion. PLoS One 2017;12:e0187213. [Crossref] [PubMed]
- Akagi R. Purification and Characterization of Cysteine Aminotransferase from Rat-Liver Cytosol. Acta Medica Okayama 1982;36:187-97. [PubMed]
- Wen YD, Wang H, Zhu YZ. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid Med Cell Longev 2018;2018:4010395. [Crossref] [PubMed]
- Shibuya N, Mikami Y, Kimura Y, et al. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J Biochem 2009;146:623-6. [Crossref] [PubMed]
- Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 2014;20:770-82. [Crossref] [PubMed]
- Pratt DS, Kaplan MM. Primary care: Evaluation of abnormal liver-enzyme results in asymptomatic patients. New Engl J Med 2000;342:1266-71. [Crossref] [PubMed]
- Wieme RJ, Demeulenaere L. Enzyme assays in liver disease. J Clin Pathol Suppl (Assoc Clin Pathol) 1970;4:51-9. [Crossref] [PubMed]
- Gores GJ, Herman B, Lemasters JJ. Plasma membrane bleb formation and rupture: a common feature of hepatocellular injury. Hepatology 1990;11:690-8. [Crossref] [PubMed]
- Schwartz P, Piper HM, Spahr R, et al. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol 1984;115:349-61. [PubMed]
- Pappas NJ Jr. Increased rat liver homogenate, mitochondrial, and cytosolic aspartate aminotransferase activity in acute carbon tetrachloride poisoning. Clin Chim Acta 1980;106:223-9. [Crossref] [PubMed]
- Edgar AD, Tomkiewicz C, Costet P, et al. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicol Lett 1998;98:13-23. [Crossref] [PubMed]
- Josekutty J, Iqbal J, Iwawaki T, et al. Microsomal Triglyceride Transfer Protein Inhibition Induces Endoplasmic Reticulum Stress and Increases Gene Transcription via Ire1 alpha/cJun to Enhance Plasma ALT/AST. J Biol Chem 2013;288:14372-83. [Crossref] [PubMed]
- Pol S, Nalpas B, Vassault A, et al. Hepatic activity and mRNA expression of aspartate aminotransferase isoenzymes in alcoholic and nonalcoholic liver disease. Hepatology 1991;14:620-5. [PubMed]
- Moriyama T, Tamura S, Nakano K, et al. Laboratory and clinical features of abnormal macroenzymes found in human sera. Biochim Biophys Acta 2015;1854:658-67. [Crossref] [PubMed]
- Remaley AT, Wilding P. Macroenzymes: biochemical characterization, clinical significance, and laboratory detection. Clin Chem 1989;35:2261-70. [Crossref] [PubMed]
- Lee M, Vajro P, Keeffe EB. Isolated aspartate aminotransferase elevation: think macro-AST. Dig Dis Sci 2011;56:311-3. [Crossref] [PubMed]
- Moriyama T, Nobuoka M, Makino M. Incidence and properties of aspartate aminotransferase-immunoglobulin complexes in patients with a high serum aspartate to alanine aminotransferase ratio. Clin Chim Acta 1990;190:47-56. [Crossref] [PubMed]
- Shen H, Damcott C, Shuldiner SR, et al. Genome-wide association study identifies genetic variants in GOT1 determining serum aspartate aminotransferase levels. J Hum Genet 2011;56:801-5. [Crossref] [PubMed]
- Ueland PM, Ulvik A, Rios-Avila L, et al. Direct and Functional Biomarkers of Vitamin B6 Status. Annu Rev Nutr 2015;35:33-70. [Crossref] [PubMed]
- Ray L, Nanda SK, Chatterjee A, et al. A comparative study of serum aminotransferases in chronic kidney disease with and without end-stage renal disease: Need for new reference ranges. Int J Appl Basic Med Res 2015;5:31-5. [Crossref] [PubMed]
- Sette LH, Lopes EP. The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 2015;70:346-9. [Crossref] [PubMed]
- McKenzie J, Fisher BM, Jaap AJ, et al. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40-4. [Crossref] [PubMed]
- Arndt V, Brenner H, Rothenbacher D, et al. Elevated liver enzyme activity in construction workers: prevalence and impact on early retirement and all-cause mortality. Int Arch Occup Environ Health 1998;71:405-12. [Crossref] [PubMed]
- Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004;328:983. [Crossref] [PubMed]
- Kim HC, Kang DR, Nam CM, et al. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage - Korea Medical Insurance Corporation Study. Stroke 2005;36:1642-7. [Crossref] [PubMed]
- Elinav E, Ackerman Z, Maaravi Y, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 2006;54:1719-24. [Crossref] [PubMed]
- Goessling W, Massaro JM, Vasan RS, et al. Aminotransferase Levels and 20-Year Risk of Metabolic Syndrome, Diabetes, and Cardiovascular Disease. Gastroenterology 2008;135:1935-44. [Crossref] [PubMed]
- Monami M, Bardini G, Lamanna C, et al. Liver enzymes and risk of diabetes and cardiovascular disease: Results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism 2008;57:387-92. [Crossref] [PubMed]
- Lee TH, Kim WR, Benson JT, et al. Serum aminotransferase activity and mortality risk in a United States community. Hepatology 2008;47:880-7. [Crossref] [PubMed]
- Fulks M, Stout RL, Dolan VF. Using Liver Enzymes as Screening Tests to Predict Mortality Risk. J Insur Med 2008;40:191-203. [PubMed]
- Hernaez R, Yeh HC, Lazo M, et al. Elevated ALT and GGT predict all-cause mortality and hepatocellular carcinoma in Taiwanese male: a case-cohort study. Hepatol Int 2013;7:1040-9. [Crossref] [PubMed]
- Koehler EM, Sanna D, Hansen BE, et al. Serum liver enzymes are associated with all-cause mortality in an elderly population. Liver Int 2014;34:296-304. [Crossref] [PubMed]
- McCallum L, Panniyammakal J, Hastie CE, et al. Longitudinal Blood Pressure Control, Long-Term Mortality, and Predictive Utility of Serum Liver Enzymes and Bilirubin in Hypertensive Patients. Hypertension 2015;66:37-43. [Crossref] [PubMed]
- Kunutsor SK, Bakker SJL, Kootstra-Ros JE, et al. Inverse linear associations between liver aminotransferases and incident cardiovascular disease risk: The PREVEND study. Atherosclerosis 2015;243:138-47. [Crossref] [PubMed]
- Zoppini G, Cacciatori V, Negri C, et al. The aspartate aminotransferase-to-alanine aminotransferase ratio predicts all-cause and cardiovascular mortality in patients with type 2 diabetes. Medicine (Baltimore) 2016;95:e4821. [Crossref] [PubMed]
- Ravel V, Streja E, Molnar MZ, et al. Association of aspartate aminotransferase with mortality in hemodialysis patients. Nephrol Dial Transplant 2016;31:814-22. [Crossref] [PubMed]
- Steininger M, Winter MP, Reiberger T, et al. De-Ritis Ratio Improves Long-Term Risk Prediction after Acute Myocardial Infarction. J Clin Med 2018;7:474. [Crossref] [PubMed]
- Choi KM, Han K, Park S, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: A nationwide population-based cohort study. Sci Rep 2018;8:3764. [Crossref] [PubMed]
- Alexander KS, Zakai NA, Lidofsky SD, et al. Non-alcoholic fatty liver disease, liver biomarkers and stroke risk: The Reasons for Geographic and Racial Differences in Stroke cohort. PLoS One 2018;13:e0194153. [Crossref] [PubMed]
- Ndrepepa G, Holdenrieder S, Cassese S, et al. Aspartate aminotransferase and mortality in patients with ischemic heart disease. Nutr Metab Cardiovasc Dis 2020;30:2335-42. [Crossref] [PubMed]
- Lee H, Shin DW, Lee TH, et al. Association Between Change in Serum Aminotransferase and Mortality: A Nationwide Cohort Study in Korea. Medicine (Baltimore) 2016;95:e3158. [Crossref] [PubMed]
- Kunutsor SK, Apekey TA, Seddoh D, et al. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol 2014;43:187-201. [Crossref] [PubMed]
- Rahmani J, Miri A, Namjoo I, et al. Elevated liver enzymes and cardiovascular mortality: a systematic review and dose-response meta-analysis of more than one million participants. Eur J Gastroenterol Hepatol 2019;31:555-62. [Crossref] [PubMed]
- Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138-53. [Crossref] [PubMed]
- Caballeria L, Pera G, Auladell MA, et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur J Gastroenterol Hepatol 2010;22:24-32. [Crossref] [PubMed]
- van den Berg EH, Amini M, Schreuder TC, et al. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS One 2017;12:e0171502. [Crossref] [PubMed]
- Mantovani A, Ballestri S, Lonardo A, et al. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Digest Dis Sci 2016;61:1246-67. [Crossref] [PubMed]
- Stepanova M, Younossi ZM. Independent Association Between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease in the US Population. Clin Gastroenterol Hepatol 2012;10:646-50. [Crossref] [PubMed]
- Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [Crossref] [PubMed]
- Ismaiel A, Dumitrascu DL. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis-Literature Review. Front Med (Lausanne) 2019;6:202. [Crossref] [PubMed]
- Sookoian S, Castano GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr 2016;103:422-34. [Crossref] [PubMed]
- Young KA, Palmer ND, Fingerlin TE, et al. Genome-Wide Association Study Identifies Loci for Liver Enzyme Concentrations in Mexican Americans: The GUARDIAN Consortium. Obesity 2019;27:1331-7. [Crossref] [PubMed]
- Porter SA, Pedley A, Massaro JM, et al. Aminotransferase Levels Are Associated With Cardiometabolic Risk Above and Beyond Visceral Fat and Insulin Resistance The Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:139. [Crossref] [PubMed]
- Kim HR, Han MA. Association between Serum Liver Enzymes and Metabolic Syndrome in Korean Adults. Int J Environ Res Public Health 2018;15:1658. [Crossref] [PubMed]
- Greber-Platzer S, Thajer A, Bohn S, et al. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr 2019;19:332. [Crossref] [PubMed]
- Piano MR. Alcohol's Effects on the Cardiovascular System. Alcohol Res 2017;38:219-41. [PubMed]
- Chiva-Blanch G, Badimon L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients 2019;12:108. [Crossref] [PubMed]
- O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health - The razor-sharp double-edged sword. J Am Coll Cardiol 2007;50:1009-14. [Crossref] [PubMed]
- Leong DP, Smyth A, Teo KK, et al. Patterns of alcohol consumption and myocardial infarction risk: observations from 52 countries in the INTERHEART case-control study. Circulation 2014;130:390-8. [Crossref] [PubMed]
- Roerecke M, Rehm J. Chronic heavy drinking and ischaemic heart disease: a systematic review and meta-analysis. Open Heart 2014;1:e000135. [Crossref] [PubMed]
- Lofthus DM, Stevens SR, Armstrong PW, et al. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coronary Artery Dis 2012;23:22-30. [Crossref] [PubMed]
- Gao M, Cheng Y, Zheng Y, et al. Association of serum transaminases with short- and long-term outcomes in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. BMC Cardiovasc Disord 2017;17:43. [Crossref] [PubMed]
- Moon J, Kang W, Oh PC, et al. Serum transaminase determined in the emergency room predicts outcomes in patients with acute ST-segment elevation myocardial infarction who undergo primary percutaneous coronary intervention. Int J Cardiol 2014;177:442-7. [Crossref] [PubMed]
- Lazo M, Rubin J, Clark JM, et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol 2015;62:841-7. [Crossref] [PubMed]
- Kubo SH, Walter BA, John DHA, et al. Liver-Function Abnormalities in Chronic Heart-Failure - Influence of Systemic Hemodynamics. Arch Intern Med 1987;147:1227-30. [Crossref] [PubMed]
- Killip T, Payne MA. High Serum Transaminase Activity in Heart Disease - Circulatory Failure and Hepatic Necrosis. Circulation 1960;21:646-60. [Crossref] [PubMed]
- Lotto V, Choi SW, Friso S. Vitamin B-6: a challenging link between nutrition and inflammation in CVD. Brit J Nutr 2011;106:183-95. [Crossref] [PubMed]
- Ndrepepa G. Alkaline phosphatase and cardiovascular disease. J Lab Precis Med 2017;2:83. [Crossref]
- Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol 1995;43:405-8. [PubMed]
- Huang JW, Yen CJ, Pai MF, et al. Association between serum aspartate transaminase and homocysteine levels in hemodialysis patients. Am J Kidney Dis 2002;40:1195-201. [Crossref] [PubMed]
- Lopes EP, Sette LH, Sette JB, et al. Serum Alanine Aminotransferase Levels, Hematocrit Rate and Body Weight Correlations before and after Hemodialysis Session. Clinics 2009;64:941-5. [Crossref] [PubMed]
- Cohen GA, Goffinet JA, Donabedian RK, et al. Observations on Decreased Serum Glutamic Oxalacetic Transaminase (Sgot) Activity in Azotemic Patients. Ann Intern Med 1976;84:275-80. [Crossref] [PubMed]
- Ndrepepa G, Kastrati A. Alanine aminotransferase—a marker of cardiovascular risk at high and low activity levels. J Lab Precis Med 2019;4:29. [Crossref]
Cite this article as: Ndrepepa G. Aspartate aminotransferase and cardiovascular disease—a narrative review. J Lab Precis Med 2021;6:6.


