
Pleural fluid investigations for pleural infections
Introduction
Pleural infection is not a new disease. Hippocrates (460–377 BC) described pleurisy and its progression to empyema if left untreated and pioneered one of the first methods of drainage of the intercostal space. Pliny the Elder (23–79 AD) described an interesting success story of pleural drainage. When Publius Cornelius Rufus was convinced that he was dying from an empyema and decided to charge into battle, he was fortuitously struck by an arrow in the chest which helped drain his effusion and improved his condition. Proactive management and drainage of pus with dedicated instruments progressed in the 16th century, predominantly lead by Ambrosie Paré (1510–1590 AD). Further emphasis on early diagnosis of pleural infection began in the 19th century, aided by the invention of the stethoscope (1).
Centuries later, pleural infection continues to be a common problem worldwide, accounting for significant morbidity and mortality. The incidence is rising; particularly vulnerable are the elderly, immunocompromised and hospitalised patients. Despite advances in the diagnosis of pleural infection and paradigm shifts in its management, the disease is still associated with alarming short- and intermediate-term mortality rates.
Terminology
Multiple terms exist to describe the spectrum of pleural cavity infection. A parapneumonic effusion is one that develops following a pneumonic process, usually adjacent to an area of pneumonia.
A simple or uncomplicated parapneumonic effusion is one with free flowing and non-infected fluid. A complicated parapneumonic effusion (CPE) can develop when microbes translocate from the lung parenchyma to infect the pleural tissue/fluid, and is often defined by surrogate biochemical criteria [such as low pleural fluid pH and/glucose, elevated lactate dehydrogenase (LDH)] as well as neutrophilia. CPEs are often characterized by adhesions or septations and resultant loculated fluid collections. Empyema is conventionally referred to as frank pus in the pleural cavity (2,3) but contemporary definition sometimes includes non-purulent effusions where bacteria are identified by culture or gram-staining.
‘Pleural infection’ is a term used in most multicentre clinical trials and incorporates both ‘complicated parapneumonic effusion’ and ‘empyema’, on the basis that both subtypes typically require pleural fluid drainage for patients to improve.
Pathogenesis
The pathogenesis of pleural infection in humans remain unclear. In a murine model of Streptococcus pneumoniae, development of empyema involves bacterial invasion of the pleura, the release of proinflammatory cytokines [e.g., interleukin (IL)-6, IL-8 and tumour necrosis factor (TNF)-α] by pleural mesothelial cells, and subsequent pleural influx of neutrophils, the predominant leukocyte subtype seen within the fluids (4). This separates pleural infection from lymphocyte-predominant effusions, such as tuberculous, malignant or cardiac failure related pleural effusions, and are useful in their differentiation (4,5). The rate of progression through the stages of pleural infection vary among patients and may be influenced by the inciting organism, host immunity and comorbidities.
The initial ‘exudative phase’ of the parapneumonic effusion is characterised by an ‘uncomplicated’ parapneumonic effusion with a pH >7.2, glucose >3.3 mmol/L and LDH less than 3 times upper limit of its normal serum concentration. Bacteria is not usually identified in the pleural fluid at this stage. Management with antibiotic therapy is sufficient without evacuation of the effusion (5).
Left untreated, bacteria can translocate into the pleural space and the fluid becomes progressively loculated on imaging. Biological activities of bacteria and neutrophils result in increased glucose consumption and production of lactic acid and carbon dioxide. The fluid will therefore become increasingly infected (pH <7.2, glucose <3.3 mmol/L) and bacteria may be detected by culture. The inflammation also leads to complex alteration of the balance between pro- and anti-fibrotic states within the pleura and reduced fibrinolysis (related to elevations in plasminogen activator inhibitors). A more complex parapneumonic effusion eventually forms, with septations and loculations, termed the ‘fibrinopurulent phase’.
An ‘organising stage’ follows, characterised by fibroblast growth into the pleura (likely mediated by growth factors such as platelet-derived growth factors and transforming growth factor-β), producing a thick pleural rind, which prevents lung expansion (2,6).
Bacteria can also invade the pleural cavity through other routes. Haematogenous spread during septicaemia, translocation of skin organisms after penetrating injury or iatrogenic (during pleural procedures), and transdiaphragmatic migration of intraabdominal infections are known to occur (7).
Epidemiology
An estimated 4 million patients are admitted worldwide with pneumonia each year. Up to 57% develop a parapneumonic effusion, with higher incidences in males than females (3,8). Approximately 10% will progress to CPEs or empyema (5,9). In a study of 1269 patients with pneumonia, the development of pleural infection was associated with alcoholism, intravenous drug use and several biochemical abnormalities on admission including hypoalbuminaemia (<30 g/L), hyponatraemia (<130 mmol/L), thrombocytosis (>400×109/L) and high inflammatory markers [C-reactive protein (CRP) >100 mg/L] (9).
Pleural infection is estimated to occur in over 80,000 patients per year in the USA and UK with an estimated annual cost of $US500 million in the USA alone (8,10).
The incidences of pleural infection continue to increase worldwide in all (but particularly the over 65 years old) age groups. In population-based studies, the incidence of pleural infection increases with age and are higher in those with pre-existing comorbid conditions and in hospitalised patients with pneumonia, where it occurs in up to 20–40%, with 5–10% progressing to empyema (2,11-13). A recent systematic review found that 72% (median, IQR 58–83%) of patients with pleural infection had comorbid conditions especially respiratory (~20%) and cardiac (~19%) diseases in addition to diabetes (~17%) (14). The effect of multivalent pneumococcal vaccines on incidence of pleural infection has been a subject of many reports with some suggesting a phenomenon of replacement of serotypes covered by the vaccines with more invasive serotypes (13,15-17) thus causing a rise in empyema.
The presence of pleural effusions in patients presenting to hospital with pneumonia significantly increased their likelihood of needing hospitalization and their length of stay. Importantly those who presented with a pleural effusion had a mortality 2.6 times higher than pneumonia patients who did not have an effusion at presentation, even adjusting for known mortality prediction factors (e.g., CURB-65) (18). Patients with pleural infection often require protracted hospitalization with a median stay of 19 (IQR 13–27) days as found in a recent systematic review of worldwide studies (14).
In hospital or 30-day mortality has been reported to be 4–8% following pleural infection, but is as high as 20% in those with confirmed empyema, particularly in the elderly (11,12,14,19,20). Mortality rates are high in patients with positive bacterial isolates cultured from pleural fluid; a large Australian study (n=601) reported an in-hospital mortality of 17% and a one-year mortality of 32% (21). Patients with bilateral effusions had a 7-fold (and unilateral effusion a 3-fold) higher 30-day mortality than those without an effusion (22). Mortality from pleural infections tends to be higher in the elderly, immunocompromised individuals and those with comorbid conditions (22).
Conventional mortality prediction scores for pneumonia (e.g., CURB-65, pneumonia severity index and SMART-COP) cannot be reliably extended to patients with pleural infection (9). The RAPID (Renal, Age, Purulence of fluid, Infection source, Dietary factors) score is a new clinical risk score specifically derived for pleural infection using data from the MIST trial-1 and has been validated using the MIST 2 cohort and in a multinational (PILOT) study (23). Older age (especially >70 years) was the most powerful predictor of mortality. Higher urea level, hospital-acquired pleural infection and low blood albumin levels were associated with higher mortality risk (23). Interestingly, patients with non-purulent fluid had worse outcome than those with pus. It is unclear at present though what, if any, role these stratification tools will impact care.
Bacteriology of pleural infection
Despite the close relationship with pneumonia, their microbiology differs significantly. Streptococcus anginosus group of bacteria were the most common microbial cause of pleural infection in UK series though they are seldom considered as causative agents of pneumonia. Conversely, many common bacterial causes of pneumonia (e.g., Haemophilus influenzae) and pathogens in atypical pneumonia (e.g., Mycoplasma, Legionella, etc.) are seldom reported in pleural infection (24). The ability of bacteria to proliferate in the acidic and hypoxic environment of the infected pleural cavity may contribute to this disparity (25).
The microbial profile of pleural infection has changed significantly since the introduction of antibiotics and vaccines. Before the discovery of antibiotic, S. pneumoniae predominated (60–70%) but it now only accounts for ~11% of cases. Staphylococcus aureus has been reported as the most common causative agent in some series (8,26). Causative bacteria are different depending on the setting of infection (community-acquired or hospital-acquired), geographical location, and the age group of patients (27,28).
Community- vs. hospital-acquired pleural infection
The microbiology of community- and hospital-acquired pleural infections are distinctively different. A review by Bedawi et al. in 2018 summarised data from three studies on pleural infection. Viridans streptococci (including Streptococcus milleri and other oral commensals from Streptococcus genus) (25%), S. pneumoniae (23.8%), S. aureus (15.4%), Enterobacteriaceae (7.5%) and Pseudomonas spp. (3.2%) were the most common bacteria in community-acquired pleural infection. The most common hospital-acquired aerobic bacteria were S. aureus (42%), Enterobacteriaceae (13.6%), viridans streptococci (9%), Pseudomonas spp. (6.5%) and Klebsiella spp. (6%), and were associated with worse outcome in pleural infections (29). Anaerobic bacteria were reported in 18% and 11% of the community- and hospital-acquired pleural infections respectively.
Geographical location
Causative organisms of pleural infection vary among reports from different parts of the world. S. milleri have been found as the most common causative bacteria of pleural infection in reports from the UK, Australia, New Zealand and Denmark (8,21,25,30). S. milleri, otherwise known as the S. anginosus group (SAG), consists of three species, S. anginosus, S. constellatus and S. intermedius. SAG is normally found in the normal flora of the oral cavity, upper respiratory, gastrointestinal and urogenital tracts (31-33).
S. aureus, on the other hand, was the most common bacterial cause in a large report from the USA of 157,094 hospitalized cases of pleural infection (16). SAG was uncommon in that series. Reports from Taiwan and South Korea showed S. pneumoniae as the most common causative agent, and also reported higher percentage of K. pneumoniae compared to other regions (28,34,35).
A recent systematic review attempted to show the differences in common bacteriology of pleural infection according to the latitude of the regions: S. pneumoniae being the most common microorganism in tropical countries (e.g., Singapore, Saudi Arabia and Mexico), S. aureus in the subtropics (e.g., South East Asia, Middle East, southern Europe and southern USA) and viridans streptococci (including SAG) in temperate regions (North America and Europe) (26).
Age groups
The microbial profile of pleural infection in paediatric and adult patients vary significantly. S. pneumoniae is the predominate cause (85%) of pleural infection in children (8,36-40). S. pyogenes was another organism noted in up to 11% of paediatric pleural infections (39). As discussed above, S. aureus and viridans streptococci group (including SAG) are the most common aerobic cause of pleural infection in adults. They are followed by Pseudomonas spp., Enterobacteriaceae group and S. pneumoniae (26).
Tuberculous pleural effusion
The World Health Organization estimated that 9.6 million people worldwide suffered from tuberculosis (TB). The incidence of TB effusion varies largely among published series (typically from 5% to 25%), making TB one of the most common causes of pleural effusions in endemic areas (41). TB can cause different types of pleural effusions. The vast majority is a result of hypersensitivity reaction of the pleura to the mycobacterial protein and the actual mycobacterial load in the pleura is very low. It is believed that the mycobacteria may have been present in peripheral lung tissues and ruptured into the pleura inducing an effusion. Histological series from pre-antibiotics era suggested that the effusion will settle spontaneously even without treatment, but more than half the subjects will develop active TB afterwards. Hence treatment is important.
TB empyema is a different and rare entity and should not be confused with the much more common tuberculous pleural effusions. TB empyema is often characterized by bronchopleural fistula and resultant polymicrobial infection including TB and other bacteria (e.g., S. aureus). This complex infection results typically in a very thickened pleura (fibrothorax) and multiloculated pleural fluid that pose management challenges with high morbidity.
Other microorganisms causing pleural infection
Fungal pleural infection is uncommon, accounting for 1–3% of total cases worldwide, but is associated with higher mortality and morbidity (26,42). Candida spp., especially Candida albicans, are the most common causative fungi reported and typically seen in immunosuppressed patients (27,42). Aspergillus spp., can also cause pleural infection, particularly in lung transplant patients (42). Examples of other bacteria reported to cause pleural infection include Mycobacterium abscessus, Pasteurella multocida, non-typhoidal salmonella and Nocardia spp. (42).
Initial diagnostic evaluation
The diagnosis of pleural infection may be delayed as symptoms of pleural infection are not dissimilar to that of the underlying pneumonia. Persistent fevers or raised inflammatory markers in the context of a non-resolving pneumonia may aid the diagnosis; however in many cases, symptoms are more indolent, with fatigue and weight loss predominating, especially in the elderly or those with anaerobic infections (19,43). Better access to imaging modalities, e.g., bedside ultrasound and computed tomography (CT) scanning, have allowed earlier identification of pleural effusions.
Imaging
Chest radiography often provides the first identification of a parapneumonic effusion. When infected, the fluid frequently becomes loculated. Separating pleural fluid from underlying lung consolidation or atelectasis can be difficult. Pleural ultrasound is valuable in detecting pleural fluid with high sensitivity and in separating it from underlying consolidated lung or elevated hemidiaphragm. Pleural ultrasound can detect septations or loculations to guide drainage procedures (27,44,45) and is recommended by clinical guidelines for management of pleural effusions. Patients with septated effusions on ultrasonography have longer hospitalisation and more likely require fibrinolytic therapy or surgery (46). Contrast-enhanced thoracic CT can identify pleural fluid as well as provide valuable information on the underlying pneumonia (± abscess formation), and other potential abnormalities, e.g., subdiaphragmatic collections (Figures 1 and 2) (47). It also helps clarify alternative differentials such as malignancy.
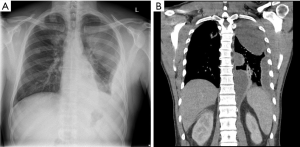
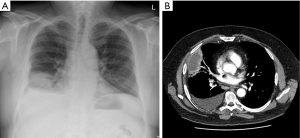
Pleural fluid sampling and analyses
Pleural fluid sampling is an important step in patients with respiratory tract infection and a pleural effusion both to establish a diagnosis and to guide management. Routine workup to establish evidence of systemic infection should include assessment of serum infection markers such as peripheral blood leucocytosis and CRP, as well as blood (and sputum if appropriate) cultures. Elevated serum procalcitonin, which is correlated with increased probability of bacterial infection, is not a reliable marker to rule-in or rule-out pleural infection. Dixon et al found no superior role of procalcitonin over CRP and leukocyte counts in the diagnosis of bacterial pleural infection in a study of 425 patients (48). None of these markers are specific in the diagnosis of pleural infection alone (48).
Presence of microbes from gram-stain and/or culture of pleural fluid defines pleural infection, but such investigations have a poor yield of <50% in most studies. The absence of microbes therefore does not exclude infection. The low culture yield is likely multifactorial. First, patients usually have received broad spectrum antibiotics prior to fluid sampling. Second, bacteria may become unviable from collection or transport issues before reaching the laboratory. Direct inoculation of blood culture bottles at bedside have been shown to increase yield in several series, particularly of anaerobic organisms. In one study use of blood culture bottle increased the proportional of patients with identifiable pathogens from 38% to 59% compared to standard culture alone (49). Third, pleural fluid may not be the most sensitive site for capturing bacteria. Murine experiments of S. pneumoniae pleuropulmonary infections showed abundance of bacteria in the pleural tissues (4,50). Ultrasound-guided pleural biopsy before chest drain insertion was performed in 20 patients with pleural infection in the AUDIO study and showed a yield of 45% (versus 20% with pleural fluid culture and 10% by blood culture) (50). Interestingly 75% of patients with positive pleural biopsy cultures had received antibiotics, suggesting the procedure is still worthwhile after commencement of antimicrobial therapy and testify to poor antibiotic penetration of pleural tissues.
Efforts to apply advanced technology to increase culture yield have not been particularly successful to date. Pleural fluid cell free DNA concentration has been shown to be higher in those with exudative over transudative effusions and concentrations are high in patients with parapneumonic effusion compared to those without. One study demonstrated that at the optimal cut-off value of 6,740 ng/mL, the test has a sensitivity of 87.5% and specificity of 80.6% for pleural infection (51). Current data are insufficient to support the use of pleural fluid cell free DNA for clinical use.
Polymerase chain reaction (PCR) to amplify and detect the 16S ribosomal RNA gene (which is present in all bacteria) from pleural fluid samples have shown confusing results (52,53). Maskell et al. found that in 34% of samples (107 of 316 patients), conventional cultures were positive but PCR was not (25). Another difficulty with micro-organisms identified with PCR is to determine if the microbe is a bystander or genuine causative agent. PCR, unlike cultures, will also detect non-viable microbes such as those already killed by antibiotics. Multiplex PCR, with results generally available within hours, is able to detect multiple pre-specified pathogens in a single nucleic acid experiment. This has been applied in a study to detect the more common pathogens associated with pneumonia and pleural infection (54). This approach risks missing organisms not included in the panel and thus should only be considered in addition to standard bacterial culture. For these reasons, PCR of pleural fluid remains experimental rather than routine practice.
The high false negative culture rate, and the need to await culture results make the use of surrogate markers for pleural infection necessary (25). Parapneumonic effusions should be exudates by Light’s criteria and cell counts should reveal a neutrophil predominance. Lymphocyte-rich fluid should raise concern of alternative diagnoses, including tuberculous pleuritis or non-infective causes. Measures of metabolic activities (e.g., acidic pH, low glucose and high LDH levels) in the fluid are the common surrogate markers used. These metabolic markers progress as fluid transit from simple to complicated parapneumonic phases. A CPE is often defined as presence of any of the followings: low pleural fluid pH (<7.20) or glucose concentration (<3.3 mmol/L), high pleural fluid LDH level (e.g., >1,000 IU/L) (2,5,27). An alkaline pleural fluid pH can occur with infection from urease-producing organisms, e.g., Proteus mirabilis, which hydrolyses urea to ammonia (55,56).
Accuracy of pleural fluid pH in this context is important, and is dependent on sample collection method, with most reliable results available when collected in a heparinised blood gas syringe. However residual air in collection tube will artificially raise the pH and carried over lignocaine (which is acidic) can reduce pH (57,58). Pleural fluid pH remains relatively stable at room temperature for up to around 4 hours, beyond which the values may not be trustworthy (57,58). Alternatively, a low pleural fluid glucose and high pleural fluid LDH level are useful in supporting the diagnosis of pleural infection. Variations in fluid appearances and biochemistry among different locules have been shown in a small series (59).
Other biomarkers, including pleural fluid procalcitonin and CRP have been investigated for their role in identifying pleural infections. None have been proven to be effective tests and are not recommended for routine use in this context. Pleural fluid procalcitonin has been found to have low sensitivity (62%) and specificity (71%) for differentiating parapneumonic effusion from other causes (60).
Novel pleural fluid diagnostic markers such as presepsin and soluble triggering receptor expressed in myeloid cells-1 (sTREM-1) have shown to be elevated in pleural infection. Presepsin is a protein which is released from the surface of various immune cells in response to pathogens and is reported to be increased specifically in the blood of patients with sepsis. A recent study has demonstrated that at a cut off of 754 pg/mL, presepsin had a diagnostic sensitivity of 90.9% and a specificity of 74.4% for diagnosing empyema (61).
The triggering receptor expressed in myeloid cells-1 (TREM-1) is shed by the membrane of activated phagocytes and is involved in the inflammatory response. A meta-analysis demonstrated sTREM-1 had a sensitivity of 78% and specificity was 84%. For the diagnosis of bacterial pleural effusion, however studies are lacking regarding whether it can differentiate between parapneumonic effusion and empyema (62).
More recently, pleural fluid soluble urokinase-type plasminogen activator receptor (suPAR) has been shown to be associated with loculated pleural effusions and may better predict the need for chest tube drainage, intrapleural fibrinolytic therapy or thoracic surgery compared with conventional pleural fluid biomarkers (63). This promising data however requires further prospective validation which should encompass usual serum and pleural fluid biomarkers to establish the full clinical utility of suPAR (for a detailed discussion, please see recent editorial by Idell and Lee) (64).
Chylous effusions
For macroscopically turbid effusions, differentiation of an empyema from a lipid effusion (chylothorax or pseudochylothorax) is important. After centrifugation of the turbid fluid, empyema typically shows a clear supernatant with dense sediment whereas lipid effusions will remain turbid (Figure 3). Empyema fluid are neutrophil predominant whereas lipid effusions are lymphocyte rich. Chylous effusions are often exudates but transudative chyloascites or chylothorax can occur as a result of concurrent other aetiologies, such as liver cirrhosis or cardiac failure.
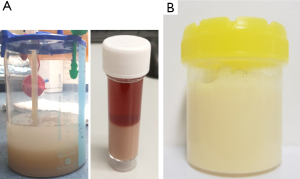
Presence of chylomicrons, defines a chylothorax. In their absence, high pleural fluid triglyceride content with a lower cholesterol level (<5.18 mmol/L) is useful. An effusion is definite/highly likely a chylothorax if pleural fluid triglyceride level exceeds 1.24 mmol/L and is suspicious of a chylothorax if triglyceride of 0.6 mmol/L. It is important to remember that the appearance of chylothorax may vary; and may not be turbid in a fasting or malnourished patient (65). Pseudochylothorax on the other hand has a high cholesterol content (66) and contain cholesterol crystals.
Tuberculous effusions
Investigations for tuberculous pleuritis should be initiated when clinically indicated. Pleural fluid is typically serous and contains very few viable mycobacteria, hence <10% will have acid fast bacilli (AFB) on fluid smear and mycobacterial cultures are positive in only a fraction (<50%) of cases (67). Nucleic acid amplification tests allow rapid detection of mycobacterium TB complex in a variety of tissue, however the sensitivity of the test on pleural fluid remains relatively low (68). Pleural tissue biopsy has a significantly higher likelihood of providing a diagnosis (69–97%) through finding AFB on microscopy, culture of the tissue (69) or, more commonly, the presence of caseating granuloma on histological examination. A randomized trial of thoracoscopic versus percutaneous blind pleural biopsies found a yield of 100% vs. 79% respectively, suggesting that the latter is a reasonable bedside test and, if it fails to provide an answer, thoracoscopy should be considered (70).
Many surrogate pleural fluid biochemical tests have been tried to increase yield of tuberculous pleuritis in a minimally invasive manner. Adenosine deaminase (ADA) is an enzyme of the lymphocytes and its activity is increased during tuberculous pleuritis. Pleural fluid ADA levels are therefore raised in practically all tuberculous effusions and with a threshold of 40±4 IU/L, it has a sensitivity of 93% and specificity of 90% in a recent meta-analysis (71,72). An elevated ADA is used in some endemic regions as a confirmatory test, in the clinically setting of a patient with lymphocytic pleural effusion and compatible history and chest imaging for TB. ADA is cheap and relatively easy to perform with a fast turnaround time and can be reliably used even in stored samples (73). ADA has been shown to be valid in immunocompromised hosts and paediatric populations (74,75). However, it must be recognized that elevated pleural fluid ADA level also occurs commonly with other (e.g., bacterial) pleural infections, rheumatological disorders and, occasionally, malignant pleural effusions. Diagnosis relying purely on pleural fluid ADA also does not provide information on mycobacterial resistance. Therefore, ADA is best used as a supportive rather than definitive diagnostic test. Restricting testing of ADA to only lymphocytic effusions help to reduce false negative diagnosis from bacterial CPE/empyema (which are neutrophilic). Testing for ADA subsets also serves that purpose but is expensive and not easily available. In non-endemic regions, negative pleural fluid ADA is a very useful rule-out test for TB and other causes for a lymphocytic effusion should be sought (76).
Interferon gamma levels in pleural fluid is raised in tuberculous pleural effusions and many studies have confirmed a similar diagnostic performance (77,78). However, interferon gamma essays are significantly more expensive than ADA and the latter is preferred especially in resource limited regions. It must be emphasized that interferon gamma releasing assays however have no role in the diagnosis of tuberculous effusions (76). Promising early data have found IL-27 as another potential surrogate marker though larger studies are needed (68).
TB empyema has a very different presentation. Imaging will show features of thickened pleura and multiloculated fluid that is difficult to evacuate and is purulent. Culture of TB empyema has a significantly higher yield of mycobacteria as well as other bacteria such as S. aureus.
Treatment of pleural infection
The mainstay of treatment for pleural infection is antimicrobial therapy and drainage of pleural fluid as outlined below. Detailed discussion is outside the scope of this review and can be found elsewhere (27,79).
Intravenous antibiotics
Antibiotics are often used empirically given the low yield of cultures. International guidelines recommend the use of penicillins combined with β-lactamase inhibitors, Metronidazole or Cephalosporins for empiric treatment of pleural infection (27). Clindamycin or Meropenem provide a reasonable alternative and methicillin resistant S. aureus (MRSA) cover should be included if clinically appropriate (79,80). It should be emphasized that very limited information exists on pleural pharmacokinetics of commonly used antibiotics after their systemic delivery. Standard practice is largely based on clinicians’ ‘best guess’.
Dexamethasone hastened clinical recovery in a small randomized controlled trial (RCT) of paediatric parapneumonic effusions (81). Its benefits in adult patients are being examined in a pilot RCT (82).
Fluid evacuation
Chest tube drainage is usually needed for CPE and empyema. A delay to drainage of >2 days correlated with increased 90-day mortality in a large Danish study (83). Imaging-guided drain insertion is valuable especially in loculated effusions. Drain size (larger or smaller than 15 Fr) did not affect outcome in a large but non-randomized observational series (84). Multiple tubes may be required to evacuate non-communicating locules. About 20% of patients failed to improve with antibiotics and chest tube drainage in large RCTs, and necessitated additional interventions to aid fluid removal (19,20).
Combined intrapleural tissue plasminogen activator (tPA) and deoxyribonuclease (DNAse) therapy has revolutionized clinical practice since a landmark RCT and subsequent large open-labelled series demonstrated its efficacy in improving fluid output, radiographic clearance and length of hospitalization, leaving <10% of patients needing surgical referral. It is hypothesized that tPA breaks pleural loculations and DNase reduces fluid viscosity. Fibrinolytics also induces significant pleural fluid formation, via a monocyte chemotactic protein-1 (MCP-1) pathway, which may provide a lavage of the infected pleural cavity (85,86). Complications of tPA/DNase therapy are acceptable (87). Pleural bleeding requiring blood transfusion occur in ~3% of patients and no systemic or fatal bleeding have been reported in most series. Dose de-escalation studies are underway to establish the lowest effective dose of tPA (88,89).
Video-assisted thoracoscopic surgery (VATS) with decortication is needed if the above measures fail (27), although the optimal timing of surgery remains debated (90). Post-operative complications including pain, persistent pleural and/or wound infection, bleeding and prolonged air leak (91-93).
Future directions
Two millennia since Hippocrates’ work, pleural infection remains a major illness. Our understanding of how and why some patients with pneumonia develop pleural complications is critical to designing strategies to avoid secondary pleural infections. The parapneumonic fluid encompasses a range of presentations from a simple effusion to complex multiloculated collections. Individualized therapy based on fluid biochemical activities has been postulated.
The RAPID (Renal, Age, Purulence of fluid, Infection source, Dietary factors) score is well validated and permits clinical risk stratification. Increasing age, elevated urea, low albumin, presence of hospital-acquired infection, and non-purulence fluid were associated with poorer outcomes including mortality and increased length of hospital stay (23). Mortality of pleural infection at 3 or 6 months are high, even after the acute infection has resolved. It is increasingly realised that pleural infection reflects advancing age and comorbidity—which are the most predictive risks of mortality in the RAPID score. Studies are urgently needed to establish the cause of death so that targeted prevention can be attempted.
Antibiotics are key to treatment but their pleural penetration has largely unknown, especially in patients with pleural thickening/loculations. Intrapleural antibiotics delivery has attracted much clinical interests but has not been formally studied.
The majority of the literature on pleural infection focussed on community acquired diseases, whereas the latest epidemiological data suggested that hospital acquired cases are several-fold more common and are associated with significantly worse outcome. This ‘untapped’ area requires urgent attention.
Acknowledgments
The authors would also like to thank Dr. Ken Chan, Prince of Wales Hospital Hong Kong for contributing to Figure 3A.
Funding: YCGL is an Australian Medical Research Future Fund Practitioner Fellow and receives grant funding from the NHMRC, iCARE Dust Disease Board, and Sir Charles Gairdner Research Advisory Committee.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Zhi-De Hu) for the series “Pleural Effusion Analysis” published in the Journal of Laboratory and Precision Medicine. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form, available at http://dx.doi.org/10.21037/jlpm-2021-01. The series “Pleural Effusion Analysis” was commissioned by the editorial office without any funding or sponsorship. YCGL is an honorary adviser for Lung Therapeutics Inc. All authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Light RW, Lee YCG. Textbook of Pleural Diseases. 3rd Edition. CRC Press; 2016.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007;45:1480-6. [Crossref] [PubMed]
- Wilkosz S, Edwards LA, Bielsa S, et al. Characterization of a new mouse model of empyema and the mechanisms of pleural invasion by Streptococcus pneumoniae. Am J Respir Cell Mol Biol 2012;46:180-7. [Crossref] [PubMed]
- Light RW, Girard WM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med 1980;69:507-12. [Crossref] [PubMed]
- Tucker T, Komissarov A, Florova G, et al. Pleural fibrosis. In: Light RW, Lee YCG, editors. Textbook of Pleural Diseases. 3rd Edition. USA: CRC Press; 2016:67-79.
- Tu CY, Chen CH. Spontaneous bacterial empyema. Curr Opin Pulm Med 2012;18:355-8. [Crossref] [PubMed]
- Lisboa T, Waterer GW, Lee YC. Pleural infection: changing bacteriology and its implications. Respirology 2011;16:598-603. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Murray MP, et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009;64:592-7. [Crossref] [PubMed]
- Bedawi EO, Rahman NM. Pleural infection: moving from treatment to prevention. In: Maskell NA, Laursen CB, Lee YCG, et al. editors. Pleural Disease (ERS Monograph). Sheffield: European Respiratory Society; 2020:155-71.
- Søgaard M, Nielsen RB, Nørgaard M, et al. Incidence, length of stay, and prognosis of hospitalized patients with pleural empyema: a 15-year Danish nationwide cohort study. Chest 2014;145:189-92. [Crossref] [PubMed]
- Shen HN, Lu CL, Li CY. Epidemiology of pleural infections in Taiwan from 1997 through 2008. Respirology 2012;17:1086-93. [Crossref] [PubMed]
- Finley C, Clifton J, Fitzgerald JM, et al. Empyema: an increasing concern in Canada. Can Respir J 2008;15:85-9. [Crossref] [PubMed]
- Cargill TN, Hassan M, Corcoran JP, et al. A systematic review of comorbidities and outcomes of adult patients with pleural infection. Eur Respir J 2019;54:1900541 [Crossref] [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population-based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [Crossref] [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [Crossref] [PubMed]
- Muñoz-Almagro C, Jordan I, Gene A, et al. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis 2008;46:174-82. [Crossref] [PubMed]
- Dean NC, Griffith PP, Sorensen JS, et al. Pleural effusions at first ED encounter predict worse clinical outcomes in patients with pneumonia. Chest 2016;149:1509-15. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Brims F, Popowicz N, Rosenstengel A, et al. Bacteriology and clinical outcomes of patients with culture-positive pleural infection in Western Australia: A 6-year analysis. Respirology 2019;24:171-8. [Crossref] [PubMed]
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia?. Arch Intern Med 1996;156:2206-12. [Crossref] [PubMed]
- Corcoran JP, Psallidas I, Gerry S, et al. Prospective validation of the RAPID clinical risk prediction score in adult patients with pleural infection: the PILOT study. Eur Respir J 2020;56:2000130 [Crossref] [PubMed]
- McCauley L, Dean N. Pneumonia and empyema: causal, casual or unknown. J Thorac Dis 2015;7:992-8. [PubMed]
- Maskell NA, Batt S, Hedley EL, et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 2006;174:817-23. [Crossref] [PubMed]
- Hassan M, Cargill T, Harriss E, et al. The microbiology of pleural infection in adults: a systematic review. Eur Respir J 2019;54:1900542 [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Bedawi EO, Hassan M, McCracken D, et al. Pleural infection: a closer look at the etiopathogenesis, microbiology and role of antibiotics. Expert Rev Respir Med 2019;13:337-47. [Crossref] [PubMed]
- Bedawi EO, Hassan M, Rahman NM. Recent developments in the management of pleural infection: a comprehensive review. Clin Respir J 2018;12:2309-20. [Crossref] [PubMed]
- Meyer CN, Rosenlund S, Nielsen J, et al. Bacteriological aetiology and antimicrobial treatment of pleural empyema. Scand J Infect Dis 2011;43:165-9. [Crossref] [PubMed]
- Jiang S, Li M, Fu T, et al. Clinical characteristics of infections caused by Streptococcus anginosus group. Sci Rep 2020;10:9032. [Crossref] [PubMed]
- Laupland KB, Ross T, Church DL, et al. Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin Microbiol Infect 2006;12:224-30. [Crossref] [PubMed]
- Asam D, Spellerberg B. Molecular pathogenicity of Streptococcus anginosus. Mol Oral Microbiol 2014;29:145-55. [Crossref] [PubMed]
- Park CK, Oh HJ, Choi HY, et al. Microbiological characteristics and predictive factors for mortality in pleural infection: a single-center cohort study in Korea. PLoS One 2016;11:e0161280 [Crossref] [PubMed]
- Lin YT, Chen TL, Siu LK, et al. Clinical and microbiological characteristics of community-acquired thoracic empyema or complicated parapneumonic effusion caused by Klebsiella pneumoniae in Taiwan. Eur J Clin Microbiol Infect Dis 2010;29:1003-10. [Crossref] [PubMed]
- Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax 2004;59:522-5. [Crossref] [PubMed]
- Langley JM, Kellner JD, Solomon N, et al. Empyema associated with community-acquired pneumonia: a Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis 2008;8:129. [Crossref] [PubMed]
- Hernández-Bou S, García-García JJ, Esteva C, et al. Pediatric parapneumonic pleural effusion: epidemiology, clinical characteristics, and microbiological diagnosis. Pediatr Pulmonol 2009;44:1192-200. [Crossref] [PubMed]
- Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J 2011;30:289-94. [Crossref] [PubMed]
- Amin M, Yousef Pour S, Navidifar T. Detection of the major bacterial pathogens among children suffering from empyema in Ahvaz city, Iran. J Clin Lab Anal 2019;33:e22855 [Crossref] [PubMed]
- Zhai K, Lu Y, Shi HZ. Tuberculous pleural effusion. J Thorac Dis 2016;8:E486-94. [Crossref] [PubMed]
- Corcoran JP, Wrightson JM, Belcher E, et al. Pleural infection: past, present, and future directions. Lancet Respir Med 2015;3:563-77. [Crossref] [PubMed]
- Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993;16:S248-55. [Crossref] [PubMed]
- Marchetti G, Arondi S, Baglivo F, et al. New insights in the use of pleural ultrasonography for diagnosis and treatment of pleural disease. Clin Respir J 2018;12:1993-2005. [Crossref] [PubMed]
- Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest 2013;143:532-8. [Crossref] [PubMed]
- Chen KY, Liaw YS, Wang HC, et al. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med 2000;19:837-43. [Crossref] [PubMed]
- Rosenstengel A, Lee YCG. Pleural infection-current diagnosis and management. J Thorac Dis 2012;4:186-93. [PubMed]
- Dixon G, Lama-Lopez A, Bintcliffe OJ, et al. The role of serum procalcitonin in establishing the diagnosis and prognosis of pleural infection. Respir Res 2017;18:30. [Crossref] [PubMed]
- Menzies SM, Rahman NM, Wrightson JM, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax 2011;66:658-62. [Crossref] [PubMed]
- Psallidas I, Kanellakis NI, Bhatnagar R, et al. A pilot feasibility study in establishing the role of ultrasound-guided pleural biopsies in pleural infection (The AUDIO Study). Chest 2018;154:766-72. [Crossref] [PubMed]
- Santotoribio JD, Cabrera-Alarcon JL, Batalha-Caetano P, et al. Pleural fluid cell-free DNA in parapneumonic pleural effusion. Clin Biochem 2015;48:1003-5. [Crossref] [PubMed]
- Insa R, Marín M, Martín A, et al. Systematic use of universal 16S rRNA gene polymerase chain reaction (PCR) and sequencing for processing pleural effusions improves conventional culture techniques. Medicine (Baltimore) 2012;91:103-10. [Crossref] [PubMed]
- Saglani S, Harris KA, Wallis C, et al. Empyema: the use of broad range 16S rDNA PCR for pathogen detection. Arch Dis Child 2005;90:70-3. [Crossref] [PubMed]
- Franchetti L, Schumann DM, Tamm M, et al. Multiplex bacterial polymerase chain reaction in a cohort of patients with pleural effusion. BMC Infect Dis 2020;20:99. [Crossref] [PubMed]
- Pine JR, Hollman JL. Elevated pleural fluid pH in Proteus mirabilis empyema. Chest 1983;84:109-11. [Crossref] [PubMed]
- Isenstein D, Honig E. Proteus vulgaris empyema and increased pleural fluid pH. Chest 1990;97:511. [Crossref] [PubMed]
- Rahman NM, Mishra EK, Davies HE, et al. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Respir Crit Care Med 2008;178:483-90. [Crossref] [PubMed]
- Bowling M, Lenz P, Chatterjee A, et al. Perception versus reality: the measuring of pleural fluid pH in the United States. Respiration 2012;83:316-22. [Crossref] [PubMed]
- Maskell NA, Gleeson FV, Darby M, et al. Diagnostically significant variations in pleural fluid pH in loculated parapneumonic effusions. Chest 2004;126:2022-4. [Crossref] [PubMed]
- He C, Wang B, Li D, et al. Performance of procalcitonin in diagnosing parapneumonic pleural effusions: A clinical study and meta-analysis. Medicine (Baltimore) 2017;96:e7829 [Crossref] [PubMed]
- Watanabe N, Ishii T, Kita N, et al. The usefulness of pleural fluid presepsin, C-reactive protein, and procalcitonin in distinguishing different causes of pleural effusions. BMC Pulm Med 2018;18:176. [Crossref] [PubMed]
- Summah H, Tao LL, Zhu YG, et al. Pleural fluid soluble triggering receptor expressed on myeloid cells-1 as a marker of bacterial infection: a meta-analysis. BMC Infect Dis 2011;11:280. [Crossref] [PubMed]
- Arnold DT, Hamilton FW, Elvers KT, et al. Pleural fluid suPAR levels predict the need for invasive management in parapneumonic effusions. Am J Respir Crit Care Med 2020;201:1545-53. [Crossref] [PubMed]
- Idell S, Lee YCG. suPAR surprises as a biomarker of invasive outcomes in pleural infection. Am J Respir Crit Care Med 2020;201:1470-2. [Crossref] [PubMed]
- Maldonado F, Hawkins FJ, Daniels CE, et al. Pleural fluid characteristics of chylothorax. Mayo Clin Proc 2009;84:129-33. [Crossref] [PubMed]
- Huggins JT. Chylothorax and cholesterol pleural effusion. Semin Respir Crit Care Med 2010;31:743-50. [Crossref] [PubMed]
- Vorster MJ, Allwood BW, Diacon AH, et al. Tuberculous pleural effusions: advances and controversies. J Thorac Dis 2015;7:981-91. [PubMed]
- Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med 2016;22:367-77. [Crossref] [PubMed]
- Shaw JA, Ahmed L, Koegelenberg CFN. Effusions related to TB. In: Maskell NA, Laursen CB, Lee YCG et al., editors. Pleural Disease (ERS Monograph). Sheffield: European Respiratory Society; 2020:172-92.
- Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003;22:589-91. [Crossref] [PubMed]
- Valdés L, San José E, Alvarez D, et al. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 1996;9:747-51. [Crossref] [PubMed]
- Aggarwal AN, Agarwal R, Sehgal IS, et al. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One 2019;14:e0213728 [Crossref] [PubMed]
- Lee YC, Rogers JT, Rodriguez RM, et al. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest 2001;120:356-61. [Crossref] [PubMed]
- Baba K, Hoosen AA, Langeland N, et al. Adenosine deaminase activity is a sensitive marker for the diagnosis of tuberculous pleuritis in patients with very low CD4 counts. PLoS One 2008;3:e2788 [Crossref] [PubMed]
- Merino JM, Carpintero I, Alvarez T, et al. Tuberculous pleural effusion in children. Chest 1999;115:26-30. [Crossref] [PubMed]
- Hooper CE, Lee YC, Maskell NA. Interferon-gamma release assays for the diagnosis of TB pleural effusions: hype or real hope? Curr Opin Pulm Med 2009;15:358-65. [Crossref] [PubMed]
- Meldau R, Peter J, Theron G, et al. Comparison of same day diagnostic tools including Gene Xpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med 2014;14:58. [Crossref] [PubMed]
- Jiang J, Shi HZ, Liang QL, et al. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest 2007;131:1133-41. [Crossref] [PubMed]
- Teixeira LR, Sasse SA, Villarino MA, et al. Antibiotic levels in empyemic pleural fluid. Chest 2000;117:1734-9. [Crossref] [PubMed]
- Niwa T, Nakamura A, Kato T, et al. Pharmacokinetic study of pleural fluid penetration of carbapenem antibiotic agents in chemical pleurisy. Respir Med 2006;100:324-31. [Crossref] [PubMed]
- Tagarro A, Otheo E, Baquero-Artigao F, et al. Dexamethasone for parapneumonic pleural effusion: a randomized, double-blind, clinical trial. J Pediatr 2017;185:117-23.e6. [Crossref] [PubMed]
- Fitzgerald DB, Waterer GW, Read CA, et al. Steroid therapy and outcome of parapneumonic pleural effusions (STOPPE): Study protocol for a multicenter, double-blinded, placebo-controlled randomized clinical trial. Medicine (Baltimore) 2019;98:e17397 [Crossref] [PubMed]
- Meyer CN, Armbruster K, Kemp M, et al. Pleural infection: a retrospective study of clinical outcome and the correlation to known etiology, co-morbidity and treatment factors. BMC Pulm Med 2018;18:160. [Crossref] [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [Crossref] [PubMed]
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014;11:1419-25. [Crossref] [PubMed]
- Kanellakis NI, Wrightson JM, Hallifax R, et al. Biological effect of tissue plasminogen activator (t-PA) and DNase intrapleural delivery in pleural infection patients. BMJ Open Respir Res 2019;6:e000440 [Crossref] [PubMed]
- Roy B, Teh MC, Kuok YJ, et al. Bronchopleural communication following intrapleural doses of tPA/DNase for empyema. Respirol Case Rep 2020;8:e00646 [Crossref] [PubMed]
- Popowicz N, Bintcliffe O, De Fonseka D, et al. Dose de-escalation of intrapleural tissue plasminogen activator therapy for pleural infection. The alteplase dose assessment for pleural infection therapy project. Ann Am Thorac Soc 2017;14:929-36. [Crossref] [PubMed]
- McClune JR, Wilshire CL, Gorden JA, et al. Safety and efficacy of intrapleural tissue plasminogen activator and DNase during extended use in complicated pleural space infections. Can Respir J 2016;2016:9796768 [Crossref] [PubMed]
- Schweigert M, Solymosi N, Dubecz A, et al. Surgical management of pleural empyema in the very elderly. Ann R Coll Surg Engl 2012;94:331-5. [Crossref] [PubMed]
- Divisi D, Gabriele F, Barone M, et al. Clinical history and surgical management of parapneumonic empyema what is the role of video-assisted thoracoscopic surgery (VATS)? Video-assist Thorac Surg 2017;2:65. [Crossref]
- Jagelavicius Z, Jovaisas V, Mataciunas M, et al. Preoperative predictors of conversion in thoracoscopic surgery for pleural empyema. Eur J Cardiothorac Surg 2017;52:70-5. [Crossref] [PubMed]
- Lardinois D, Gock M, Pezzetta E, et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6. [Crossref] [PubMed]
Cite this article as: Roy B, Shak HJ, Lee YCG. Pleural fluid investigations for pleural infections. J Lab Precis Med 2021;6:12.


