
Hypoglycaemia associated with critical illness and hormone deficiencies: a narrative review
Introduction
Glucose homeostasis in the human body depends on a variety of gluco-regulatory organs including pancreas, liver, adrenals, kidneys, pituitary, and hypothalamus, and is also under the strict control by multiple hormones, viz. insulin, glucagon, catecholamines, cortisol, and growth hormone (GH) (1). Given the multi-organ and multi-hormonal regulation of glucose, hypoglycaemia which is defined as blood glucose levels less than 4 mmol/L is likely to happen due to significant derangements of any of these organs and hormone systems (2). Spontaneous hypoglycaemia develops when the utilization of glucose exceeds its production from both exogenous (dietary) and endogenous (liver and kidney) sources. The tissues that are involved in glucose utilization are brain, obligatory glycolytic tissues such as red blood cells and renal medullae, and insulin sensitive tissues especially, the skeletal muscles (3). Spontaneous hypoglycaemia results from a variety of causes including critical illness and hormonal deficiencies. In this review, we aim to go through the available literature to elaborate the pathophysiology, clinical features, and the management of spontaneous hypoglycaemia in adults caused by critical illnesses and hormone deficiencies.
We searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) database for articles in English language published from 1930 to September 2020, and selected papers on the basis of quality, and relevance for this review. The following keywords/MeSH terms were used for identifying appropriate citations: “spontaneous hypoglycaemia”, “glucose homeostasis”, “critical illness”, “sepsis”, “renal failure”, “liver failure”, “heart failure”, “respiratory failure”, “hypothermia”, “malnutrition”, “intoxication”, “circadian rhythm”, “hormone deficiencies”, “adrenal insufficiency”, “hypothyroidism”, “growth hormone deficiency”, “glucagon deficiency”, “catecholamine deficiency” and “hypopituitarism”. We further filtered the number of articles identified by the keyword search by appropriate use of the Boolean operators “AND”/“OR” when necessary. We gave particular emphasis on evidence-based guidelines and high-quality studies while writing this narrative review. We present the article in accordance with the narrative review checklist provided (available at http://dx.doi.org/10.21037/jlpm-2020-ash-01).
Epidemiology of spontaneous hypoglycaemia
In a retrospective cohort study done using electronic emergency medical service (EMS) patient record system in Finland, spontaneous hypoglycaemia was found to be a common cause of hypoglycaemia, accounting for 41.6% of all cases of hypoglycaemia and among patients with spontaneous hypoglycaemia, 23.2% were found to have serious hypoglycaemia (defined as blood glucose value <3 mmol/L) (4). The estimated annual incidence of spontaneous hypoglycaemia was 1,082 cases per 100,000 inhabitants. The commonest aetiologies for spontaneous hypoglycaemia encountered by the EMS were alcohol abuse (41.4%; 95% CI: 39.8–42.9), hypothermia (17.2%; 95% CI: 16.0–18.4), malnutrition (17.0%; 95% CI: 15.8–18.2), intoxication (13.5%; 95% CI: 12.4–14.6), and infections (14.3%; 95% CI: 13.3–15.5) (4). Less common causes for spontaneous hypoglycaemia were renal failure, liver failure, heart failure, malignancies, and endocrine problems. In patients with spontaneous hypoglycaemia, the mortality at 1 year varied based on the age and aetiology of hypoglycaemia (4). Patients with renal failure, liver failure, cardiac failure and malignancies have nearly 50% mortality at 1 year. The mortality at 1 year for those presenting with infection was nearly 20%, hypothermia and malnutrition was nearly 15% and alcohol abuse was nearly 6% (4).
In another retrospective study using a national inpatient database from 2008 to 2012 in Japan, spontaneous hypoglycaemia accounted for 20–30% of hospitalizations with hypoglycaemia, at a rate of 3.8 per 10,000 admissions (5). An estimated 5,000–7,000 cases were admitted annually with hypoglycaemia. The common aetiologies for spontaneous hypoglycaemia in these inpatients were malignancies (15.8%), cerebrovascular diseases (13%), pneumonia (11.2%), renal failure (8.6%), and heart failure (7.8%). The study showed that inpatients with spontaneous hypoglycaemia had an increased mortality rate compared to inpatients with diabetic hypoglycaemia, when the observations from this study was compared with the data from another study (6) done by the same group in diabetic subjects (14.9% versus 3.8%) (5).
Spontaneous hypoglycaemia and the iatrogenic hypoglycaemia have notably different pathogenesis and prognostic implications. In a large retrospective cohort study on patients hospitalised for acute myocardial infarction between 2000 and 2005 in the United States, those who developed spontaneous hypoglycaemia had increased mortality, as compared to those who developed hypoglycaemia following insulin initiation (7). The investigators proposed that those who developed spontaneous hypoglycaemia were having higher disease burden and comorbidities, both of which were thought to be the reasons for hypoglycaemia and increased mortality. They considered hypoglycaemia as a marker of poor outcome, rather than the mediator of higher mortality. The study, however, was underpowered to detect mortality in subjects with iatrogenic hypoglycaemia.
A retrospective cohort study in hospitalized non-critically ill patients also made a similar observation that the spontaneous hypoglycaemia was associated with increased mortality with a hazard ratio (HR) of 2.62 (95% CI: 1.97–3.47, P<0.001), as compared to the iatrogenic hypoglycaemia (HR: 1.06, 95% CI: 0.74–1.52, P=0.749) (8). The study also observed that, once adjusted for comorbidities, the association between spontaneous hypoglycaemia and mortality was eliminated (HR: 1.11, 95% CI: 0.76–1.64, P=0.582). Thus, in the general medical ward with non-critically ill patients, spontaneous hypoglycaemia as such is not associated with increased mortality, and it is just as a marker of disease burden (co-morbidities). Similar observation was also noted among patients with sepsis associated spontaneous hypoglycaemia (9).
A large retrospective study among hospitalised patients in Israel showed that hypoglycaemia, which is common in the geriatric population, was a marker or poor health and general deterioration without any direct impact on survival (10). Elderly patients are prone to develop spontaneous hypoglycaemia due to polypharmacy, frailty, multiple organ failure, malnutrition, malignancies, and underlying dementia. The proposed pathogenic mechanisms for increased hypoglycaemia risk in the elderly population are impaired glucagon and epinephrine release in response to hypoglycaemia and reduced awareness of the autonomic symptoms of hypoglycaemia (11).
Hypoglycaemia associated with critical illness
Many patients develop hyperglycaemia in response to acute illness as a stress response. In critically ill patients without diabetes, the stress hyperglycaemia that occurs, up to a certain level, is beneficial by increasing the glucose supply to the tissues at times hypoperfusion (12). A retrospective study using intensive care unit (ICU) records from Germany showed that severe hyperglycaemia (>11.1 mmol/L or 200 mg/dL) in critically ill patients without diabetes managed in ICU had high mortality [odds ratio (OR) 2.15; 95% CI: 1.79–2.57] (12). Similarly, hypoglycaemia (≤3.9 mmol/L or 70 mg/dL) was also associated with high ICU mortality (OR 2.94; 95% CI: 2.28–3.80). In the subset of patients with diabetes mellitus, severe hyperglycaemia (defined as blood sugar value>11.1 mmol/L) was not associated with increased ICU mortality (OR 1.05; 95% CI: 0.68–1.62), whereas iatrogenic hypoglycaemia was associated with high ICU mortality (OR 4.71; 95% CI: 2.60–8.55) (12).
As the serum glucose level of <3.0 mmol/L (<54 mg/dL; clinically significant hypoglycaemia) is associated with neuroglycopenia, and arrhythmia (13), the investigators recalculated the ICU mortality in patients having type 2 diabetes mellitus (T2DM) with blood glucose levels between 3–3.9 mmol/L and <3 mmol/L (14). They observed that the association between hypoglycaemia and mortality did not remain significant after correction for Acute Physiology and Chronic Health Evaluation II (APACHE2) score [OR 1.18; 95% CI: 0.46–3.02] when the blood glucose levels were between 3 and 3.9 mmol/L, whereas it remained significant after correction for APACHE2 (OR 8.26; 95% CI: 2.41–28.29) and lactate (OR 4.85; 95% CI: 1.61–14.57) when the glucose levels were <3 mmol/L (14).
In a recent retrospective cohort study from the United States, hospital mortality did not differ between iatrogenic versus spontaneous hypoglycaemia (OR 1.22, 95% CI: 0.77–1.93) in critically ill patients (15). In another retrospective cohort study from two ICUs in Melbourne and Sydney [2000–2004], there was a proportionate increase in hospital mortality related to the severity of hypoglycaemia and that both spontaneous as well as iatrogenic hypoglycaemia were associated with increased mortality risk (16). A study among all hospitalised patients showed that both iatrogenic hypoglycaemia as well as spontaneous hypoglycaemia were associated with increased mortality (17). Moreover, spontaneous hypoglycaemia was associated with greater mortality risk compared to iatrogenic hypoglycaemia.
In conclusion, among hospitalised non-critically ill and critically ill patients, those who develop spontaneous hypoglycaemia have at least equal or even greater mortality risk when compared to those who develop iatrogenic hypoglycaemia, and in these patients, hypoglycaemia is only a marker of comorbidity, not directly contributing to the mortality risk.
Glycaemic targets in noncritically and critically ill patients
As spontaneous and iatrogenic hypoglycaemia are associated with increased mortality, close glucose monitoring is indicated in all critically ill patients with or without diabetes mellitus. The nondiabetic patients should maintain glucose in the range 3.9–11.1 mmol/L (12). However, in patients with diabetes, a glucose level of >11.1 mmol/L might not cause any immediate consequences, but glucose levels <3.9 mmol/L should be avoided. The American Diabetes Association (ADA 2020) recommends a target glucose of 7.8–10.0 mmol/L (140–180 mg/dL) for majority of critically ill and noncritically ill patients (18). More stringent glucose target of 6.1–7.8 mmol/L (110–140 mg/dL) in critically ill postsurgical or post-cardiac surgery patients is desirable, if achievable without significant hypoglycaemia. The ADA also recommends a less stringent target of >10 mmol/L (180 mg/dL) in patients with terminal illness or severe comorbidities, and in the inpatient settings where frequent glucose monitoring or close nursing supervision is not feasible (18).
Pathogenesis of hypoglycaemia associated with critical illness
Critical illnesses and multiple organ failure result in reduction in gluconeogenesis, and the metabolic stress increases the glucose utilization by the tissues (2). Additionally critical illness is associated with suboptimal nutrition and insufficient provision of glucose. Failure of multiple organs like liver and kidney, associated with dysfunction of endocrine glands such as adrenal and pituitary may also contribute to hypoglycaemia associated with critical illness (2). Table 1 gives the common causes of hypoglycaemia that should not be missed in any critically ill patient. In critical illness, hypoglycaemia is deleterious as it increases the systemic inflammatory response, induces neuroglycopenia, limits the glucocorticoid response to stress, impairs the sympathetic responsiveness, induces cerebral vasodilatation, and also through other unidentified mechanisms (19-22). Moreover, hypoglycaemia could significantly worsen the critical illness-induced neurocognitive dysfunction (23), and prolong the length of stay in ICU independent of the severity of illness, thereby increasing the heath care expenses (24).
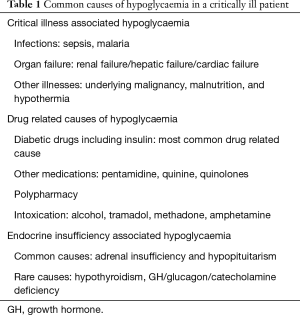
Full table
Sepsis including malaria
Stress hyperglycaemia following infection is a physiological response secondary to increase in insulin resistance and catecholamine release (25). However, hypoglycaemia can be a sign of overwhelming sepsis with worse outcomes, and is much more common than previously recognized (26). The hypoglycaemia in sepsis can be due to increased glucose utilisation by macrophage-rich tissues such as liver, lung, spleen, ileum, and skin (27), depletion in glycogen stores (26), decrease in hepatic glucose output associated with decreased sensitivity to counterregulatory hormones, or adrenal insufficiency associated with increase in metabolic demand (25). The common pathogens causing hypoglycaemia, even in people without diabetes mellitus, include Streptococcus pneumoniae, Haemophilus influenzae type b, Streptococcus pyogenes, and Escherichia coli (26,28).
A retrospective cohort study on subjects with radiologically proven pneumonia showed that those who developed hypoglycaemia at presentation had 3 times higher 30-day mortality than those with normal plasma glucose (27 vs. 8.6), after adjusting for severity of illness and other potential confounders like presence of diabetes mellitus (29). Thus, while managing community acquired pneumonia, the patients presenting with hypoglycaemia should be closely monitored even when adult pneumonia specific scoring systems including CURB-65 or Pneumonia Severity Index suggest that these patients can be discharged as these systems do not consider hypoglycaemia as a major risk factor for mortality.
A retrospective analysis of patients with Escherichia coli bacteraemia revealed that nearly 4.6% developed hypoglycaemia and the incidence was nearly equal in diabetic and nondiabetic individuals (28). Those who developed hypoglycaemia during bacteraemia had a poor prognosis, and 4.7-fold higher mortality, as compared to those without hypoglycaemia, especially in those with renal and hepatic dysfunction. In a retrospective cohort study of patients with gram negative bacteraemia, a proportionate rise in mortality was observed depending on the deviation in blood glucose from a central value associated with lowest mortality (30). Greater deviation of blood glucose from the central value (8.6 mmol/L or 155 mg/dL) was associated with higher mortality.
Hypoglycaemia with blood glucose in the range of 2.2–3.9 mmol/L or 40–70 mg/dL has been shown to be associated with nearly 2-fold increased mortality in critically ill patients (31). Mild hypoglycaemia in patients with sepsis has been proven to be independently associated with increased in-hospital mortality (OR 3.43; 95% CI: 1.51–7.82) (32). Moreover, even a single event was an independent risk factor (OR 2.98; 95% CI: 1.10–8.09) for in-hospital mortality. Kaplan-Meier analysis showed that hypoglycaemia was significantly associated with decreased 1-year survival among these patients with sepsis. Mild hypoglycaemia was also associated with higher ICU-related complications as well (32).
Hypoglycaemia and lactic acidosis can occur in patients with severe infection from plasmodium falciparum malaria (33). The pathogenic mechanisms for hypoglycaemia are decreased glucose production, increased peripheral uptake of glucose, increased anaerobic glycolysis and quinine-induced hyperinsulinemia. However, there is no dose relationship between quinine and occurrence of hypoglycaemia (34). Pathogenesis of lactic acidosis is anaerobic glucose metabolism caused by microvascular sequestration of parasitized red blood cells that reduces the blood flow to tissues (33). Hypoglycaemia is an independent risk factor for mortality in falciparum malaria (OR 3.45; 95% CI: 2.30–5.16) (34) Thus, severe malaria cases should have regular glucose monitoring to detect hypoglycaemia, and once detected it should be treated with intravenous glucose (33).
Renal failure
There are various pathogenic mechanisms for hypoglycaemia in patients with renal failure. These mechanisms include calorie deprivation, decreased glycogen stores secondary to anorexia, vomiting, or protein restriction (2), lack of precursors of gluconeogenesis (alanine) secondary to muscle wasting (35), impaired renal gluconeogenesis secondary to reduced renal mass, impaired glycogenolysis, decreased renal clearance of insulin and hypoglycaemic agents (35), and decreased insulin degradation in peripheral tissues (36). Few other mechanisms include coexistent heart failure, liver failure or recurrent infections, endocrine dysfunction with deficient counterregulatory hormones like glucagon and catecholamines (35), and impaired hepatorenal glucose reciprocity secondary to uremic acidosis (37). Dialysis predisposes to hypoglycaemia as it is associated with a chronic state of malnutrition (35). The post-dialysis hypoglycaemia is due to glucose-induced hyperinsulinemia caused by the high glucose content in the dialysate.
The hepatorenal glucose reciprocity is defined as the reciprocal changes in hepatic and renal glucose release to maintain normoglycaemia (38). Thus, any decrease in glucose release by kidney or liver is associated with a compensatory increase in glucose release by liver or kidney so as to prevent hypoglycaemia. The primary regulator of gluconeogenesis in liver is glucagon and that of kidney is catecholamines. As renal failure is frequently associated with autonomic nervous system dysfunction, the renal failure-related hypoglycaemia is not associated with catecholamine release and adrenergic symptoms (35). Instead, neuroglycopenic symptoms predominate in such cases. However, the severity and duration of neuroglycopenic manifestations are highly variable. Hypoglycaemia should be suspected if any patient with renal failure exhibits any change in mental or neurologic status (35).
Patients with chronic kidney disease (CKD) with or without diabetes are at increased risk of developing hypoglycaemia and associated mortality (36). The incidence of hypoglycaemia was 10.72 versus 5.33 per 100 patient-months among diabetics with CKD versus without CKD. Similarly, the incidence of hypoglycaemia was 3.46 versus 2.23 per 100 patient-months among non-diabetes patients with CKD versus without CKD (36). Among patients with end stage renal disease (ESRD), hypoglycaemia accounts for up to 3.6% of all admissions (39). ESRD cases who develop hypoglycaemia during hospitalization had poor prognosis with a mortality rate of nearly 30%. Hypoglycaemia in patients with ESRD could be caused by malnutrition, alcohol abuse, multiple organ failure, infections, drugs or hypoadrenalism (40). Nearly 20% of ESRD cases on dialysis with hypotension have biochemical diagnosis of adrenal insufficiency (41) After excluding adrenal insufficiency, most of the cases of hypoinsulinaemic hypoglycaemia in ESRD patients are multifactorial due to malnutrition, infection, multiorgan dysfunction, or drugs (quinolones, pentamidine, quinine, trimethoprim-sulfamethoxazole, or megestrol acetate) (40).
Most of the other causes of hypoglycaemia in patients with renal failure are associated with undetectable insulin levels. When associated with high insulin levels, consider coexistence of insulinoma (42) or nesidioblastosis (43). There is no established biochemical criterion for diagnosis of hyperinsulinaemic hypoglycaemia in patients with renal failure (43). The fasting insulin and C-peptide levels are higher in renal failure cases (decreased clearance) when compared to individuals with normal kidney function, and these levels will come down by 50% following haemodialysis (44). The diagnostic work up for hypoinsulinaemic hypoglycaemia in renal failure is difficult due to the challenges in the interpretation of insulin and C-peptide levels.
The treatment of hyperkalaemia with glucose-insulin regimen might result in hypoglycaemia in 13% of ESRD cases (45). In order to avoid this, some recommend reducing the dose of intravenous regular insulin to 5–6 units (rather than 10 units) given along with the standard dose of 25 grams of glucose (50 mL of 50% glucose) (42). During haemodialysis, glucose-free dialysate predisposes to development of hypoglycaemia. In patients with post-dialysis hypoglycaemia, some dialysis providers consider using a higher dialysate glucose concentration, although this might have unfavourable metabolic effects including hyperglycaemia and hyperinsulinism, in patients on chronic hemodialysis (46).
Treatment of multifactorial hypoglycaemia that occurs in ESRD can be with 15 grams of oral glucose, or intravenous glucose (if unable to take oral glucose) or with intramuscular glucagon 1 mg (if no intravenous access) (42). Glucagon may not be effective in malnourished ESRD cases due to the lack of hepatic glycogen reserve. In those ESRD cases with refractory hypoglycaemia in which nutritional interventions, optimization of dialysis and medication adjustments have not ameliorated the spontaneous hypoglycaemia, diazoxide that inhibits the insulin release by binding to ATP-sensitive potassium channels in the β-cells can be tried (47).
Liver failure
Patients with acute liver failure as well acute decompensated liver cirrhosis can present with hypoglycaemia, in whom hypoglycaemia can mask the symptoms of hepatic encephalopathy or vice versa (48). The pathogenesis of spontaneous hypoglycaemia in patients with liver disease could be decreased glycogen stores, gluconeogenesis, glycogenolysis, and impaired insulin uptake as well as degradation by the hepatocytes resulting in hyperinsulinism (49). An analysis of retrospective data showed that among patients with acute decompensation of liver cirrhosis, those who developed hypoglycaemia had increased risk of ICU admissions and increased in-hospital mortality (50). However, it was unclear whether hypoglycaemia was just a marker of severity of decompensated liver disease or contributed directly to the excess mortality risk. In a retrospective cross-sectional study among hypoglycaemic hospitalized patients, the mortality risk was 4.4-fold higher among those with concurrent liver cirrhosis than those without (51).
Hypoglycaemia can be seen in a variety of liver diseases including ischaemic hepatitis resulting from hypoperfusion due to heart failure (52), or post-operative state (53), toxic hepatitis from paracetamol overdosage (54), or as an atypical early presentation of hepatocellular carcinoma (55). Though the King’s College criteria is used as a prediction tool to list patients for emergency liver transplantation in paracetamol-induced toxic hepatitis, a recent study showed that a combination of hypoglycaemia, lactic acidosis, and coagulopathy is a better predictor of mortality, or indication for transplantation compared to the King’s College criteria (54). There are rare paradoxical reports of hypoglycaemia secondary to use of octreotide in patients with liver cirrhosis (56), though octreotide is useful in the treatment of hypoglycaemia induced by sulfonylurea (57), as well as levofloxacin (58).
Heart failure
The primary mechanism in the pathogenesis of hypoglycaemia in patients with heart failure is congestive hepatopathy (59). Various other patterns of hepatic involvement in heart failure apart from congestive hepatopathy include hepatic fibrosis, hepatic cirrhosis, and ischaemic hepatitis (60). The gastrointestinal involvement in heart failure is characterised by gut oedema secondary to systemic volume overload. Gut oedema can lead to bacterial translocation into the systemic circulation, systemic inflammation, and heart failure progression (60). Heart failure related spontaneous hypoglycaemia is mediated by impaired hepatic gluconeogenesis and glycogenolysis along with decreased intestinal absorption of glucose (61). Moreover, liver failure is associated with reduced insulin degradation and shunting of portal venous blood into the systemic circulation leading to hyperinsulinism that augments the risk of hypoglycaemia (62). As hypoglycaemia can be a presenting symptom of heart failure, this possibility should be considered in the differential diagnosis of spontaneous hypoglycaemia, especially in the elderly (63).
Respiratory failure
In a study of 5,365 medical, surgical, and cardiac ICU patients, mechanical ventilation was identified as an independent risk factor for the development of severe hypoglycaemia (<2.2 mmol/L) along with other independent risk factors including diabetes, tight glycaemic control using the intensive insulin therapy, septic shock, renal failure, and severity of illness (64). This study observed severe hypoglycaemia as an independent predictor of mortality, and among severely hypoglycaemic patients, mechanical ventilation independently predicted mortality.
A study of 596 post-cardiac surgery intensive care patients observed that hypoglycaemia was an independent determinant for tracheostomy (OR 21.6) and respiratory failure necessitating prolonged mechanical ventilation (OR 1.4) (65). The proposed mechanism for high tracheostomy rate was neurological consequences of hypoglycaemia that impaired the respiratory function and delayed the weaning from ventilation which increased the need for tracheostomy. The study also revealed that postoperative patients with hypoglycaemia had increased requirement for mechanical haemodynamic support including intra-aortic balloon pump (IABP), indicating hypoglycaemia mediated cardiac dysfunction.
Respiratory failure that accompanies pneumococcal infections with overwhelming sepsis could be associated hypoglycaemia (66). Hence, patients with severe chest sepsis and impending respiratory failure should have their glucose levels monitored to avoid missing hypoglycaemia that can worsen the outcome. On the contrary, hypoglycaemia might cause respiratory failure in adults and children (67,68) with or without diabetes. Moreover, severe hypoglycaemia can rarely present as noncardiogenic pulmonary oedema mediated by the sympathetic stimulation and catecholamine surge, much like neurogenic pulmonary oedema (69).
Hypothermia
A study that examined the association between spontaneous hypoglycaemia and seasonal variation revealed that severe hypoglycaemia and sepsis occurred significantly more often in the winter months than in summer (30.6% vs. 19.6% and 24.5% vs. 5.9%, respectively) (70). Patients treated with therapeutic hypothermia after cardiac arrest demonstrated improved neurologic outcomes and decreased mortality (71). Therapeutic hypothermia is known to reduce the insulin secretion and increase insulin resistance. Therefore, hyperglycaemia is often seen during initiation of therapeutic hypothermia and hypoglycaemia is seen during rewarming (71). Similar response is seen during rewarming after accidental hypothermia (prolonged avalanche burial), in which spontaneous hypoglycaemia and respiratory failure were observed (72).
Malnutrition
A cross-sectional study that evaluated the association between Nutritional Risk Screening 2002 (NRS2002) score, an indicator of malnutrition, and incidence of hypoglycaemia revealed that higher NRS2002 scores are associated with increased incidence of hypoglycaemia (OR 1.982, 95% CI: 1.056–3.718), regardless of age, sex and diabetes status (73). Moreover, when NRS2002 is combined with hypoalbuminemia, the overall sensitivity improved from 0.55 to 0.71, but specificity reduced from 0.63 to 0.46. The combination is associated with increased hypoglycaemia incidence, regardless of diabetes status (74). Patients with malnutrition related hypoglycaemia is associated with prolonged duration of hospitalisation and increased mortality (30-day and 1-year mortality), regardless of diabetes status (75).
Elderly patients with malnutrition are at high risk for hypoglycaemia due to depleted glycogen stores (76). At least 2% of patients on palliative care develop hypoglycaemia and nearly 25–60% of them are asymptomatic, without autonomic or neuroglycopenic symptoms (77). Hence it is possible that hypoglycaemia might go unnoticed. A shared decision-making between patient and caregiver is needed in these patients regarding optimal management. Patients with anorexia nervosa are at risk of developing hypoglycaemia due to depleted glycogen stores, impaired glycogenolysis and gluconeogenesis (78). Reactive hypoglycaemia can be seen during early refeeding phase in these patients and acute liver failure can be potentially fatal.
Intoxication
Alcohol is known to cause hypoglycaemia by impairment of gluconeogenesis, increasing the blood flow to pancreatic islets, and thereby stimulating the late phase of insulin secretion, and by reducing the counter-regulatory hormone production (79). Tramadol intoxication can cause hypoglycaemia by either serotonin or µ-opioid receptor (MOR) mediated stimulation of insulin secretion, reduction in hepatic gluconeogenesis, enhancement of insulin sensitivity and glucose utilisation by the liver and skeletal muscle (80). Another pathogenic mechanism of tramadol-induced hypoglycaemia is N-methyl-D-aspartate receptor (NMDAR) antagonism. Methadone use also can cause a dose-dependent hypoglycaemia via MOR stimulation. Other opioids such as morphine, fentanyl, levorphanol, or oxycodone did not show significant association with hypoglycaemia (80). Amphetamine and methamphetamine (Ecstasy) cause hypoglycaemia both in animal models (81) and humans (82), by inappropriate insulin secretion. During the COVID-19 pandemic, many countries used hydroxychloroquine by virtue of its effect against macrophage activation. When the drug levels are high it might result in intracellular alkalinisation, inactivation of insulinase, increased insulin levels and hypoglycaemia (83). Figure 1 summarizes the diagnostic approach to hypoglycaemia in critically ill patients.
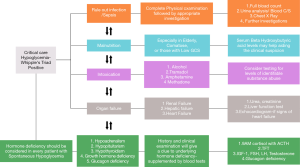
Hypoglycaemia associated with hormone deficiencies
Circadian rhythm and implications on hypoglycaemia
In healthy nondiabetic subjects, plasma insulin levels remain constant overnight with a modest transient rise in insulin secretion just before dawn to suppress the hepatic glucose output and to prevent hyperglycaemia (84). In healthy nondiabetic subjects with a stable sleep-wake cycles, the serum cortisol levels begin to rise by 04:00 hours, reach a peak between 07:00 and 09:00 hours, and then fall for the rest of the day to reach the nadir (<50% of the morning cortisol levels) between 22:00 and 02:00 hours (85,86). Similarly, GH exhibits a circadian rhythm with the peak in the second half of night (84).
As lower levels of cortisol and GH are associated with improved insulin sensitivity, the insulin sensitivity is highest between 02:00 and 04:00 hours. Thereafter, the increase in counterregulatory hormones especially the GH is responsible for the increase in insulin resistance occurring after 04:00 hours in healthy subjects. It is important to note that healthy subjects will not develop hyperglycaemia (dawn phenomenon) despite an increased insulin resistance as they secrete extra insulin to prevent it. However, patients with type 1 and type 2 diabetes do exhibit dawn phenomenon (84). Patients with adrenal insufficiency and type 1 diabetes mellitus (T1DM) are at highest risk of hypoglycaemia between 02:00 and 04:00 hours, due to various reasons. The Insulin sensitivity being at its peak, coupled with the lowest serum cortisol levels at this period experienced by patients on standard hydrocortisone supplementation—leads to nocturnal hypoglycaemia (87).
Adrenal insufficiency
Primary adrenal insufficiency is a life-threatening condition characterized by insufficient secretion of glucocorticoids with or without insufficient mineralocorticoids and androgens. It is associated with a rise in adrenocorticotropic hormone (ACTH) and/or renin. Adrenal insufficiency due to hypothalamic-pituitary disease (central hypoadrenalism) can be either secondary adrenal insufficiency due to pituitary diseases, or tertiary adrenal insufficiency due to diseases of hypothalamus, or ACTH suppression by endogenous (Cushing’s syndrome) or exogenous cortisol excess. Central hypoadrenalism accounts for ~60% and primary adrenal insufficiency for ~40% of all adrenal insufficiency cases (88).
The diagnosis of adrenal insufficiency is frequently delayed due to its rarity and non-specific presentation such as weakness, fatigue, postural dizziness, musculoskeletal pain, weight loss, abdominal pain, and mood disorders. Among T1DM patients, 15–30% have autoimmune thyroid disease (AITD), 4–9% coeliac disease, and 0.5% Addison’s disease (89). On the other hand, among Addison’s disease patients, nearly 50% have AITD and up to 14% have T1DM (90). The estimated prevalence for the combination of T1DM and Addison’s disease is 20 cases per million (90).
Hypoglycaemia can occur in patients with undiagnosed or undertreated hypoadrenalism, though it is not the common presentation of hypoadrenalism in patients with or without T1DM (91). Cortisol deficiency increases the insulin sensitivity whereas cortisol excess worsens the insulin resistance. The enhanced insulin sensitivity due to cortisol deficiency increases the peripheral utilisation of glucose, and suppresses the gluconeogenesis to decrease the hepatic glucose output causing hypoglycaemia (92). Whilst hypoglycaemia related to hypoadrenalism is common in children than in adults, several cases of nocturnal and fasting hypoglycaemia are reported in adults despite steroid replacement. As hypoadrenalism is one of the treatable causes of hypoglycaemia, it should be considered as a differential diagnosis of unexplained recurrent hypoglycaemia in conjunction with a decline in insulin requirements in patients on diabetes treatment. There should be a low threshold for appropriate investigations (93).
The gold standard investigation to establish the diagnosis is short synacthen test. However, 09:00 AM cortisol can be used as a screening test, which could reduce the number of unnecessary synacthen tests by 21%. Synacthen test provides no additional information if the 09:00 AM cortisol is <100 nmol/L or >500 nmol/L (94). Even though adrenal antibodies can be used to screen for Addison's disease, this test lacks specificity that only 15% of T1DM patients who were tested positive for adrenal antibodies have biochemical evidence of Addison’s disease. Furthermore, negative adrenal antibody tests does not exclude Addison’s disease (95). Though routine determination of adrenal antibody levels is not recommended, it can be used in T1DM patients over the age of 18 years with clinical symptoms of adrenal insufficiency or once in every 5 years if there is adrenal insufficiency in the first-degree relatives (96). Once hypoadrenalism is diagnosed, further hypoglycaemic episodes can be prevented by strict adherence to the sick day rules (97).
Hypopituitarism
Loss of counter-regulatory hormones (GH and ACTH) secondary to many types of pituitary insult can result in an increased sensitivity to insulin, sudden decrease in insulin requirements, hypoglycaemia, or even complete amelioration of diabetes. This is known as the Houssay phenomenon (98). Various causes for hypopituitarism include neoplasms such as pituitary adenoma, craniopharyngioma, and meningioma; cysts like Rathke’s cleft, arachnoid, epidermoid, and dermoid cysts; treatment of sellar, parasellar, and hypothalamic diseases with surgery or radiotherapy; inflammatory or infiltrative diseases like lymphocytic hypophysitis, hemochromatosis, granulomatosis with polyangiitis, sarcoidosis, and Langerhans cell histiocytosis; pituitary apoplexy; Sheehan’s syndrome (postpartum pituitary necrosis); infections like bacterial, fungal, parasitic, tuberculosis or syphilis; head injury; empty sella; and idiopathic hypopituitarism (99). Recurrent or refractory hypoglycaemia with or without hyponatremia is described in relation to many of the causes of hypopituitarism.
In patients with hypopituitarism, the sequential failure of pituitary hormones is in the following order: GH, followed by LH and FSH, and finally TSH and ACTH. In general, ACTH is the last pituitary hormone to be lost (100). Hence, patients with secondary adrenal insufficiency is characterized by other hormone deficiencies. One exception to this rule is lymphocytic hypophysitis where ACTH deficiency, seen in 65%, is an early and frequent feature (101,102). The short synacthen test that is used in the diagnosis of primary adrenal insufficiency can show a physiological increase in cortisol early during the course of secondary adrenal insufficiency. However, absence of ACTH drive causes adrenal dysfunction over time, and the cortisol response is usually lost in ~6 weeks (98). Hypoglycaemia is more common in patients with hypopituitarism compared to primary adrenal insufficiency, as the former is associated with concomitant GH and ACTH insufficiency (103). Hypopituitarism can also cause hyponatraemia secondary to inappropriate vasopressin secretion. However, as the zona glomerulosa of adrenal glands is mostly preserved, signs such hyperkalaemia, hypotension, and dehydration will be less prominent as the case of primary adrenal failure.
When a patient without the diagnosis of hypoadrenalism presents with adrenal crisis, certain clinical clues might help in differentiating primary adrenal insufficiency from central hypoadrenalism. Patients with primary adrenal insufficiency have the characteristic skin hyperpigmentation particularly in areas exposed to mechanical shear stress including palmar creases, nipples, scars, and oral mucosa, whereas central hypoadrenalism is associated with alabaster-like pale skin (88). Abdominal symptoms such as abdominal pain, tenderness, guarding, nausea, and vomiting are particularly common in primary adrenal insufficiency than central hypoadrenalism. Patients with central hypoadrenalism have symptoms and signs suggestive of pituitary or hypothalamic pathology (headache, ophthalmoplegia, or field defects) or coexisting anterior pituitary hormone deficiencies.
In a medically unstable patient with provisional diagnosis of hypoadrenalism, it is unsafe to delay treatment pending investigations. In these situations, the advice is to collect blood sample for cortisol and ACTH measurement and treat with intravenous hydrocortisone while awaiting results. Anterior pituitary hormone profile should be done in medically stable patients with suspected central hypoadrenalism with or without hypopituitarism. This include 9 AM paired cortisol and ACTH followed by a short synacthen test; TSH and free T4; insulin like growth factor-1 (IGF-1) and prolactin; LH, FSH and testosterone or oestradiol. In patients with central hypoadrenalism, the synacthen test will have a diagnostic value only after 3–6 weeks of an acute pituitary insult like pituitary surgery, apoplexy, or traumatic brain injury (104). This is the minimal time for the adrenal atrophy to happen following ACTH deficiency. As short synacthen test cannot be used in these settings, the alternative is to use insulin tolerance test (ITT), which can assess both the ACTH production from pituitary gland as well as the steroid response from adrenal gland at the same time (105). Furthermore, the ITT has the advantage that the GH axis can be simultaneously assessed (104). On the flip side, ITT cannot be used in patients with ischaemic heart disease, cerebrovascular disease, and seizures (99).
Hyperprolactinaemia is associated with reduced insulin sensitivity. Though dopaminergic agonists improved the insulin sensitivity, they did not increase the hypoglycaemia (106). Hypoglycaemia is not uncommon with pituitary disorders, nevertheless it is extremely rare with hypothalamic disorders. Hypopituitarism poses a therapeutic challenge in patient management, as many important principles are involved as given in the Table 2 (99).

Full table
Hypothyroidism
Hypothyroidism is one of the uncommon causes of hypoglycaemia. Various mechanisms involved in the development of hypoglycaemia included decreased appetite, decreased insulin clearance, delayed gastric emptying, decreased portal venous flow, decreased intestinal glucose absorption, decreased hepatic glucose output due to reduction in glycogenolysis and gluconeogenesis, and finally, reduction in glucagon secretion and its action on hepatocytes (107). The improvement in hypoglycaemia once thyroid functions are normalized following treatment with levothyroxine is a point in favour of this concept. However, some researchers consider that presence of hypoglycaemia in a patient with hypothyroidism should suggest possibility of underlying hypopituitarism (108). Blunting of hypothalamic-pituitary-adrenal axis (109) and GH secretion (110) has been observed in hypothyroidism cases which provides the mechanistic evidence for the hypoglycaemia in these patients.
GH deficiency
GH induces insulin resistance as it antagonises the hepatic and peripheral effects of insulin on glucose metabolism, and insulin resistance is a defence against prolonged hypoglycaemia (111). Furthermore, insulin resistance mediates the development of stress hyperglycaemia during fasting or inflammatory illness, and the dawn phenomenon (112). GH deficiency has varying impact on glucose homeostasis at various stages of life. GH deficient children tend to be insulin sensitive and develop spontaneous hypoglycaemia due to reduced hepatic glucose production (113), whereas GH deficient adults who are not receiving GH replacement are insulin resistant, probably due to increased adiposity, reduced lean body mass (LBM), and impaired exercise tolerance (114). The insulin resistance in GH deficient adults will exhibit temporary deterioration when GH replacement is initiated. Patients with GH excess (acromegaly) are consistently insulin resistant despite increased LBM and decreased fat mass and become more insulin sensitive after proper therapy (113).
In children, GH deficiency is a well described treatable cause of hypoglycaemia whereas it is a rare cause of hypoglycaemia in the adults. Mecasermin is a recombinant human IGF-1 that is used in the treatment of children with growth failure resulting from primary IGF-1 deficiency, or for cases of GH gene deletion with neutralising antibodies to GH that make therapy with GH ineffective. Hypoglycaemia is a well-recognised side effect and care should be taken to administer the drug with food (115).
Glucagon deficiency
Glucagon is the main counter-regulatory hormone to insulin secreted in response to hypoglycaemia. In healthy individuals, a fall in plasma glucose level causes a decrease in insulin secretion that signals a rise in glucagon secretion, associated with catecholamine secretion. Glucagon is more important than catecholamine in the counterregulatory response to hypoglycaemia as studies have shown that catecholamine cannot prevent hypoglycaemia in the presence of complete glucagon deficiency state (116). Glucagon increases the glucose levels by increasing the glycogenolysis and gluconeogenesis which increases the hepatic glucose output. The glucagon also decreases the glycolysis and glycogenesis (117). The isolated glucagon deficiency resulting in spontaneous hypoglycaemia can occur in conditions including autoimmune α-cell failure (118), and post-pancreatectomy (119).
Catecholamine deficiency
Catecholamine excess states like pheochromocytoma and paraganglioma are associated with hyperglycaemic states including diabetic ketoacidosis, and hyperosmolar hyperglycaemic state. The pathogenetic mechanisms include decreased glucose uptake (via desensitization of β-adrenoreceptors), impaired insulin secretion, increased lipolysis, and increased hepatic insulin resistance (via pro-inflammatory state) (120). Resection of these tumours might result in reactive hypoglycaemia in the immediate post-operative period. There was no significant difference between adrenal and extra-adrenal pheochromocytomas regarding the incidence of post-operative hypoglycaemia, though it is more common in pheochromocytomas that are adrenaline producing and is associated with von Hippel-Lindau disease (121,122).
Mechanisms for post-operative hypoglycaemia are enhanced insulin secretion secondary to sudden catecholamine withdrawal or use of β-blocker; impaired glucagon response to hypoglycaemia in presence of catecholamine excess; or preoperative α-blockade without β-blockade (119). Pheochromocytoma cases can develop hypoglycaemia even before surgical removal and the proposed mechanisms include enhanced consumption of glucose by the rapidly dividing tumor cells (123), or enhanced insulin release by the action of catecholamine on the β-adrenoreceptors (124).
Summary and conclusions
Whilst the presence of spontaneous hypoglycaemia is clearly associated with increased mortality independent of any other confounding factors, it is considered as a sign of poor physiological reserve and hence poor outcome rather than a cause and effect phenomenon. Occurrence of hypoglycaemia in a non-diabetic cohort is associated with increased mortality as compared to those with diabetes. Spontaneous hypoglycaemia can result from severe sepsis, overwhelming infection as well as multiorgan failures including renal, hepatic, and cardiac failure. A variety of endocrinopathies leading to hypoglycaemia adds to the diagnostic conundrum. The daunting task of evaluation of hypoglycaemia is made simple by a systematic approach to clinical evaluation and diligent investigations as outlined. In most of the situations, hypoglycaemia in non-diabetic individuals should be considered as a poor prognostic indicator necessitating closer observation in the intensive care unit. Clinicians should be more alert and have low threshold for specific evaluation and management of spontaneous hypoglycaemia which can improve the patient outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rousseau Gama) for the series “Adult Spontaneous Hypoglycaemia” published in Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/jlpm-2020-ash-01
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jlpm-2020-ash-01). The series “Adult Spontaneous Hypoglycaemia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cryer PE. Glucose homeostasis and hypoglycaemia, in Williams Textbook of Endocrinology, 13th edn. By Melmed S, Polonsky KS, Larsen PR, et al. ed. (Elsevier, New York), 2015:1582-607.
- Kandaswamy L, Raghavan R, Pappachan JM. Spontaneous hypoglycaemia: diagnostic evaluation and management. Endocrine 2016;53:47-57. [Crossref] [PubMed]
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycaemic disorders: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009;94:709-28. [Crossref] [PubMed]
- Vihonen H, Kuisma M, Nurmi J. Hypoglycaemia without diabetes encountered by emergency medical services: a retrospective cohort study. Scand J Trauma Resusc Emerg Med 2018;26:12. [Crossref] [PubMed]
- Sako A, Yasunaga H, Matsui H, et al. Hospitalization with hypoglycaemia in patients without diabetes mellitus: A retrospective study using a national inpatient database in Japan, 2008-2012. Medicine (Baltimore) 2017;96:e7271. [Crossref] [PubMed]
- Sako A, Yasunaga H, Matsui H, et al. Hospitalization for hypoglycaemia in Japanese diabetic patients: A retrospective study using a national inpatient database, 2008-2012. Medicine (Baltimore) 2015;94:e1029. [Crossref] [PubMed]
- Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycaemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556-64. [Crossref] [PubMed]
- Boucai L, Southern WN, Zonszein J. Hypoglycaemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011;124:1028-35. [Crossref] [PubMed]
- Bellomo R, Egi M. Hypoglycaemia in sepsis: biomarker, mediator, or both? Crit Care Med 2011;39:2367-9. [Crossref] [PubMed]
- Kagansky N, Levy S, Rimon E, et al. Hypoglycaemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med 2003;163:1825-9. [Crossref] [PubMed]
- Meneilly GS, Cheung E, Tuokko H. Altered responses to hypoglycaemia of healthy elderly people. J Clin Endocrinol Metab 1994;78:1341-8. [PubMed]
- Wernly B, Jirak P, Lichtenauer M, et al. Hypoglycaemia but not hyperglycemia is associated with mortality in critically ill patients with diabetes. Med Princ Pract 2019;28:186-92. [Crossref] [PubMed]
- Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738-47. [Crossref] [PubMed]
- Wernly B, Jung C. Reply to the letter to the editor "hypoglycaemia and mortality in critically ill patients with type 2 diabetes". Med Princ Pract 2020;29:100. [Crossref] [PubMed]
- Saliba L, Cook CH, Dungan KM, et al. Medication-induced and spontaneous hypoglycaemia carry the same risk for hospital mortality in critically ill patients. J Crit Care 2016;36:13-7. [Crossref] [PubMed]
- Egi M, Bellomo R, Stachowski E, et al. Hypoglycaemia and outcome in critically ill patients. Mayo Clin Proc 2010;85:217-24. [Crossref] [PubMed]
- Garg R, Hurwitz S, Turchin A, et al. Hypoglycaemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care 2013;36:1107-10. [Crossref] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin Diabetes 2020;38:10-38. [Crossref] [PubMed]
- Schlenk F, Graetz D, Nagel A, et al. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care 2008;12:R9. [Crossref] [PubMed]
- Inouye KE, Chan O, Yue JT, et al. Effects of diabetes and recurrent hypoglycemia on the regulation of the sympathoadrenal system and hypothalamo-pituitary-adrenal axis. Am J Physiol Endocrinol Metab 2005;288:E422-9. [Crossref] [PubMed]
- Herlein JA, Morgan DA, Phillips BG, et al. Antecedent hypoglycaemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology 2006;147:2781-8. [Crossref] [PubMed]
- Diéguez G, Fernandez N, Garcia JL, et al. Role of nitric oxide in the effects of hypoglycaemia on the cerebral circulation in awake goats. Eur J Pharmacol 1997;330:185-93. [Crossref] [PubMed]
- Duning T, van den Heuvel I, Dickmann A, et al. Hypoglycaemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care 2010;33:639-44. [Crossref] [PubMed]
- Krinsley J, Schultz MJ, Spronk PE, et al. Mild hypoglycaemia is strongly associated with increased intensive care unit length of stay. Ann Intensive Care 2011;1:49. [Crossref] [PubMed]
- Furukawa M, Kinoshita K, Yamaguchi J, et al. Sepsis patients with complication of hypoglycaemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med 2019;14:539-48. [Crossref] [PubMed]
- Miller SI, Wallace RJ Jr, Musher DM, et al. Hypoglycaemia as a manifestation of sepsis. Am J Med 1980;68:649-54. [Crossref] [PubMed]
- Maitra SR, Wojnar MM, Lang CH. Alterations in tissue glucose uptake during the hyperglycemic and hypoglycaemic phases of sepsis. Shock 2000;13:379-85. [Crossref] [PubMed]
- Alamgir S, Volkova NB, Peterson MW. Prognostic value of low blood glucose at the presentation of E. coli bacteremia. Am J Med 2006;119:952-7. [Crossref] [PubMed]
- Mortensen EM, Garcia S, Leykum L, et al. Association of hypoglycaemia with mortality for subjects hospitalized with pneumonia. Am J Med Sci 2010;339:239-43. [Crossref] [PubMed]
- Peralta G, Sanchez MB, Garrido JC, et al. Altered blood glucose concentration is associated with risk of death among patients with community-acquired Gram-negative rod bacteremia. BMC Infect Dis 2010;10:181. [Crossref] [PubMed]
- Krinsley JS, Schultz MJ, Spronk PE, et al. Mild hypoglycaemia is independently associated with increased mortality in the critically ill. Crit Care 2011;15:R173. [Crossref] [PubMed]
- Park S, Kim DG, Suh GY, et al. Mild hypoglycaemia is independently associated with increased risk of mortality in patients with sepsis: a 3-year retrospective observational study. Crit Care 2012;16:R189. [Crossref] [PubMed]
- Planche T, Dzeing A, Ngou-Milama E, et al. Metabolic complications of severe malaria. Curr Top Microbiol Immunol 2005;295:105-36. [Crossref] [PubMed]
- Ogetii GN, Akech S, Jemutai J, et al. Hypoglycaemia in severe malaria, clinical associations, and relationship to quinine dosage. BMC Infect Dis 2010;10:334. [Crossref] [PubMed]
- Arem R. Hypoglycaemia associated with renal failure. Endocrinol Metab Clin North Am 1989;18:103-21. [Crossref] [PubMed]
- Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycaemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1121-7. [Crossref] [PubMed]
- Alsahli M, Gerich JE. Hypoglycaemia in patients with diabetes and renal disease. J Clin Med 2015;4:948-64. [Crossref] [PubMed]
- Meyer C, Dostou JM, Gerich JE. Role of the human kidney in glucose counterregulation. Diabetes 1999;48:943-8. [Crossref] [PubMed]
- Gianchandani RY, Neupane S, Iyengar JJ, et al. Pathophysiology and management of hypoglycaemia in end-stage renal disease patients: A review. Endocr Pract 2017;23:353-62. [Crossref] [PubMed]
- Gosmanov AR, Gosmanova EO, Kovesdy CP. Evaluation and management of diabetic and non-diabetic hypoglycaemia in end-stage renal disease. Nephrol Dial Transplant 2016;31:8-15. [Crossref] [PubMed]
- Arregger AL, Cardoso EM, Zucchini A, et al. Adrenocortical function in hypotensive patients with end stage renal disease. Steroids 2014;84:57-63. [Crossref] [PubMed]
- Foppiani L, Panarello S, Filauro M, et al. Insulinoma and Chronic Kidney Disease: An Uncommon Conundrum Not to Be Overlooked. Clin Med Insights Endocrinol Diabetes 2017;10:1179551417742620. [Crossref] [PubMed]
- Lozano-Melendez E, Aguilar-Soto M, Graniel-Palafox LE, et al. Adult nesidioblastosis in chronic kidney disease. Case Rep Endocrinol 2019;2019:7640384. [Crossref] [PubMed]
- Jørgensen MB, Idorn T, Knop FK, et al. Clearance of glucoregulatory peptide hormones during haemodialysis and haemodiafiltration in non-diabetic end-stage renal disease patients. Nephrol Dial Transplant 2015;30:513-20. [Crossref] [PubMed]
- Apel J, Reutrakul S, Baldwin D. Hypoglycaemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J 2014;7:248-50. [Crossref] [PubMed]
- Raimann JG, Kruse A, Thijssen S, et al. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transplant 2012;27:1559-68. [Crossref] [PubMed]
- Mesmar B, Kristan M, Satyarengga M, et al. The use of diazoxide in the management of spontaneous hypoglycaemia in patients with ESRD. CEN Case Rep 2020;9:271-7. [Crossref] [PubMed]
- Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med 2015;19:27-33. [Crossref] [PubMed]
- Samson RI, Trey C, Timme AH, et al. Fulminating hepatitis with recurrent hypoglycaemia and hemorrhage. Gastroenterology 1967;53:291-300. [Crossref]
- Pfortmueller CA, Wiemann C, Funk GC, et al. Hypoglycaemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care 2014;29:316.e7-12. [Crossref] [PubMed]
- Su YJ, Lai YC, Liao CJ. Hazardous factors besides infection in hypoglycaemia. Biomed Rep 2017;6:480-4. [Crossref] [PubMed]
- Nomura T, Keira N, Urakabe Y, et al. Chronic pericardial constriction induced severe ischemic hepatitis manifesting as hypoglycaemic attack. Circ J 2009;73:183-6. [Crossref] [PubMed]
- Chung K, Bang S, Kim Y, et al. Intraoperative severe hypoglycaemia indicative of post-hepatectomy liver failure. J Anesth 2016;30:148-51. [Crossref] [PubMed]
- Levine M, Stellpflug SJ, Pizon AF, et al. Hypoglycaemia and lactic acidosis outperform King's College criteria for predicting death or transplant in acetaminophen toxic patients. Clin Toxicol (Phila) 2018;56:622-5. [Crossref] [PubMed]
- Tsai CY, Chou SC, Liu HT, et al. Persistent hypoglycaemia as an early, atypical presentation of hepatocellular carcinoma: A case report and systematic review of the literature. Oncol Lett 2014;8:1810-4. [Crossref] [PubMed]
- Abell SK, Teng J, Dowling A, et al. Prolonged life-threatening hypoglycaemia following dose escalation of octreotide LAR in a patient with malignant polysecreting pancreatic neuroendocrine tumour. Endocrinol Diabetes Metab Case Rep 2015;2015:140097. [Crossref] [PubMed]
- Glatstein M, Scolnik D, Bentur Y. Octreotide for the treatment of sulfonylurea poisoning. Clin Toxicol (Phila) 2012;50:795-804. [Crossref] [PubMed]
- Watson MR, Ward CT, Prabhakar A, et al. Successful use of octreotide therapy for refractory levofloxacin-induced hypoglycaemia: A case report and literature review. Case Rep Crit Care 2019;2019:3560608. [Crossref] [PubMed]
- Tumulty PA, Mellinkoff SM. Hypoglycaemia in congestive heart failure. Diabetes 1958;7:147. [Crossref] [PubMed]
- Sundaram V, Fang JC. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016;133:1696-703. [Crossref] [PubMed]
- Drah M, Ghose RR. Hypoglycaemia and heart failure. Postgrad Med J 1992;68:304. [Crossref] [PubMed]
- Khoury H, Daugherty T, Ehsanipoor K. Spontaneous hypoglycaemia associated with congestive heart failure attributable to hyperinsulinism. Endocr Pract 1998;4:94-5. [Crossref] [PubMed]
- Hajji R, Elleuch M, Derbali F, et al. Persistent hypoglycaemia revealing severe heart failure. J Gerontol Geriatr 2016;5:3. [Crossref]
- Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007;35:2262-7. [Crossref] [PubMed]
- D'Ancona G, Bertuzzi F, Sacchi L, et al. Iatrogenic hypoglycemia secondary to tight glucose control is an independent determinant for mortality and cardiac morbidity. Eur J Cardiothorac Surg 2011;40:360-6. [Crossref] [PubMed]
- Jan IS, Tsai TH, Chen JM, et al. Hypoglycaemia associated with bacteremic pneumococcal infections. Int J Infect Dis 2009;13:570-6. [Crossref] [PubMed]
- Baig MA, Ali S, Rasheed J, et al. Severe hypoglycaemia in a nondiabetic patient leading to acute respiratory failure. J Natl Med Assoc 2006;98:1362-4. [PubMed]
- Luber S, Meldon S, Brady W. Hypoglycaemia presenting as acute respiratory failure in an infant. Am J Emerg Med 1998;16:281-4. [Crossref] [PubMed]
- Mishriki YY. Hypoglycemia-induced neurogenic-type pulmonary edema: an underrecognized association. Endocr Pract 2004;10:429-31. [Crossref] [PubMed]
- Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Seasonal variations of severe hypoglycaemia in patients with type 1 diabetes mellitus, type 2 diabetes mellitus, and non-diabetes mellitus: clinical analysis of 578 hypoglycaemia cases. Medicine (Baltimore) 2014;93:e148. [Crossref] [PubMed]
- Haase KK, Grelle JL, Khasawneh FA, et al. Variability in glycemic control with temperature transitions during therapeutic hypothermia. Crit Care Res Pract 2017;2017:4831480. [Crossref] [PubMed]
- Strapazzon G, Nardin M, Zanon P, et al. Respiratory failure, and spontaneous hypoglycaemia during noninvasive rewarming from 24.7°C (76.5°F) core body temperature after prolonged avalanche burial. Ann Emerg Med 2012;60:193-6. [Crossref] [PubMed]
- Leibovitz E, Adler H, Giryes S, et al. Malnutrition risk is associated with hypoglycaemia among general population admitted to internal medicine units. Results from the MENU study. Eur J Clin Nutr 2018;72:888-93. [Crossref] [PubMed]
- Khanimov I, Ditch M, Adler H, et al. Prediction of Hypoglycemia During Admission of Non-Critically Ill Patients: Results from the MENU Study. Horm Metab Res 2020;52:660-8. [Crossref] [PubMed]
- Leibovitz E, Khanimov I, Wainstein J, et al. Documented hypoglycaemia is associated with poor short- and long-term prognosis among patients admitted to general internal medicine departments. Diabetes Metab Syndr 2019;13:222-6. [Crossref] [PubMed]
- Anno T, Kaneto H, Shigemoto R, et al. Hypoinsulinemic hypoglycemia triggered by liver injury in elderly subjects with low body weight: case reports. Endocrinol Diabetes Metab Case Rep 2018;2018:17-0155. [Crossref] [PubMed]
- Kok VC, Lee PH. Management of hypoglycaemia in nondiabetic palliative care patients: a prognosis-based approach. Palliat Care 2016;10:1-5. [PubMed]
- Mehler PS, Brown C. Anorexia nervosa - medical complications. J Eat Disord 2015;3:11. [Crossref] [PubMed]
- Hammerstedt H, Chamberlain SL, Nelson SW, et al. Alcohol-related hypoglycaemia in rural Uganda: socioeconomic and physiologic contrasts. Int J Emerg Med 2011;4:5. [Crossref] [PubMed]
- Makunts T, , Andrew U, Atayee RS, et al. Retrospective analysis reveals significant association of hypoglycemia with tramadol and methadone in contrast to other opioids. Sci Rep 2019;9:12490. [Crossref] [PubMed]
- Zhang Y, Shu G, Bai Y, et al. Effect of methamphetamine on the fasting blood glucose in methamphetamine abusers. Metab Brain Dis 2018;33:1585-97. [Crossref] [PubMed]
- Carrera P, Iyer VN. Profound hypoglycaemia with ecstasy intoxication. Case Rep Emerg Med 2015;2015:483153. [Crossref] [PubMed]
- Moro D, Gastaldi G, Zekry D, et al. Severe hypoglycemia in a nondiabetic old woman treated with a single oral dose of hydroxychloroquine for covid-19. J Geriatr Med Gerontol 2020;6:91.
- Porcellati F, Lucidi P, Bolli GB, et al. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care 2013;36:3860-2. [Crossref] [PubMed]
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016;101:364-89. [Crossref] [PubMed]
- Oprea A, Bonnet NCG, Polle O, et al. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab 2019;10:2042018818821294. [Crossref] [PubMed]
- Meyer G, Hackemann A, Reusch J, et al. Nocturnal hypoglycaemia identified by a continuous glucose monitoring system in patients with primary adrenal insufficiency (Addison's Disease). Diabetes Technol Ther 2012;14:386-8. [Crossref] [PubMed]
- Simpson H, Tomlinson J, Wass J, et al. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond) 2020;20:371-8. [Crossref] [PubMed]
- Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab 2006;91:1210-7. [Crossref] [PubMed]
- Løvås K, Husebye ES. High prevalence and increasing incidence of Addison's disease in western Norway. Clin Endocrinol (Oxf) 2002;56:787-91. [Crossref] [PubMed]
- Passanisi S, Timpanaro T, Lo Presti D, et al. Recurrent hypoglycaemia in type-1 diabetes mellitus may unravel the association with Addison's disease: a case report. BMC Res Notes 2014;7:634. [Crossref] [PubMed]
- Christiansen JJ, Djurhuus CB, Gravholt CH, et al. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab 2007;92:3553-9. [Crossref] [PubMed]
- Tanaka S, Abe M, Kohno G, et al. A single episode of hypoglycemia as a possible early warning sign of adrenal insufficiency. Ther Clin Risk Manag 2020;16:147-53. [Crossref] [PubMed]
- Le Roux CW, Meeran K, Alaghband-Zadeh J. Is a 0900-h serum cortisol useful prior to a short synacthen test in outpatient assessment? Ann Clin Biochem 2002;39:148-50. [Crossref] [PubMed]
- Likhari T, Magzoub S, Griffiths MJ, et al. Screening for Addison's disease in patients with type 1 diabetes mellitus and recurrent hypoglycaemia. Postgrad Med J 2007;83:420-1. [Crossref] [PubMed]
- Kakleas K, Soldatou A, Karachaliou F, et al. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev 2015;14:781-97. [Crossref] [PubMed]
- Arlt W. Society for Endocrinology Clinical Committee. Society for endocrinology endocrine emergency guidance: Emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect 2016;5:G1-G3. [Crossref] [PubMed]
- Pramanik S, Bhattacharjee R, Mukhopadhyay P, et al. Lesson of the month 2: Houssay phenomenon - hypopitutarism leading to remission of diabetes. Clin Med (Lond) 2016;16:294-6. [Crossref] [PubMed]
- Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016;101:3888-921. [Crossref] [PubMed]
- Grossman AB. Clinical Review#: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab 2010;95:4855-63. [Crossref] [PubMed]
- Caturegli P, Newschaffer C, Olivi A, et al. Autoimmune hypophysitis. Endocr Rev 2005;26:599-614. [Crossref] [PubMed]
- Lee P, Chrysostomou A, Tress B, et al. Lymphocytic hypophysitis: a rare cause of hypoglycaemia in a man with type 2 diabetes mellitus. Intern Med J 2005;35:254-7. [Crossref] [PubMed]
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014;383:2152-67. [Crossref] [PubMed]
- Crowley RK, Argese N, Tomlinson JW, et al. Central hypoadrenalism. J Clin Endocrinol Metab 2014;99:4027-36. [Crossref] [PubMed]
- Mukherjee JJ, de Castro JJ, Kaltsas G, et al. A comparison of the insulin tolerance/glucagon test with the short ACTH stimulation test in the assessment of the hypothalamo-pituitary-adrenal axis in the early post-operative period after hypophysectomy. Clin Endocrinol (Oxf) 1997;47:51-60. [Crossref] [PubMed]
- Auriemma RS, De Alcubierre D, Pirchio R, et al. Glucose abnormalities associated to prolactin secreting pituitary adenomas. Front Endocrinol (Lausanne) 2019;10:327. [Crossref] [PubMed]
- Saif A. Polyglandular dysfunction in patients with type 1 diabetes: Recurrent hypoglycaemia is an alarming symptom. Clin Diabetes 2016;34:113-4. [Crossref] [PubMed]
- Saleh M, Grunberger G. Hypoglycemia: An excuse for poor glycemic control? Clin Diabetes 2001;19:161-7. [Crossref]
- Johnson EO, Kamilaris TC, Calogero AE, et al. Effects of short- and long-duration hypothyroidism on function of the rat hypothalamic-pituitary-adrenal axis. J Endocrinol Invest 2013;36:104-10. [PubMed]
- Katz HP, Youlton R, Kaplan SL, et al. Growth and growth hormone. 3. Growth hormone release in children with primary hypothyroidism and thyrotoxicosis. J Clin Endocrinol Metab 1969;29:346-351. [Crossref] [PubMed]
- Nishad R, Mukhi D, Menon RK, et al. Growth hormone and metabolic homeostasis. EMJ Diabet 2018;6:78-87.
- Møller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152-77. [Crossref] [PubMed]
- Haymond MW, Karl I, Weldon VV, et al. The role of growth hormone and cortisone on glucose and gluconeogenic substrate regulation in fasted hypopituitary children. J Clin Endocrinol Metab 1976;42:846-56. [Crossref] [PubMed]
- Castillo AR, de Souza AL, Alegre SM, et al. Insulin sensitivity is not decreased in adult patients with hypopituitarism without growth hormone replacement. Front Endocrinol (Lausanne) 2019;10:534. [Crossref] [PubMed]
- Bhattacharya S, Kalra S, Dutta D, et al. The interplay between pituitary health and diabetes mellitus - the need for 'hypophyseo-vigilance'. Eur Endocrinol 2020;16:25-31. [Crossref] [PubMed]
- Abs R, Verbist L, Moeremans M, et al. Hypoglycaemia owing to inappropriate glucagon secretion treated with a continuous subcutaneous glucagon infusion system. Acta Endocrinol (Copenh) 1990;122:319-22. [Crossref] [PubMed]
- Quesada I, Tuduri E, Ripoll C, et al. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 2008;199:5-19. [Crossref] [PubMed]
- Starke AA, Valverde I, Bottazzo GF, et al. Glucagon deficiency associated with hypoglycaemia and the absence of islet cell antibodies in the polyglandular failure syndrome before the onset of insulin-dependent diabetes mellitus: a case report. Diabetologia 1983;25:336-9. [Crossref] [PubMed]
- Boden G, Master RW, Rezvani I, et al. Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. J Clin Invest 1980;65:706-16. [Crossref] [PubMed]
- Ronen JA, Gavin M, Ruppert MD, et al. Glycemic disturbances in pheochromocytoma and paraganglioma. Cureus 2019;11:e4551. [PubMed]
- Araki S, Kijima T, Waseda Y, et al. Incidence and predictive factors of hypoglycaemia after pheochromocytoma resection. Int J Urol 2019;26:273-7. [Crossref] [PubMed]
- Butz JJ, Yan Q, McKenzie TJ, et al. Perioperative outcomes of syndromic paraganglioma and pheochromocytoma resection in patients with von Hippel-Lindau disease, multiple endocrine neoplasia type 2, or neurofibromatosis type 1. Surgery 2017;162:1259-69. [Crossref] [PubMed]
- Habra MA, Nunez R, Chuang H, et al. Fatal hypoglycemia in malignant pheochromocytoma: direct glucose consumption as suggested by (18)F-2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging. Endocrine 2010;37:209-12. [Crossref] [PubMed]
- Thonangi RP, Bhardwaj M, Kulshreshtha B. A case report of reactive hypoglycaemia in a patient with pheochromocytoma and its review of literature. Indian J Endocrinol Metab 2014;18:234-7. [Crossref] [PubMed]
Cite this article as: Fernandez CJ, Rajgopal RK, Pappachan JM. Hypoglycaemia associated with critical illness and hormone deficiencies: a narrative review. J Lab Precis Med 2021;6:7.


