Diagnostic accuracy of endostatin for malignant pleural effusion: a systematic review and meta-analysis
Introduction
Pleural effusion (PE) is a common sign in clinical practice with various etiologies. It was reported in previous studies that malignant PE (MPE) accounts for 20% to 50% of PE (1,2). Diagnosing MPE in PE patients with unknown etiology is a challenge for clinicians. The gold standard for diagnosing MPE is the closed pleural biopsy and medical thoracoscopy followed by pathological examination (3). However, both of those tools are invasive and observer-dependent (4,5). Thus, it is of great value to develop non-invasive and objective diagnostic tools for MPE.
Tumor biomarkers in PE represent a diagnostic tool for MPE (6,7). To date, accumulated studies have been conducted to investigate the diagnostic accuracy of tumor biomarkers for MPE. The diagnostic accuracy of conventional tumor biomarkers, including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 15-3, CA19-9, CA 125, cytokeratin fragment 19 (CYFRA 21-1), and neuron-specific enolase (NSE), has been widely studied. However, the evidence from meta-analyses indicates that these tumor biomarkers’ diagnostic accuracy is unsatisfactory, with sensitivities around 0.50 and specificity around 0.90 (8-10). Therefore, it is essential to develop novel tumor biomarkers that can improve the diagnostic accuracy of conventional tumor biomarkers or replace them (7).
Previous studies reported that endostatin, an endogenous angiogenesis inhibitor, increased in MPE and represented a potential diagnostic biomarker for MPE (11). Several studies have investigated the diagnostic accuracy of PE endostatin for MPE, but the results were heterogeneous. Therefore, we conducted this systematic review to determine the accuracy of endostatin for MPE. We present the following article in accordance with the PRISMA-DTA reporting checklist (12) (available at http://dx.doi.org/10.21037/jlpm-20-91).
Methods
Literature retrieval
The study was not registered before performing. Two databases, the PubMed and Web of Science, were searched to identify eligible studies. The last search date was June 1, 2020. The search algorithm in PubMed is ("Endostatins"[nm] or endostatin or endostar) and (pleural or pleurisy or pleura or pleurae or pleuritis). A similar strategy was used in Web of Science. We also manually searched the references listed at the end of the review in this field and eligible studies.
Inclusion and exclusion criteria
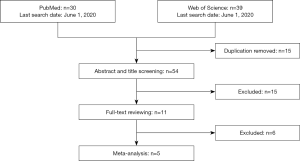
We included the studies investigating PE endostatin for MPE. The exclusion criteria were: (I) animal studies; (II) review, case report, and editorial; (III) conference abstract; (IV) studies with insufficient detail to construct a two-by-two table for meta-analysis. In the first round of study selection, two reviewers independently screened the title and abstract of searched studies to identify obviously irrelevant studies. In the second round, a full-text review was performed to identify eligible studies.
Data extraction and quality assessment
Data extraction was performed by two reviewers independently. The data extracted were: name of the first author, country, year, component of control, sample sizes of MPE and non-MPE, study design (prospective or retrospective), consecutive enrollment, blinded test and interpretation, the reference standard for MPE diagnosis, endostatin assay, cutoff used for diagnosis, sensitivity, and specificity. We constructed a two-by-two table for each eligible study with the sensitivity, specificity, and sample sizes of MPE and non-MPE. The two-by-two table contains the numbers of true positive (TP), false negative (FN), true negative (TN), and false positive (FP).
The revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used to assess the eligible studies’ quality (13).
Statistical analysis
All statistical analyses were performed with the MetaDTA, an online tool for meta-analysis (14). A bivariate model was used to pool the sensitivities and specificities of included studies (15). A summary receiver operating characteristic (sROC) curve, which is threshold independent, was used to measure endostatin’s overall diagnostic performance (16). I2. was used to determine the heterogeneity across the eligible studies [13]. P<0.05 was considered statistically significant.
Results
Summary of the eligible studies
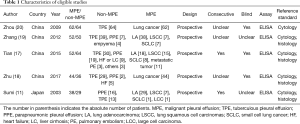
Figure 1 is a flowchart of study selection. Finally, five studies (11,17-20) with 248 MPEs and 243 non-MPEs were included. Characteristics of the eligible studies are summarized in Table 1. Four of the eligible studies were performed in China (17-20). The remaining one was performed in Japan (11). All eligible studies are prospective design and determine PE endostatin with ELISA. In four of the eligible studies, MPE was caused by lung cancer (11,18-20), while the remaining one study included patients with metastatic tumors (17). The disease profile of non-MPE was various, including tuberculous pleural effusion (TPE) (11,17-20), parapneumonic pleural effusion (PPE) (11,17-19), heart failure (HF) (17,18), liver cirrhosis (LC) (17), and pulmonary embolism (PE) (17). Patients in the two studies were consecutively enrolled (11,17), while the remaining three studies did not report whether patients were consecutively enrolled (18-20). Two studies did not report whether the PE endostatin results were masked to the clinicians who made the final diagnosis (11,19).
Full table
Diagnostic performance and quality assessment of eligible studies
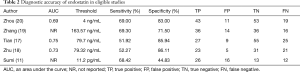
The diagnostic accuracy of endostatin in eligible studies is listed in Table 2. Two of the eligible studies did not report the area under the receiver operating characteristic (ROC) curve (AUC). In the remaining three studies, the AUC of endostatin ranged from 0.69 to 0.75. Diagnostic thresholds adopted by the eligible studies ranged from 0.0112 to 163.57 ng/mL. The eligible studies’ sensitivities ranged from 51.92% to 69.30%, and specificities ranged from 44.83% to 83.00%.
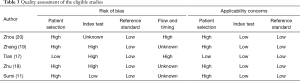
The quality assessment of the included studies is shown in Table 3. Four of the five eligible studies (11,18-20) have patient selection bias because their disease profile was not representative. One study only included TPE as control (20), and some studies were two-gate design (21). The three studies’ index test domain was labeled as high because the diagnostic threshold was not prespecified (17-19). A previous study has revealed that a data-driven threshold may overestimate an index test’s diagnostic accuracy, especially in studies with small sample size (22). Two of the eligible studies were marked as high regarding the flow and timing domain because of partial verification bias (17,20). In partial verification design, not all subjects receive reference standard (23). This type of design may introduce bias because some patients with target disease may be missed. The remaining three studies were labeled as unknown because partial verification bias was not indicated in the report.
Full table
Full table
AUC, an area under the curve; NR, not reported; TP, true positive; FP, false positive; TN, true negative; FN, false negative.
Meta-analysis
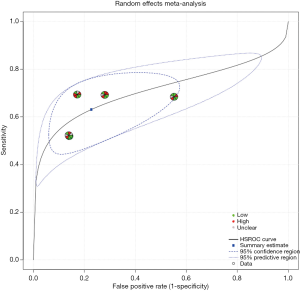
Figure 2 is a forest plot of endostatin. Pooled sensitivity and specificity of endostatin were 0.63 (95% CI: 0.55–0.71) and 0.77 (95% CI: 0.63–0.87), respectively. Positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were was 2.78 (95% CI: 1.49–5.43) and 0.48 (95% CI: 0.34–0.72), respectively. The diagnostic odds ratio (DOR) was 0.50 (95% CI: 2.08–16.07). The sROC curve of endostatin is shown in Figure 3. Heterogeneity across the eligible studies measured by I2 was 82% (95% CI: 60–100%). AUC of the sROC was 0.71 (95% CI: 0.67–0.75).
Discussion
In this study, we performed a systematic review and meta-analysis to ascertain the diagnostic accuracy of endostatin for MPE. With five included studies, we found that the pooled sensitivity and specificity of endostatin for diagnosing MPE were 0.63 and 0.77, respectively. The area under the sROC curve was 0.71. These results do not support PE endostatin as a useful diagnostic biomarker for MPE.
Compared with a previous meta-analysis published in 2015 (17), our study has some advantages. The first advantage is we searched the Web of Science database and included the studies published after 2015. The second advantage is that our study’s quality assessment tool is QUADAS-2, which is more comprehensive than the first version of QUADAS (13).
Sensitivity and specificity are two main indicators to measure an index test’s diagnostic accuracy, and both of them are greatly affected by the adopted threshold of the index test (24). By contrast, the AUC of an index test is threshold independent, and it is a better metric to estimate the diagnostic accuracy of an index test. This study found that the AUC of endostatin was only 0.71, indicating that endostatin’s diagnostic accuracy is low. NLR and PLR are two indicators used for ruling in or ruling out of target disease. It is widely accepted that PLR >10 or NLR<0.1 is strong evidence to rule in or rule out target diagnosis, respectively (25). In this study, the PLR and NLR were 2.78 and 0.48, respectively, indicating that endostatin, when used alone, is insufficient to rule in or rule out MPE. Taken together, the current evidence does not support using PE endostatin for diagnosing MPE.
Currently, some studies have been performed to investigate the diagnostic accuracy of PE biomarkers for MPE. According to the published meta-analyses, these markers’ diagnostic sensitivities were around 50%, and the specificities were about 90% (8,9,26,27). Their AUCs were around 0.80. By contrast, this study found that endostatin has a sensitivity of 63%, a specificity of 77%, and an AUC of 0.71, suggesting that endostatin’s diagnostic accuracy is inferior to the conventional biomarkers.
This meta-analysis has some limitations. First, the small sample size is the major weakness of this systematic review and meta-analysis. Therefore, the results of this meta-analysis were not sufficiently robust. Second, we could not perform meta-regression to explore the heterogeneity sources. Third, we did not perform publication bias analysis because of the small sample size.
In conclusion, our results do not support PE endostatin as a useful diagnostic marker for MPE. Given the small sample size of this meta-analysis, further studies with a large sample size are needed to validate this study’s findings.
Acknowledgments
Funding: This work was supported by the Qimeng Project of the Affiliated Hospital of Inner Mongolia Medical University (FYQMJH2020031).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Laboratory and Precision Medicine for the series “Pleural Effusion Analysis”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-20-91
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-20-91). The series “Pleural Effusion Analysis” was commissioned by the editorial office without any funding or sponsorship. ZDH served as the unpaid Guest Editor of the series and serves as an unpaid executive editor of the Journal of Laboratory and Precision Medicine from Nov 2016 to Oct 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study does not require any ethical approval and individual consent because this is a systematic review and meta-analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [Crossref] [PubMed]
- Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration 2015;90:251-5. [Crossref] [PubMed]
- Wei Y, Shen K, Lv T, et al. Comparison between closed pleural biopsy and medical thoracoscopy for the diagnosis of undiagnosed exudative pleural effusions: a systematic review and meta-analysis. Transl Lung Cancer Res 2020;9:446-58. [Crossref] [PubMed]
- Ferreiro L, Toubes ME, San Jose ME, et al. Advances in pleural effusion diagnostics. Expert Rev Respir Med 2020;14:51-66. [Crossref] [PubMed]
- Porcel JM, Azzopardi M, Koegelenberg CF, et al. The diagnosis of pleural effusions. Expert Rev Respir Med 2015;9:801-15. [Crossref] [PubMed]
- Bhatnagar R, Maskell N. Pleural fluid biochemistry - old controversies, new directions. Ann Clin Biochem 2014;51:421-3. [Crossref] [PubMed]
- Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis 2018;12:1753466618808660. [Crossref] [PubMed]
- Nguyen AH, Miller EJ, Wichman CS, et al. Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res 2015;166:432-9. [Crossref] [PubMed]
- Liang QL, Shi HZ, Qin XJ, et al. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax 2008;63:35-41. [Crossref] [PubMed]
- Zhu J, Feng M, Liang L, et al. Is neuron-specific enolase useful for diagnosing malignant pleural effusions? evidence from a validation study and meta-analysis. BMC Cancer 2017;17:590. [Crossref] [PubMed]
- Sumi M, Kagohashi K, Satoh H, et al. Endostatin levels in exudative pleural effusions. Lung 2003;181:329-34. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Freeman SC, Kerby CR, Patel A, et al. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol 2019;19:81. [Crossref] [PubMed]
- Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [Crossref] [PubMed]
- Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002;21:1237-56. [Crossref] [PubMed]
- Tian P, Shen Y, Feng M, et al. Diagnostic accuracy of endostatin for malignant pleural effusion: A clinical study and meta-analysis. Postgrad Med 2015;127:529-34. [Crossref] [PubMed]
- Zhu YY, Wu HM, Liu RY. Diagnostic Values of sVEGFR-1 and Endostatin in Malignant Pleural Effusions in Patients with Lung Cancer. Clin Lab 2017;63:1371-8. [Crossref] [PubMed]
- Zhang Y, Yu LK, Xia N. Evaluation of serum and pleural levels of endostatin and vascular epithelial growth factor in lung cancer patients with pleural effusion. Asian Pac J Trop Med 2012;5:239-42. [Crossref] [PubMed]
- Zhou WB, Bai M, Jin Y. Diagnostic value of vascular endothelial growth factor and endostatin in malignant pleural effusions. Int J Tuberc Lung Dis 2009;13:381-6. [PubMed]
- Rutjes AW, Reitsma JB, Vandenbroucke JP, et al. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 2005;51:1335-41. [Crossref] [PubMed]
- Leeflang MM, Moons KG, Reitsma JB, et al. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem 2008;54:729-37. [Crossref] [PubMed]
- de Groot JA, Bossuyt PM, Reitsma JB, et al. Verification problems in diagnostic accuracy studies: consequences and solutions. BMJ 2011;343:d4770. [Crossref] [PubMed]
- Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994;308:1552. [Crossref] [PubMed]
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168-9. [Crossref] [PubMed]
- Yang Y, Liu YL, Shi HZ. Diagnostic Accuracy of Combinations of Tumor Markers for Malignant Pleural Effusion: An Updated Meta-Analysis. Respiration 2017;94:62-9. [Crossref] [PubMed]
- Shen YC, Liu MQ, Wan C, et al. Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: A meta-analysis. Exp Ther Med 2012;3:1072-6. [Crossref] [PubMed]
Cite this article as: Yang SY, Zhao Y, Wang XR, Wu J, Yang DN, Liu CL, Hu ZD. Diagnostic accuracy of endostatin for malignant pleural effusion: a systematic review and meta-analysis. J Lab Precis Med 2021;6:5.